Evaluate of Wheat Gluten as a Protein Alternative for Fish Meal and Soy Protein Concentrate in Red Spotted Grouper Epinephelus akaara
Abstract
:1. Introduction
 × E. lanceolatus
× E. lanceolatus  [8,9,10]. However, the price of SPC often fluctuates owing to the unstable price of soybeans; thus, finding other plant protein sources is still necessary.
[8,9,10]. However, the price of SPC often fluctuates owing to the unstable price of soybeans; thus, finding other plant protein sources is still necessary.2. Materials and Methods
2.1. Ingredients and Diets
2.2. Fish Management
2.3. Sampling
2.4. Analysis
2.5. Calculation and Statistical Analysis
3. Results
3.1. Growth Performance, Feed Utilization and Morphologic Indexes
3.2. Body Compositions and Nutrient Retentions
3.3. Serum and Liver Physiological and Antioxidant Parameters
4. Discussion
 × E. fuscoguttatus
× E. fuscoguttatus  ) [45], but higher than 72.2 g kg−1 diet (incorporation with a cellulose content of ~250 g kg−1 diet) and 246 g kg−1 diet reported in E. akaara [19] and E. malabaricus [46] respectively. In this study, higher dietary starch decreased growth performance and feed utilisation in red sported grouper, which was consistent with previous studies on Nile tilapia Oreochromis niloticus [47], gibel carp Carassius auratus var. gibelio [48], grass carp Ctenopharyngodon idella [49], hybrid grouper E. fuscoguttatus
) [45], but higher than 72.2 g kg−1 diet (incorporation with a cellulose content of ~250 g kg−1 diet) and 246 g kg−1 diet reported in E. akaara [19] and E. malabaricus [46] respectively. In this study, higher dietary starch decreased growth performance and feed utilisation in red sported grouper, which was consistent with previous studies on Nile tilapia Oreochromis niloticus [47], gibel carp Carassius auratus var. gibelio [48], grass carp Ctenopharyngodon idella [49], hybrid grouper E. fuscoguttatus  × E. lanceolatus
× E. lanceolatus  [50] and largemouth bass Micropterus salmoides [51].
[50] and largemouth bass Micropterus salmoides [51].5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gatlin, D.M.; Barrows, F.T.; Brown, P.; Dabrowski, K.; Gaylord, T.G.; Hardy, R.W.; Herman, E.; Hu, G.; Krogdahl, Å.; Nelson, R.; et al. Expanding the utilization of sustainable plant products in aquafeeds: A review. Aquac. Res. 2007, 38, 551–579. [Google Scholar] [CrossRef]
- Anderson, R.L.; Wolf, W.J. Compositional changes in trypsin inhibitors, phytic acid, saponins and isoflavones related to soybean processing. J. Nutr. 1995, 125, 581–588. [Google Scholar]
- Kaushik, S.J.; Cravedi, J.P.; Lalles, J.P.; Sumpter, J.; Fauconneau, B.; Laroche, M. Partial or total replacement of fish meal by soybean protein on growth, protein utilization, potential estrogenic or antigenic effects, cholesterolemia and flesh quality in rainbow trout, Oncorhynchus mykiss. Aquaculture 1995, 133, 257–274. [Google Scholar] [CrossRef]
- Deng, J.; Mai, K.; Ai, Q.; Zhang, W.; Wang, X.; Xu, W.; Liufu, Z. Effects of replacing fish meal with soy protein concentrate on feed intake and growth of juvenile Japanese flounder, Paralichthys olivaceus. Aquaculture 2006, 258, 503–513. [Google Scholar] [CrossRef]
- Biswas, A.; Araki, H.; Sakata, T.; Nakamori, T.; Kato, K.; Takii, K. Fish meal replacement by soy protein from soymilk in the diets of red sea bream (Pagrus major). Aquac. Nutr. 2017, 23, 1379–1389. [Google Scholar] [CrossRef]
- Biswas, A.; Araki, H.; Sakata, T.; Nakamori, T.; Takii, K. Optimum fish meal replacement by soy protein concentrate from soymilk and phytase supplementation in diet of red sea bream, Pagrus major. Aquaculture 2019, 506, 51–59. [Google Scholar] [CrossRef]
- Tola, S.; Fukada, H.; Masumoto, T. Effects of feeding a fish meal-free soy protein concentrate-based diet on the growth performance and nutrient utilization of red sea bream (Pagrus major). Aquac. Res. 2019, 50, 1087–1095. [Google Scholar] [CrossRef]
- Mohd Faudzi, N.; Yong, A.S.K.; Shapawi, R.; Senoo, S.; Biswas, A.; Takii, K. Soy protein concentrate as an alternative in replacement of fish meal in the feeds of hybrid grouper, brown-marbled grouper (Epinephelus fuscoguttatus) × giant grouper (E. lanceolatus) juvenile. Aquac. Res. 2018, 49, 431–441. [Google Scholar] [CrossRef]
- Chen, Y.; Liu, W.; Ma, J.; Wang, Y.; Huang, H. Comprehensive physiological and transcriptomic analysis revealing the responses of hybrid grouper (Epinephelus fuscoguttatus♀ × E. lanceolatus♂) to the replacement of fish meal with soy protein concentrate. Fish Physiol. Biochem. 2020, 46, 2037–2053. [Google Scholar] [CrossRef]
- Wang, J.; Liang, D.; Yang, Q.; Tan, B.; Dong, X.; Chi, S.; Liu, H.; Zhang, S. The effect of partial replacement of fish meal by soy protein concentrate on growth performance, immune responses, gut morphology and intestinal inflammation for juvenile hybrid grouper (Epinephelus fuscoguttatus ♀ × Epinephelus lanceolatus ♂). Fish Shellfish Immunol. 2020, 98, 619–631. [Google Scholar] [CrossRef]
- Apper-Bossard, E.; Feneuil, A.; Wagner, A.; Respondek, F. Use of vital wheat gluten in aquaculture feeds. Aquat. Biosyst. 2013, 9, 21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pfeffer, E.; Al-Sabty, H.; Haverkamp, R. Studies on lysine requirements of rainbow trout (Oncorhynchus mykiss) fed wheat gluten as only source of dietary protein. J. Anim. Physiol. Anim. Nutr. 1992, 67, 74–82. [Google Scholar] [CrossRef]
- Davies, S.J.; Morris, P.C.; Baker, R.T.M. Partial substitution of fish meal and full-fat soya bean meal with wheat gluten and influence of lysine supplementation in diets for rainbow trout, Oncorhynchus mykiss (Walbaum). Aquac. Res. 1997, 28, 317–328. [Google Scholar] [CrossRef]
- Helland, S.J.; Grisdale-Helland, B. Replacement of fish meal with wheat gluten in diets for Atlantic halibut (Hippoglossus hippoglossus): Effect on whole-body amino acid concentrations. Aquaculture 2006, 261, 1363–1370. [Google Scholar] [CrossRef]
- Messina, M.; Piccolo, G.; Tulli, F.; Messina, C.M.; Cardinaletti, G.; Tibaldi, E. Lipid composition and metabolism of European sea bass (Dicentrarchus labrax L.) fed diets containing wheat gluten and legume meals as substitutes for fish meal. Aquaculture 2013, 376–379, 6–14. [Google Scholar] [CrossRef]
- Wang, P.; Lou, Y.; Feng, J.; He, J.; Zhu, J.; Zhou, Q. Effect of replacing fish meal with wheat gluten meal on growth, serum biochemical indexes and antioxidant enzyme activity of juvenile large yellow croaker (Larimichthys crocea). J. Fish. China 2018, 42, 733–743. [Google Scholar]
- Jiang, Y.D.; Wang, J.T.; Han, T.; Li, X.Y.; Hu, S.X. Effect of dietary lipid level on growth performance, feed utilization and body composition by juvenile red spotted grouper (Epinephelus akaara). Aquacult. Int. 2015, 23, 99–110. [Google Scholar] [CrossRef]
- Wang, J.; Jiang, Y.; Li, X.; Han, T.; Yang, Y.; Hu, S.; Yang, M. Dietary protein requirement of juvenile red spotted grouper (Epinephelus akaara). Aquaculture 2016, 450, 289–294. [Google Scholar] [CrossRef]
- Wang, J.; Li, X.; Han, T.; Yang, Y.; Jiang, Y.; Yang, M.; Xu, Y.; Harpaz, S. Effects of different dietary carbohydrate levels on growth, feed utilization and body composition of juvenile grouper Epinephelus akaara. Aquaculture 2016, 459, 143–147. [Google Scholar] [CrossRef]
- Luo, Z.; Liu, Y.J.; Mai, K.S.; Tian, L.X.; Liu, D.H.; Tan, X.Y. Optimal dietary protein requirement of grouper Epinephelus coioides juveniles fed isoenergetic diets in floating net cages. Aquac. Nutr. 2004, 10, 247–252. [Google Scholar] [CrossRef]
- Yan, X.; Yang, J.; Dong, X.; Tan, B.; Zhang, S.; Chi, S.; Yang, Q.; Liu, H.; Yang, Y. Optimum protein requirement of juvenile orange-spotted grouper (Epinephelus coioides). Sci. Rep. 2021, 11, 6230. [Google Scholar] [CrossRef]
- Yan, X.; Yang, J.; Dong, X.; Tan, B.; Zhang, S.; Chi, S.; Yang, Q.; Liu, H.; Yang, Y. The protein requirement of grouper Epinephelus coioides at grow-out stage. Aquac. Nutr. 2020, 26, 1555–1567. [Google Scholar] [CrossRef]
- Wang, Y.R.; Wang, L.; Zhang, C.X.; Song, K. Effects of substituting fishmeal with soybean meal on growth performance and intestinal morphology in orange-spotted grouper (Epinephelus coioides). Aquac. Rep. 2017, 5, 52–57. [Google Scholar] [CrossRef]
- Millamena, O.M. Replacement of fish meal by animal by-product meals in a practical diet for grow-out culture of grouper Epinephelus coioides. Aquaculture 2002, 204, 75–84. [Google Scholar] [CrossRef]
- Chen, G.; Yin, B.; Liu, H.; Tan, B.; Dong, X.; Yang, Q.; Chi, S.; Zhang, S. Effects of fishmeal replacement with cottonseed protein concentrate on growth, digestive proteinase, intestinal morphology and microflora in pearl gentian grouper (♀ Epinephelus fuscoguttatus × ♂ Epinephelus lanceolatu). Aquac. Res. 2020, 51, 2870–2884. [Google Scholar] [CrossRef]
- Huang, B.; Zhang, S.; Dong, X.; Chi, S.; Yang, Q.; Liu, H.; Tan, B.; Xie, S. Effects of fishmeal replacement by black soldier fly on growth performance, digestive enzyme activity, intestine morphology, intestinal flora and immune response of pearl gentian grouper (Epinephelus fuscoguttatus ♀ × Epinephelus lanceolatus ♂). Fish Shellfish Immunol. 2022, 120, 497–506. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Ma, J.; Huang, H.; Zhong, H. Effects of the replacement of fishmeal by soy protein concentrate on growth performance, apparent digestibility, and retention of protein and amino acid in juvenile pearl gentian grouper. PLoS ONE 2019, 14, e0222780. [Google Scholar] [CrossRef] [Green Version]
- Song, S.G.; Chi, S.Y.; Tan, B.P.; Liang, G.L.; Lu, B.Q.; Dong, X.H.; Liu, H.; Zhang, S. Effects of fishmeal replacement by Tenebrio molitor meal on growth performance, antioxidant enzyme activities and disease resistance of the juvenile pearl gentian grouper (Epinephelus lanceolatus ♂ × Epinephelus fuscoguttatus ♀). Aquac. Res. 2018, 49, 2210–2217. [Google Scholar] [CrossRef]
- Zhang, Y.; Guo, M.; Li, N.; Dong, Z.; Cai, L.; Wu, B.; Xie, J.; Liu, L.; Ren, L.; Shi, B. New insights into β-glucan-enhanced immunity in largemouth bass Micropterus salmoides by transcriptome and intestinal microbial composition. Front. Immunol. 2022, 13, 108103. [Google Scholar] [CrossRef]
- Lin, Y.H.; Lu, R.M. Dietary taurine supplementation enhances growth and nutrient digestibility in giant grouper Epinephelus lanceolatus fed a diet with soybean meal. Aquac. Rep. 2020, 18, 100464. [Google Scholar] [CrossRef]
- Park, G.S.; Takeuchi, T.; Yokoyama, M.; Seikai, T. Optimal dietary taurine level for growth of juvenile Japanese flounder Paralichthys olivaceus. Fish. Sci. 2002, 68, 824–829. [Google Scholar] [CrossRef] [Green Version]
- Lunger, A.N.; McLean, E.; Gaylord, T.G.; Kuhn, D.; Craig, S.R. Taurine supplementation to alternative dietary proteins used in fish meal replacement enhances growth of juvenile cobia (Rachycentron canadum). Aquaculture 2007, 271, 401–410. [Google Scholar] [CrossRef]
- Jirsa, D.; Davis, D.A.; Salze, G.P.; Rhodes, M.; Drawbridge, M. Taurine requirement for juvenile white seabass (Atractoscion nobilis) fed soy-based diets. Aquaculture 2014, 422–423, 36–41. [Google Scholar] [CrossRef]
- Johnson, R.B.; Kim, S.K.; Watson, A.M.; Barrows, F.T.; Kroeger, E.L.; Nicklason, P.M.; Goetz, G.W.; Place, A.R. Effects of dietary taurine supplementation on growth, feed efficiency, and nutrient composition of juvenile sablefish (Anoplopoma fimbria) fed plant based feeds. Aquaculture 2015, 445, 79–85. [Google Scholar] [CrossRef]
- de Moura, L.B.; Diógenes, A.F.; Campelo, D.A.V.; de Almeida, F.L.A.; Pousão-Ferreira, P.M.; Furuya, W.M.; Oliva-Teles, A.; Peres, H. Taurine and methionine supplementation as a nutritional strategy for growth promotion of meagre (Argyrosomus regius) fed high plant protein diets. Aquaculture 2018, 497, 389–395. [Google Scholar] [CrossRef]
- Iwashita, Y.; Suzuki, N.; Yamamoto, T.; Shibata, J.I.; Isokawa, K.; Soon, A.H.; Ikehata, Y.; Furuita, H.; Sugita, T.; Goto, T. Supplemental effect of cholyltaurine and soybean lecithin to a soybean meal-based fish meal-free diet on hepatic and intestinal morphology of rainbow trout Oncorhynchus mykiss. Fish. Sci. 2008, 74, 1083–1095. [Google Scholar] [CrossRef]
- Sohel, M.; Bolong, A.A. Effects of soybean lecithin on growth and survival of tropical tin foil barb. Ann. Vet. Anim. Sci. 2020, 7, 15–22. [Google Scholar]
- Draganovic, V.; van der Goot, A.J.; Boom, R.; Jonkers, J. Assessment of the effects of fish meal, wheat gluten, soy protein concentrate and feed moisture on extruder system parameters and the technical quality of fish feed. Anim. Feed Sci. Technol. 2011, 165, 238–250. [Google Scholar] [CrossRef]
- Storebakken, T.; Shearer, K.D.; Baeverfjord, G.; Nielsen, B.G.; Åsgård, T.; Scott, T.; De Laporte, A. Digestibility of macronutrients, energy and amino acids, absorption of elements and absence of intestinal enteritis in Atlantic salmon, Salmo salar, fed diets with wheat gluten. Aquaculture 2000, 184, 115–132. [Google Scholar] [CrossRef]
- Li, X.; Wu, X.; Dong, Y.; Gao, Y.; Yao, W.; Zhou, Z. Effects of dietary lysine levels on growth, feed utilization and related gene expression of juvenile hybrid grouper (Epinephelus fuscoguttatus ♀ × Epinephelus lanceolatus ♂). Aquaculture 2019, 502, 153–161. [Google Scholar] [CrossRef]
- Luo, Z.; Liu, Y.; Mai, K.; Tian, L.; Tan, X.; Yang, H.; Liang, G.; Liu, D. Quantitative L-lysine requirement of juvenile grouper Epinephelus coioides. Aquac. Nutr. 2006, 12, 165–172. [Google Scholar] [CrossRef]
- Luo, Z.; Liu, Y.; Mai, K.; Tian, L.; Yang, H.; Tan, X.; Liu, D. Dietary l-methionine requirement of juvenile grouper Epinephelus coioides at a constant dietary cystine level. Aquaculture 2005, 249, 409–418. [Google Scholar] [CrossRef]
- Li, P.; Mai, K.; Trushenski, J.; Wu, G. New developments in fish amino acid nutrition: Towards functional and environmentally oriented aquafeeds. Amino Acids 2009, 37, 43–53. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Øverland, M.; Xie, S.; Dong, Z.; Lv, Z.; Xu, J.; Storebakken, T. Mixtures of lupin and pea protein concentrates can efficiently replace high-quality fish meal in extruded diets for juvenile black sea bream (Acanthopagrus schlegeli). Aquaculture 2012, 354–355, 68–74. [Google Scholar] [CrossRef]
- Luo, Y.; Wu, X.; Li, W.; Jiang, S.; Lu, S.; Wu, M. Effects of different corn starch levels on growth, protein input, and feed utilization of juvenile hybrid grouper (male Epinephelus lanceolatus × female E. fuscoguttatus). N. Am. J. Aquac. 2016, 78, 168–173. [Google Scholar] [CrossRef]
- Shiau, S.Y.; Lin, Y.H. Carbohydrate utilization and its protein-sparing effect in diets for grouper (Epinephelus malabaricus). Anim. Sci. 2001, 73, 299–304. [Google Scholar] [CrossRef]
- Tran-Duy, A.; Smit, B.; van Dam, A.A.; Schrama, J.W. Effects of dietary starch and energy levels on maximum feed intake, growth and metabolism of Nile tilapia, Oreochromis niloticus. Aquaculture 2008, 277, 213–219. [Google Scholar] [CrossRef]
- Tan, Q.; Wang, F.; Xie, S.; Zhu, X.; Lei, W.; Shen, J. Effect of high dietary starch levels on the growth performance, blood chemistry and body composition of gibel carp (Carassius auratus var. gibelio). Aquac. Res. 2009, 40, 1011–1018. [Google Scholar] [CrossRef]
- Tian, L.X.; Liu, Y.J.; Yang, H.J.; Liang, G.Y.; Niu, J. Effects of different dietary wheat starch levels on growth, feed efficiency and digestibility in grass carp (Ctenopharyngodon idella). Aquac. Int. 2012, 20, 283–293. [Google Scholar] [CrossRef]
- Li, S.; Li, Z.; Zhang, J.; Sang, C.; Chen, N. The impacts of dietary carbohydrate levels on growth performance, feed utilization, glycogen accumulation and hepatic glucose metabolism in hybrid grouper (Epinephelus fuscoguttatus ♀ × E. lanceolatus ♂). Aquaculture 2019, 512, 734351. [Google Scholar] [CrossRef]
- Li, X.; Zheng, S.; Ma, X.; Cheng, K.; Wu, G. Effects of dietary starch and lipid levels on the protein retention and growth of largemouth bass (Micropterus salmoides). Amino Acids 2020, 52, 999–1016. [Google Scholar] [CrossRef] [PubMed]
- Bradshaw, C.J.; Higgins, J.; Michael, K.J.; Wotherspoon, S.J.; Hindell, M.A. At-sea distribution of female southern elephant seals relative to variation in ocean surface properties. ICES J. Mar. Sci. 2004, 61, 1014–1027. [Google Scholar] [CrossRef]
- Robaina, L.; Corraze, G.; Aguirre, P.; Blanc, D.; Melcion, J.; Kaushik, S. Digestibility, postprandial ammonia excretion and selected plasma metabolites in European sea bass (Dicentrarchus labrax) fed pelleted or extruded diets with or without wheat gluten. Aquaculture 1999, 179, 45–56. [Google Scholar] [CrossRef]


| Ingredients | Fish Meal † | Wheat Gluten ‡ | Soy Protein Concentration § |
|---|---|---|---|
| Compositions, kg−1 | |||
| Dry matter, g | 913 | 935 | 925 |
| Crude protein, g | 748 | 837 | 694 |
| Crude fat, g | 117 | 44 | 47 |
| Starch, g | - | 74 | - |
| Ash, g | 122 | 10 | 58 |
| Gross energy, MJ | 21.8 | 22.0 | 20.1 |
| Essential amino acids (EAA), g (16 g N)−1 | |||
| Arg | 5.5 | 3.6 | 7.4 |
| His | 1.7 | 2.0 | 2.5 |
| Leu | 7.0 | 7.0 | 8.1 |
| Ile | 3.8 | 3.5 | 4.6 |
| Lys | 7.3 | 1.8 | 6.7 |
| Met | 2.6 | 1.4 | 0.9 |
| Phe | 3.8 | 5.2 | 5.3 |
| Thr | 4.1 | 2.5 | 4.1 |
| Tyr | 3.2 | 3.3 | 3.5 |
| Val | 4.5 | 3.8 | 4.7 |
| Total EAA ¶ | 43.3 | 34.1 | 47.9 |
| Non-essential amino acids (NEAA), g (16 g N)−1 | |||
| Ala | 6.1 | 2.7 | 4.4 |
| Asp | 8.4 | 3.2 | 11.6 |
| Cys | 0.8 | 1.8 | 0.3 |
| Glu | 13.1 | 35.2 | 19.1 |
| Gly | 5.8 | 3.4 | 4.2 |
| Pro | 4.2 | 12.8 | 5.0 |
| Ser | 4.0 | 4.6 | 5.2 |
| Total NEAA | 42.3 | 63.8 | 49.8 |
| Total AA ¶ | 85.6 | 97.9 | 97.6 |
| Ingredients, g kg−1 | R0 | RF1 | RF2 | RF3 | RS1 | RS2 | RS3 | RFS |
|---|---|---|---|---|---|---|---|---|
| Constant feed ingredients † | 221.5 | 221.5 | 221.5 | 221.5 | 221.5 | 221.5 | 221.5 | 221.5 |
| GWT ‡ | - | 69.0 | 138.0 | 207.0 | 69.0 | 138.0 | 207.0 | 207.0 |
| Fish meal § | 200.0 | 133.0 | 66.0 | - | 200.0 | 200.0 | 200.0 | 100.0 |
| Soy protein concentrate | 214.0 | 214.0 | 214.0 | 214.0 | 142.0 | 71.0 | - | 107.0 |
| Wheat flour | 266.4 | 250.4 | 234.6 | 217.8 | 262.9 | 259.1 | 255.4 | 236.3 |
| Fish oil | 84.0 | 89.0 | 94.0 | 99.0 | 84.0 | 84.0 | 84.0 | 92.0 |
| Mono calcium Phosphate ¶ | 14.0 | 17.0 | 20.0 | 23.0 | 14.5 | 14.5 | 14.5 | 19.0 |
| L-Lysine †† | - | 3.7 | 7.4 | 11.0 | 3.1 | 6.1 | 9.1 | 10.0 |
| DL-Methionine †† | - | 0.4 | 0.8 | 1.2 | - | - | - | 0.2 |
| L-Arginine †† | - | 1.1 | 2.1 | 3.1 | 2.1 | 4.2 | 6.2 | 4.7 |
| L-Threonine †† | - | 0.8 | 1.5 | 2.3 | 0.8 | 1.5 | 2.2 | 2.2 |
| Analysed content, kg−1 | ||||||||
| Dry matter, g | 951 | 954 | 957 | 956 | 957 | 957 | 957 | 955 |
| Crude protein, g | 441 | 447 | 444 | 449 | 447 | 451 | 456 | 454 |
| Crude fat, g | 135 | 136 | 139 | 142 | 140 | 141 | 131 | 140 |
| Ash, g | 76 | 69 | 61 | 54 | 72 | 68 | 54 | 59 |
| Gross energy, MJ | 21.5 | 21.6 | 21.6 | 22.0 | 21.5 | 21.7 | 21.8 | 22.0 |
| Essential amino acid, g (16 N)−1 | ||||||||
| Arg | 6.5 | 6.5 | 6.6 | 6.4 | 6.5 | 6.3 | 6.8 | 6.2 |
| Ile | 4.1 | 3.9 | 3.9 | 3.8 | 3.9 | 3.7. | 3.7 | 3.5 |
| Leu | 7.2 | 7.0 | 7.2 | 7.0 | 7.0 | 6.7 | 6.9 | 6.6 |
| Lys | 6.1 | 6.0 | 6.1 | 6.1 | 6.1 | 5.9 | 6.2 | 5.9 |
| Met + Cys | 2.1 | 2.0 | 2.1 | 2.1 | 2.0 | 2.0 | 2.5 | 1.9 |
| Phe + Tyr | 7.4 | 7.3 | 7.7 | 7.7 | 7.1 | 6.7 | 7.3 | 6.8 |
| Thr | 3.8 | 3.8 | 3.8 | 3.7 | 3.8 | 3.7 | 3.8 | 3.5 |
| Val | 4.4 | 4.2 | 4.1 | 4.0 | 4.2 | 4.0 | 4.1 | 3.8 |
| Total essential amino acid | 41.7 | 40.7 | 41.5 | 40.7 | 40.6 | 39.0 | 41.4 | 38.2 |
| Non-essential amino acid, g (16 N)−1 | ||||||||
| Ala | 4.7 | 4.3 | 4.0 | 3.5 | 4.6 | 4.2 | 4.3 | 3.7 |
| Asp | 9.9 | 8.8 | 8.6 | 7.8 | 8.8 | 7.8 | 7.1 | 6.9 |
| Glu | 16.3 | 18.7 | 21.0 | 22.9 | 18.0 | 19.1 | 22.1 | 21.4 |
| Gly | 5.0 | 4.5 | 4.3 | 3.9 | 4.7 | 4.5 | 4.69 | 4.0 |
| Pro | 4.7 | 5.4 | 6.1 | 7.0 | 5.5 | 6.0 | 7.2 | 7.2 |
| Ser | 4.4 | 4.4 | 4.6 | 4.5 | 4.3 | 4.2 | 4.3 | 4.2 |
| Total non-essential amino acid | 45.0 | 46.0 | 48.6 | 49.6 | 46.0 | 45.7 | 49.7 | 47.3 |
| Total amino acid | 86.6 | 86.8 | 90.1 | 90.4 | 86.5 | 86.6 | 90.9 | 85.6 |
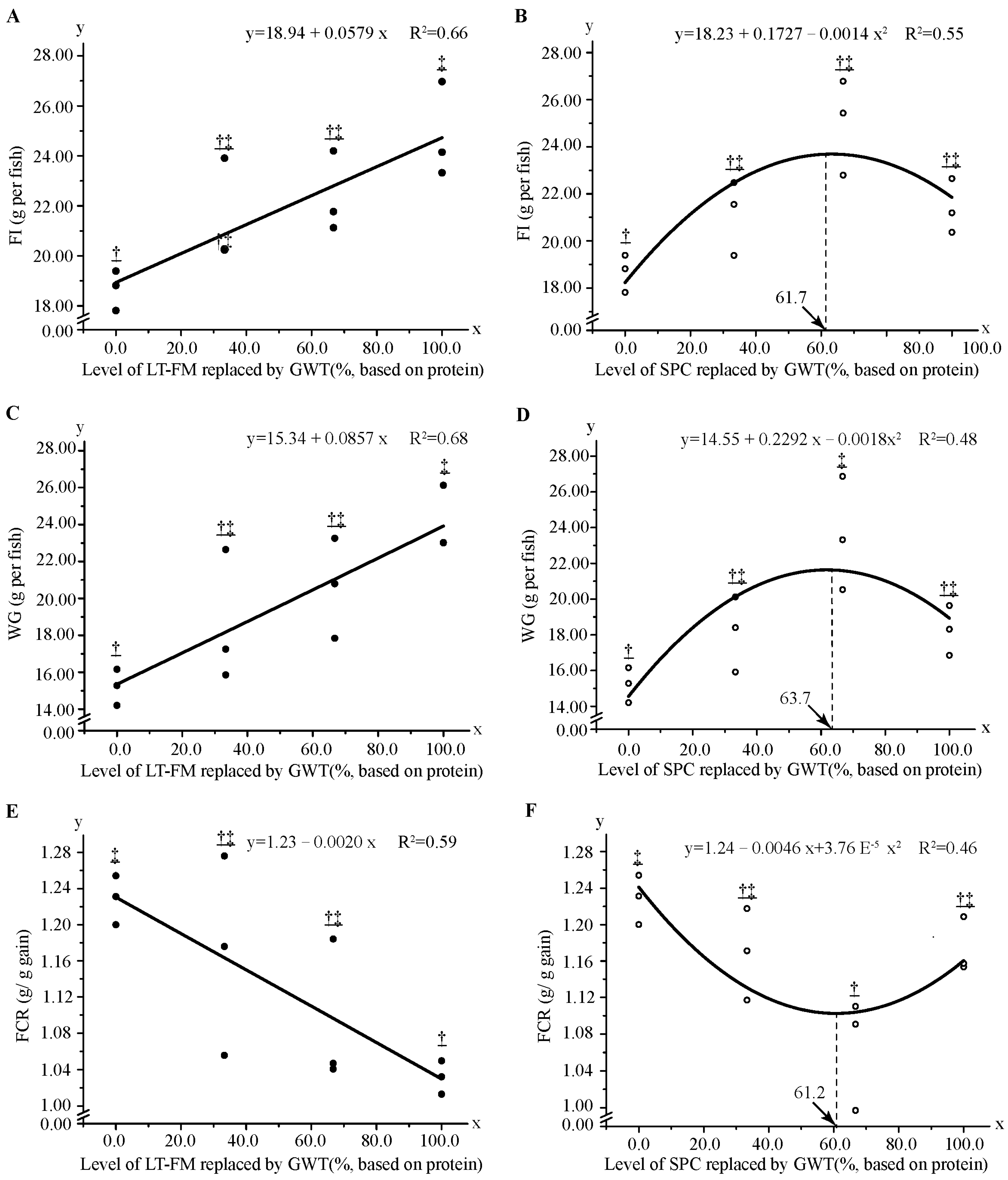
| Diets | Feed Intake, g DM Fish−1 | Weight Gain, g Fish−1 | Feed Conversion Ratio, g DM Ingested (g Gain)−1 | |||
|---|---|---|---|---|---|---|
| 0–28 Days | 0–56 Days | 0–28 Days | 0–56 Days | 0–28 Days | 0–56 Days | |
| R0 | 11.9 ± 0.28 | 18.7 ± 0.46 † | 12.2 ± 0.36 | 15.2 ± 0.57 † | 0.98 ± 0.01 | 1.23 ± 0.02 ‡ |
| RF1 | 12.4 ± 0.16 | 21.5 ± 1.22 †‡ | 12.5 ± 0.24 | 18.6 ± 2.07 †‡ | 0.99 ± 0.01 | 1.17 ± 0.06 †‡ |
| RF2 | 12.0 ± 0.04 | 22.4 ± 0.94 †‡ | 12.9 ± 0.14 | 20.6 ± 1.56 †‡ | 0.93 ± 0.01 | 1.09 ± 0.05 †‡ |
| RF3 | 12.1 ± 0.25 | 24.8 ± 1.10 ‡ | 12.5 ± 0.35 | 24.1 ± 1.04 ‡ | 0.96 ± 0.02 | 1.03 ± 0.01 † |
| RS1 | 12.3 ± 0.52 | 21.1 ± 0.92 † | 12.1 ± 0.73 | 18.2 ± 1.22 † | 1.02 ± 0.03 | 1.17 ± 0.03 †‡ |
| RS2 | 13.1 ± 0.17 | 25.0 ± 1.17 ‡ | 14.2 ± 0.33 | 23.6 ± 1.83 ‡ | 0.92 ± 0.01 | 1.07 ± 0.03 †‡ |
| RS3 | 12.4 ± 0.61 | 21.4 ± 0.67 †‡ | 12.7 ± 1.01 | 18.3 ± 0.80 †‡ | 0.98 ± 0.03 | 1.17 ± 0.02 †‡ |
| RFS | 12.7 ± 0.20 | 23.9 ± 1.59 ‡ | 13.4 ± 0.29 | 22.0 ± 2.28 †‡ | 0.95 ± 0.02 | 1.10 ± 0.05 †‡ |
| ANOVA p | 0.27 | 0.01 | 0.15 | 0.01 | 0.05 | 0.03 |
| Parameters | R0 | RF1 | RF2 | RF3 | RS1 | RS2 | RS3 | RFS | p |
|---|---|---|---|---|---|---|---|---|---|
| HSI, % | 2.08 ± 0.25 | 1.76 ± 0.25 | 2.16 ± 0.16 | 2.03 ± 0.20 | 1.76 ± 0.22 | 1.97 ± 0.20 | 2.08 ± 0.15 | 1.81 ± 0.26 | 0.61 |
| VSI, % | 13.8 ± 0.23 | 13.8 ± 0.28 | 13.4 ± 0.46 | 14.3 ± 0.35 | 13.5 ± 0.48 | 13.4 ± 0.30 | 13.2 ± 0.19 | 13.2 ± 0.39 | 0.31 |
| CF, g/cm3 | 2.72 ± 0.04 | 2.84 ± 0.08 | 2.89 ± 0.07 | 2.93 ± 0.06 | 2.80 ± 0.06 | 2.80 ± 0.04 | 2.96 ± 0.09 | 2.81 ± 0.10 | 0.27 |
| Diets | Dry Matter, g kg−1 | Proximate Composition, g kg−1 | Energy, KJ kg−1 | Retention, % | |||
|---|---|---|---|---|---|---|---|
| Protein | Lipid | Ash | Protein (PRE) | Energy (ERE) | |||
| R0 | 331 ± 5.78 | 176 ± 1.53 | 106 ± 2.85 | 49.7 ± 2.24 | 8.06 ± 0.12 | 32.7 ± 0.28 † | 34.3 ± 0.71 |
| RF1 | 326 ± 5.29 | 174 ± 5.24 | 103 ± 3.84 | 47.9 ± 2.37 | 8.43 ± 0.34 | 33.9 ± 1.66 †‡ | 34.7 ± 2.33 |
| RF2 | 333 ± 9.45 | 178 ± 2.73 | 111 ± 4.37 | 44.3 ± 2.78 | 8.26 ± 0.20 | 38.1 ± 1.17 ‡ | 38.6 ± 1.18 |
| RF3 | 324 ± 5.78 | 173 ± 2.40 | 110 ± 2.19 | 40.5 ± 1.50 | 8.24 ± 0.14 | 37.6 ± 0.68 ‡ | 39.4 ± 1.19 |
| RS1 | 335 ± 0.33 | 179 ± 1.67 | 110 ± 1.86 | 46.7 ± 0.35 | 8.30 ± 0.06 | 35.9 ± 1.20 †‡ | 36.8 ± 0.45 |
| RS2 | 327 ± 3.18 | 175 ± 0.88 | 108 ± 2.73 | 44.5 ± 1.37 | 8.19 ± 0.13 | 36.4 ± 1.04 †‡ | 38.5 ± 1.03 |
| RS3 | 333 ± 1.00 | 182 ± 0.88 | 105 ± 1.00 | 46.1 ± 1.13 | 8.17 ± 0.02 | 35.7 ± 0.74 †‡ | 35.4 ± 0.26 |
| RFS | 330 ± 4.73 | 178 ± 4.33 | 109 ± 4.70 | 43.5 ± 2.75 | 8.22 ± 0.11 | 36.5 ± 0.64 †‡ | 37.3 ± 1.69 |
| ANOVA p | 0.79 | 0.53 | 0.64 | 0.11 | 0.88 | 0.03 | 0.08 |
| Parameters | Diets | p | |||||||
|---|---|---|---|---|---|---|---|---|---|
| R0 | RF1 | RF2 | RF3 | RS1 | RS2 | RS3 | RFS | ||
| Plasma | |||||||||
| TP, g/L | 32.5 ± 1.36 | 28.7 ± 5.14 | 34.3 ± 2.17 | 30.7 ± 1.03 | 34.4 ± 1.60 | 33.2 ± 0.76 | 34.2 ± 0.27 | 32.7 ± 1.89 | 0.61 |
| TG, mmol/L | 4.20 ± 1.60 | 2.73 ± 1.10 | 2.43 ± 0.42 | 1.76 ± 0.34 | 5.04 ± 2.03 | 4.20 ± 3.03 | 4.01 ± 0.22 | 1.88 ± 0.88 | 0.70 |
| CHOL, mmol/L | 3.94 ± 0.72 | 2.61 ± 0.69 | 2.41 ± 0.26 | 1.69 ± 0.02 | 4.00 ± 0.52 | 2.72 ± 0.75 | 3.14 ± 0.11 | 2.60 ± 0.31 | 0.07 |
| GLU, mmol/L | 7.96 ± 0.32 | 6.42 ± 0.92 | 6.01 ± 0.85 | 5.51 ± 0.56 | 7.52 ± 1.92 | 6.22 ± 1.68 | 6.31 ± 0.48 | 6.39 ± 1.09 | 0.81 |
| ALT, U/L | 1.83 ± 0.88 | 7.50 ± 4.54 | 2.17 ± 0.17 | 3.17 ± 0.33 | 2.00 ± 0.29 | 3.17 ± 0.60 | 2.67 ± 0.17 | 2.67 ± 0.93 | 0.37 |
| AST, U/L | 122 ± 11.1 | 102 ± 32.5 | 142 ± 14.1 | 128 ± 15.4 | 117 ± 35.0 | 119 ± 15.2 | 132 ± 10.8 | 94.5 ± 22.3 | 0.80 |
| SOD, U/mL | 21.0 ± 0.42 | 22.4 ± 0.19 | 22.0 ± 0.44 | 22.3 ± 0.66 | 20.5 ± 0.19 | 21.7 ± 0.39 | 20.8 ± 0.22 | 21.5 ± 0.16 | 0.12 |
| Liver | |||||||||
| SOD, U/mg prot | 273 ± 3.38 ‡ | 233 ± 4.95 † | 342 ± 13.2 § | 345 ± 9.40 § | 237 ± 4.36 † | 212 ± 7.80 † | 208 ± 1.70 † | 283 ± 3.65 ‡ | <0.01 |
| MDA, nmol/mg prot | 1.74 ± 0.04 | 1.52 ± 0.02 | 2.17 ± 0.01 | 2.08 ± 0.38 | 2.77 ± 0.66 | 1.58 ± 0.01 | 1.54 ± 0.01 | 1.40 ± 0.03 | 0.05 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cheng, Y.; Wang, Y.; Dong, Z.; Storebakken, T.; Xu, G.; Shi, B.; Zhang, Y. Evaluate of Wheat Gluten as a Protein Alternative for Fish Meal and Soy Protein Concentrate in Red Spotted Grouper Epinephelus akaara. Metabolites 2023, 13, 832. https://doi.org/10.3390/metabo13070832
Cheng Y, Wang Y, Dong Z, Storebakken T, Xu G, Shi B, Zhang Y. Evaluate of Wheat Gluten as a Protein Alternative for Fish Meal and Soy Protein Concentrate in Red Spotted Grouper Epinephelus akaara. Metabolites. 2023; 13(7):832. https://doi.org/10.3390/metabo13070832
Chicago/Turabian StyleCheng, Yanbo, Yongchao Wang, Zhiyong Dong, Trond Storebakken, Guohuan Xu, Bo Shi, and Yuexing Zhang. 2023. "Evaluate of Wheat Gluten as a Protein Alternative for Fish Meal and Soy Protein Concentrate in Red Spotted Grouper Epinephelus akaara" Metabolites 13, no. 7: 832. https://doi.org/10.3390/metabo13070832
APA StyleCheng, Y., Wang, Y., Dong, Z., Storebakken, T., Xu, G., Shi, B., & Zhang, Y. (2023). Evaluate of Wheat Gluten as a Protein Alternative for Fish Meal and Soy Protein Concentrate in Red Spotted Grouper Epinephelus akaara. Metabolites, 13(7), 832. https://doi.org/10.3390/metabo13070832







