The Implications in Meat Quality and Nutrition by Comparing the Metabolites of Pectoral Muscle between Adult Indigenous Chickens and Commercial Laying Hens
Abstract
:1. Introduction
2. Materials and Methods
2.1. Birds and Sample Preparation
2.2. UHPLC-MS/MS
2.2.1. Sample Preparation
2.2.2. UHPLC-MS/MS Analysis
2.3. Oil-Red O Staining and Triglyceride Measurement
2.4. Statistical Analysis
3. Results and Discussion
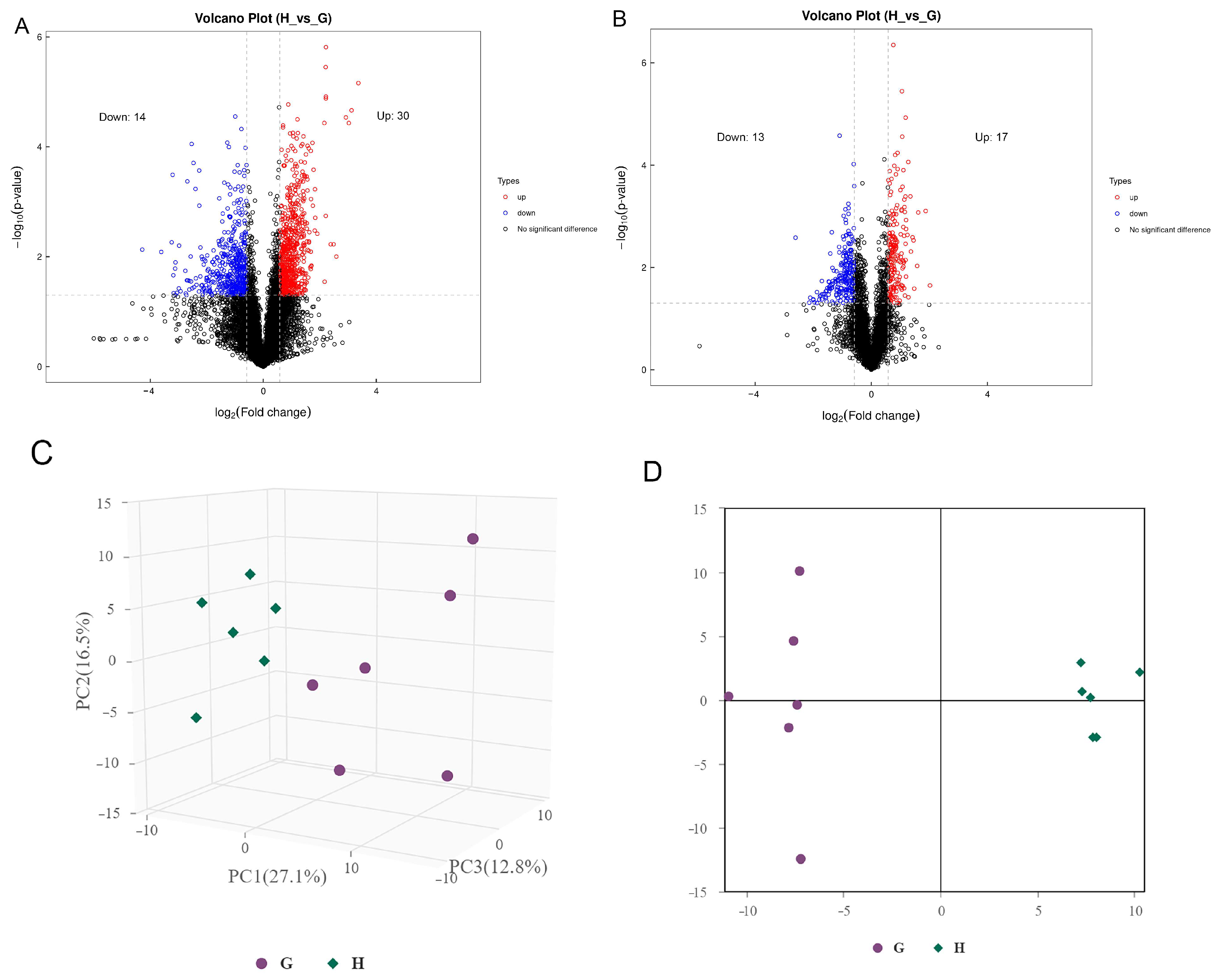
3.1. Metabolomic Composition of Guangyuan Grey Chicken and Hy-Line Grey Hen
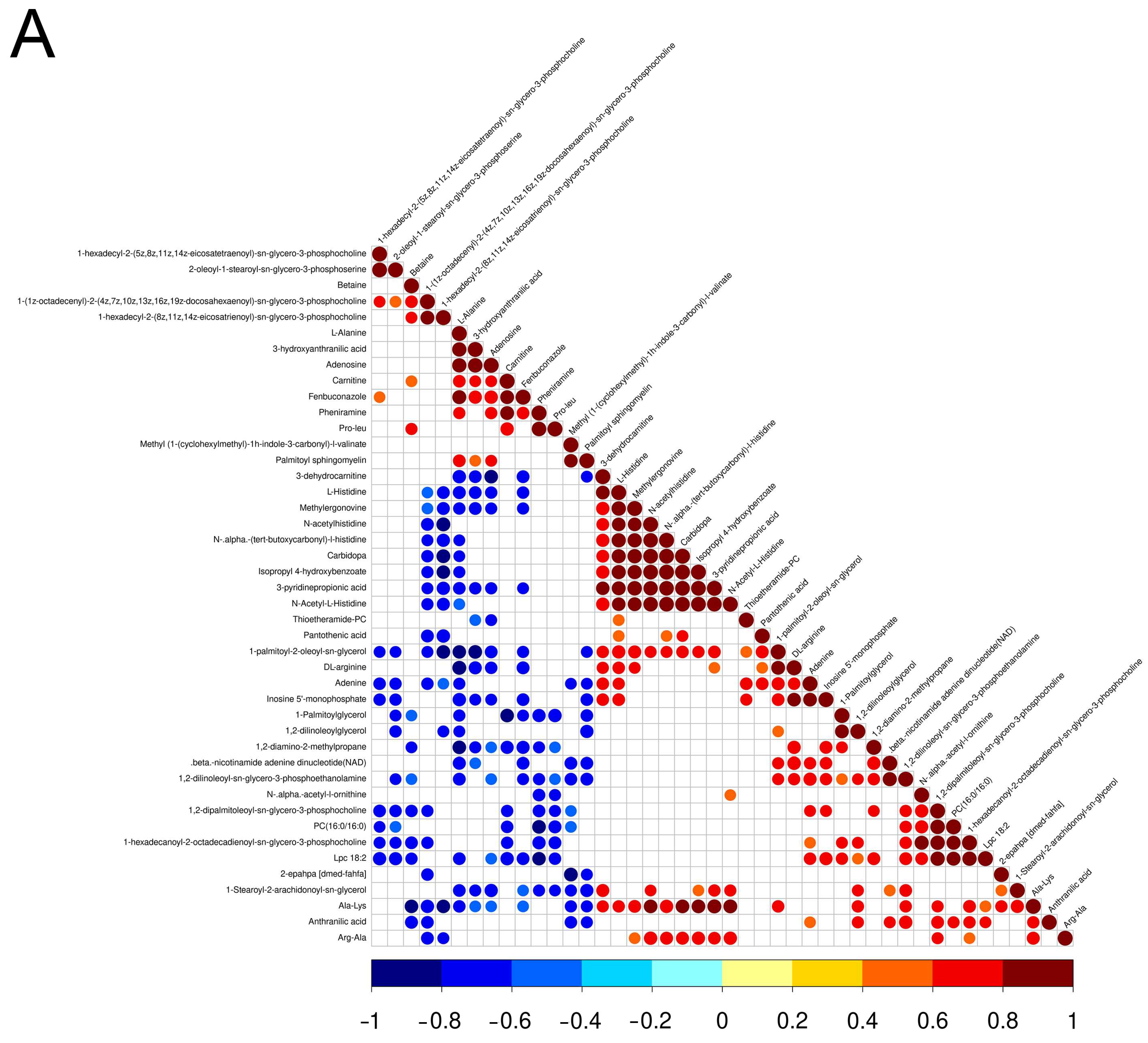
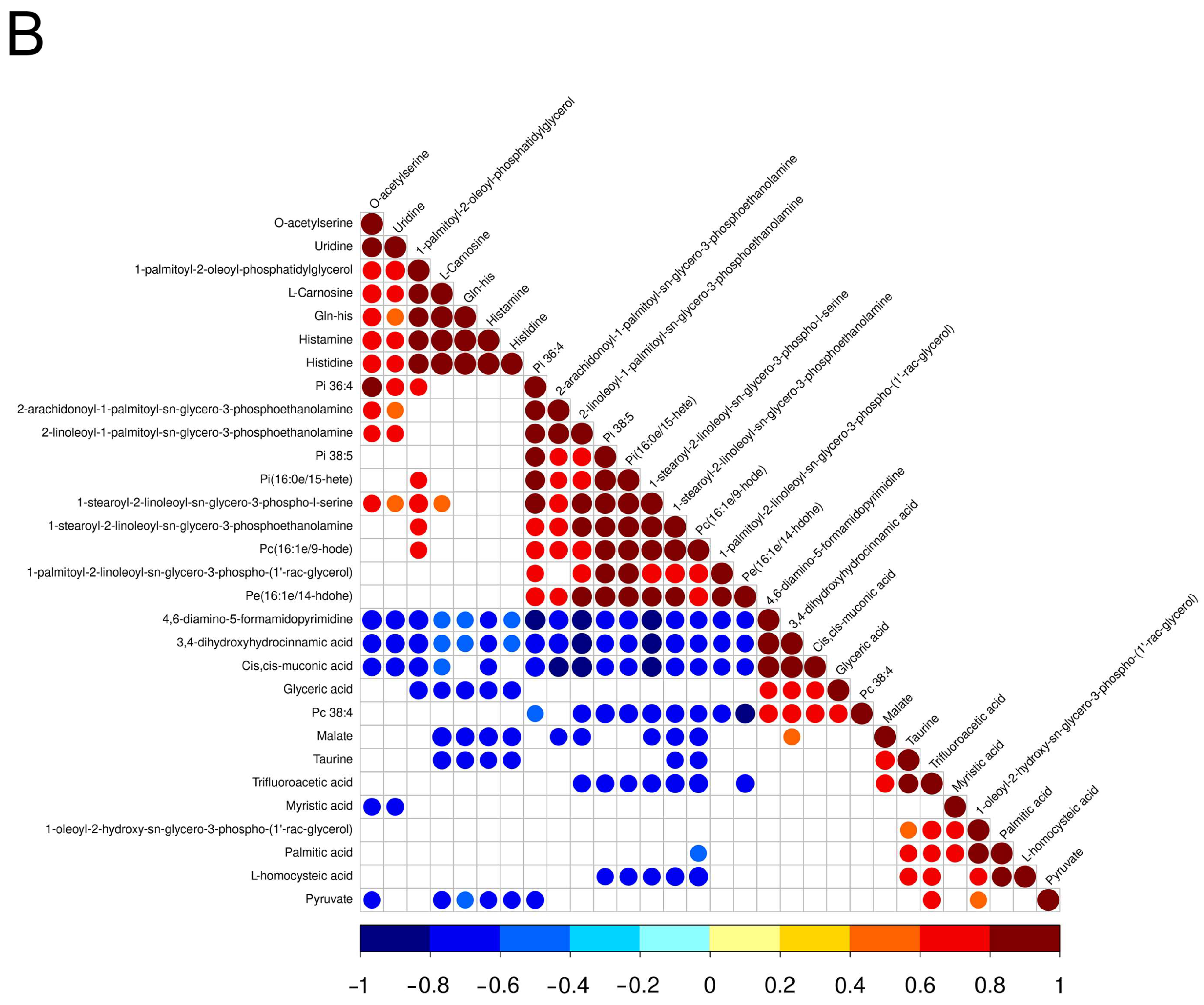
3.2. Screening and Correlation Analysis of Differential Metabolites
3.3. KEGG Pathway Analysis of Significantly Differential Metabolites
3.4. Oil Red O Staining and Triglyceride Measurement of Pectoral Muscle
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- FAOSTAT. Food Supply Quantity of Food Balances; Food and Agriculture Organization of the United Nations Statistics Division (FAOSTAT): Rome, Italy, 2020. [Google Scholar]
- Baek, K.H.; Utama, D.T.; Lee, S.G.; An, B.K.; Lee, S.K. Effects of Replacing Pork Back Fat with Canola and Flaxseed Oils on Physicochemical Properties of Emulsion Sausages from Spent Layer Meat. Asian-Australas. J. Anim. Sci. 2016, 29, 865–871. [Google Scholar] [CrossRef] [PubMed]
- Karthik, P.; Kulkarni, V.V.; Sivakumar, K. Preparation, storage stability and palatability of spent hen meal based pet food. J. Food Sci. Technol. 2010, 47, 330–334. [Google Scholar] [CrossRef] [PubMed]
- Katemala, S.; Molee, A.; Thumanu, K.; Yongsawatdigul, J. Meat quality and Raman spectroscopic characterization of Korat hybrid chicken obtained from various rearing periods. Poult. Sci. 2021, 100, 1248–1261. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Qiao, Y.; Xiao, Y.; Chen, H.; Zhao, L.; Huang, M.; Zhou, G. Differences in Physicochemical and Nutritional Properties of Breast and Thigh Meat from Crossbred Chickens, Commercial Broilers, and Spent Hens. Asian-Australas. J. Anim. Sci. 2016, 29, 855–864. [Google Scholar] [CrossRef] [PubMed]
- Fan, H.; Bhullar, K.S.; Wu, J. Spent Hen Muscle Protein-Derived RAS Regulating Peptides Show Antioxidant Activity in Vascular Cells. Antioxidants 2021, 10, 290. [Google Scholar] [CrossRef]
- Yu, W.; Field, C.J.; Wu, J. Purification and identification of anti-inflammatory peptides from spent hen muscle proteins hydrolysate. Food Chem. 2018, 253, 101–107. [Google Scholar] [CrossRef]
- Hong, H.; Fan, H.; Roy, B.C.; Wu, J. Amylase enhances production of low molecular weight collagen peptides from the skin of spent hen, bovine, porcine, and tilapia. Food Chem. 2021, 352, 129355. [Google Scholar] [CrossRef]
- Kumar, D.; Tarafdar, A.; Dass, S.L.; Pareek, S.; Badgujar, P.C. Antioxidant potential and amino acid profile of ultrafiltration derived peptide fractions of spent hen meat protein hydrolysate. J. Food Sci. Technol. 2023, 60, 1195–1201. [Google Scholar] [CrossRef]
- Esparza, Y.; Bandara, N.; Ullah, A.; Wu, J. Hydrogels from feather keratin show higher viscoelastic properties and cell proliferation than those from hair and wool keratins. Mater. Sci. Eng. C Mater. Biol. Appl. 2018, 90, 446–453. [Google Scholar] [CrossRef]
- Zubair, M.; Wu, J.; Ullah, A. Hybrid Bionanocomposites from Spent Hen Proteins. ACS Omega 2019, 4, 3772–3781. [Google Scholar] [CrossRef]
- Nicholson, J.K.; Lindon, J.C.; Holmes, E. ‘Metabonomics’: Understanding the metabolic responses of living systems to pathophysiological stimuli via multivariate statistical analysis of biological NMR spectroscopic data. Xenobiotica 1999, 29, 1181–1189. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Shi, X.; Hou, J.; Gao, S.; Chao, Y.; Ding, J.; Chen, L.; Qian, Y.; Shao, G.; Si, Y.; et al. Integrating metabolomics and network pharmacology to explore Rhizoma Coptidis extracts against sepsis-associated acute kidney injury. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2021, 1164, 122525. [Google Scholar] [CrossRef] [PubMed]
- Talmor-Barkan, Y.; Bar, N.; Shaul, A.A.; Shahaf, N.; Godneva, A.; Bussi, Y.; Lotan-Pompan, M.; Weinberger, A.; Shechter, A.; Chezar-Azerrad, C.; et al. Metabolomic and microbiome profiling reveals personalized risk factors for coronary artery disease. Nat. Med. 2022, 28, 295–302. [Google Scholar] [CrossRef] [PubMed]
- Yoo, S.A.; Park, S.E.; Seo, S.H.; Lee, H.J.; Lee, K.I.; Son, H.S. A GC-MS based metabolic profiling of fermented sausage supplemented with pineapple. Food Sci. Biotechnol. 2016, 25, 1657–1664. [Google Scholar] [CrossRef]
- Lotfy, M.M.; Sayed, A.M.; AboulMagd, A.M.; Hassan, H.M.; El Amir, D.; Abouzid, S.F.; El-Gendy, A.O.; Rateb, M.E.; Abdelmohsen, U.R.; Alhadrami, H.; et al. Metabolomic profiling, biological evaluation of Aspergillus awamori, the river Nile-derived fungus using epigenetic and OSMAC approaches. RSC Adv. 2021, 11, 6709–6719. [Google Scholar] [CrossRef]
- Zhu, G.; Wang, S.; Huang, Z.; Zhang, S.; Liao, Q.; Zhang, C.; Lin, T.; Qin, M.; Peng, M.; Yang, C.; et al. Rewiring of the Fruit Metabolome in Tomato Breeding. Cell 2018, 172, 249–261. [Google Scholar] [CrossRef]
- Tan, C.; Selamat, J.; Jambari, N.N.; Sukor, R.; Murugesu, S.; Khatib, A. Muscle and Serum Metabolomics for Different Chicken Breeds under Commercial Conditions by GC-MS. Foods 2021, 10, 2174. [Google Scholar] [CrossRef]
- Izquierdo-Garcia, J.L.; Rodriguez, I.; Kyriazis, A.; Villa, P.; Barreiro, P.; Desco, M.; Ruiz-Cabello, J. A novel R-package graphic user interface for the analysis of metabonomic profiles. BMC Bioinform. 2009, 10, 363. [Google Scholar] [CrossRef]
- Thevenot, E.A.; Roux, A.; Xu, Y.; Ezan, E.; Junot, C. Analysis of the Human Adult Urinary Metabolome Variations with Age, Body Mass Index, and Gender by Implementing a Comprehensive Workflow for Univariate and OPLS Statistical Analyses. J. Proteome Res. 2015, 14, 3322–3335. [Google Scholar] [CrossRef]
- Li, J.; Li, Z.; Ran, J.; Yang, C.; Lin, Z.; Liu, Y. LC/MS-based lipidomics to characterize breed-specific and tissue-specific lipid composition of chicken meat and abdominal fat. LWT 2022, 163, 113611. [Google Scholar] [CrossRef]
- Antonny, B.; Vanni, S.; Shindou, H.; Ferreira, T. From zero to six double bonds: Phospholipid unsaturation and organelle function. Trends Cell Biol. 2015, 25, 427–436. [Google Scholar] [CrossRef] [PubMed]
- Tasseva, G.; Bai, H.D.; Davidescu, M.; Haromy, A.; Michelakis, E.; Vance, J.E. Phosphatidylethanolamine deficiency in Mammalian mitochondria impairs oxidative phosphorylation and alters mitochondrial morphology. J. Biol. Chem. 2013, 288, 4158–4173. [Google Scholar] [CrossRef] [PubMed]
- Smokvarska, M.; Bayle, V.; Maneta-Peyret, L.; Fouillen, L.; Poitout, A.; Dongois, A.; Fiche, J.B.; Gronnier, J.; Garcia, J.; Höfte, H.; et al. The receptor kinase FERONIA regulates phosphatidylserine localization at the cell surface to modulate ROP signaling. Sci. Adv. 2023, 9, eadd4791. [Google Scholar] [CrossRef] [PubMed]
- Hur, S.J.; Choi, B.D.; Choi, Y.J.; Kim, B.G.; Jin, S.K. Quality characteristics of imitation crab sticks made from Alaska Pollack and spent laying hen meat. LWT—Food Sci. Technol. 2011, 44, 1482–1489. [Google Scholar] [CrossRef]
- Choe, J.; Kim, H.Y. Physicochemical characteristics of breast and thigh meats from old broiler breeder hen and old laying hen and their effects on quality properties of pressed ham. Poult. Sci. 2020, 99, 2230–2235. [Google Scholar] [CrossRef]
- Jeong, H.S.; Utama, D.T.; Kim, J.; Barido, F.H.; Lee, S.K. Quality comparison of retorted Samgyetang made from white semi-broilers, commercial broilers, Korean native chickens, and old laying hens. Asian-Australas. J. Anim. Sci. 2020, 33, 139–147. [Google Scholar] [CrossRef]
- Bradbury, K.E.; Skeaff, C.M.; Green, T.J.; Gray, A.R.; Crowe, F.L. The serum fatty acids myristic acid and linoleic acid are better predictors of serum cholesterol concentrations when measured as molecular percentages rather than as absolute concentrations. Am. J. Clin. Nutr. 2010, 91, 398–405. [Google Scholar] [CrossRef]
- Harvey, K.A.; Walker, C.L.; Pavlina, T.M.; Xu, Z.; Zaloga, G.P.; Siddiqui, R.A. Long-chain saturated fatty acids induce pro-inflammatory responses and impact endothelial cell growth. Clin. Nutr. 2010, 29, 492–500. [Google Scholar] [CrossRef]
- Burdock, G.A.; Carabin, I.G. Safety assessment of myristic acid as a food ingredient. Food Chem. Toxicol. Int. J. Publ. Br. Ind. Biol. Res. Assoc. 2007, 45, 517–529. [Google Scholar] [CrossRef]
- Knottnerus, S.J.G.; Bleeker, J.C.; Wust, R.C.I.; Ferdinandusse, S.; IJlst, L.; Wijburg, F.A.; Wanders, R.J.A.; Visser, G.; Houtkooper, R.H. Disorders of mitochondrial long-chain fatty acid oxidation and the carnitine shuttle. Rev. Endocr. Metab. Disord. 2018, 19, 93–106. [Google Scholar] [CrossRef]
- Adeva-Andany, M.; Lopez-Ojen, M.; Funcasta-Calderon, R.; Ameneiros-Rodriguez, E.; Donapetry-Garcia, C.; Vila-Altesor, M.; Rodriguez-Seijas, J. Comprehensive review on lactate metabolism in human health. Mitochondrion 2014, 17, 76–100. [Google Scholar] [CrossRef]
- Gray, L.R.; Tompkins, S.C.; Taylor, E.B. Regulation of pyruvate metabolism and human disease. Cell. Mol. Life Sci. 2014, 71, 2577–2604. [Google Scholar] [CrossRef] [PubMed]
- Qiu, K.; He, W.; Zhang, H.; Wang, J.; Qi, G.; Guo, N.; Zhang, X.; Wu, S. Bio-Fermented Malic Acid Facilitates the Production of High-Quality Chicken via Enhancing Muscle Antioxidant Capacity of Broilers. Antioxidants 2022, 11, 2309. [Google Scholar] [CrossRef] [PubMed]
- Yan, E.; Wang, Y.; He, L.; Guo, J.; Zhang, X.; Yin, J. Effects of Dietary L-malic Acid Supplementation on Meat Quality, Antioxidant Capacity and Muscle Fiber Characteristics of Finishing Pigs. Foods 2022, 11, 3335. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Guo, X.; Wang, N.; Lu, S.; Dong, J.; Qi, Z.; Zhou, J.; Wang, Q. Evaluation of changes in egg yolk lipids during storage based on lipidomics through UPLC-MS/MS. Food Chem. 2023, 398, 133931. [Google Scholar] [CrossRef]
- Poccia, D.; Larijani, B. Phosphatidylinositol metabolism and membrane fusion. Biochem. J. 2009, 418, 233–246. [Google Scholar] [CrossRef]
- Rosenblat, M.; Vaya, J.; Shih, D.; Aviram, M. Paraoxonase 1 (PON1) enhances HDL-mediated macrophage cholesterol efflux via the ABCA1 transporter in association with increased HDL binding to the cells: A possible role for lysophosphatidylcholine. Atherosclerosis 2005, 179, 69–77. [Google Scholar] [CrossRef]
- Shadan, S.; Holic, R.; Carvou, N.; Ee, P.; Li, M.; Murray-Rust, J.; Cockcroft, S. Dynamics of lipid transfer by phosphatidylinositol transfer proteins in cells. Traffic 2008, 9, 1743–1756. [Google Scholar] [CrossRef]
- Niknafs, S.; Fortes, M.R.S.; Cho, S.; Black, J.L.; Roura, E. Alanine-specific appetite in slow growing chickens is associated with impaired glucose transport and TCA cycle. BMC Genom. 2022, 23, 393. [Google Scholar] [CrossRef]
- Peiretti, P.G.; Medana, C.; Visentin, S.; Giancotti, V.; Zunino, V.; Meineri, G. Determination of carnosine, anserine, homocarnosine, pentosidine and thiobarbituric acid reactive substances contents in meat from different animal species. Food Chem. 2011, 126, 1939–1947. [Google Scholar] [CrossRef]
- Boldyrev, A.A.; Stvolinsky, S.L.; Fedorova, T.N.; Suslina, Z.A. Carnosine as a natural antioxidant and geroprotector: From molecular mechanisms to clinical trials. Rejuvenation Res. 2010, 13, 156–158. [Google Scholar] [CrossRef] [PubMed]
- Sugiyama, T.; Tanaka, H.; Kawai, S. Improvement of periarticular osteoporosis in postmenopausal women with rheumatoid arthritis by beta-alanyl-L-histidinato zinc: A pilot study. J. Bone Miner. Metab. 2000, 18, 335–338. [Google Scholar] [CrossRef] [PubMed]
- Feehan, J.; Hariharan, R.; Buckenham, T.; Handley, C.; Bhatnagar, A.; Baba, S.P.; de Courten, B. Carnosine as a potential therapeutic for the management of peripheral vascular disease. Nutr. Metab. Cardiovasc. Dis. 2022, 32, 2289–2296. [Google Scholar] [CrossRef]
- McFarland, G.A.; Holliday, R. Retardation of the senescence of cultured human diploid fibroblasts by carnosine. Exp. Cell Res. 1994, 212, 167–175. [Google Scholar] [CrossRef] [PubMed]
- Suwanvichanee, C.; Sinpru, P.; Promkhun, K.; Kubota, S.; Riou, C.; Molee, W.; Yongsawatdigul, J.; Thumanu, K.; Molee, A. Effects of β-alanine and L-histidine supplementation on carnosine contents in and quality and secondary structure of proteins in slow-growing Korat chicken meat. Poult. Sci. 2022, 101, 101776. [Google Scholar] [CrossRef]
- Dobkin-Bekman, M.; Naidich, M.; Pawson, A.J.; Millar, R.P.; Seger, R.; Naor, Z. Activation of mitogen-activated protein kinase (MAPK) by GnRH is cell-context dependent. Mol. Cell. Endocrinol. 2006, 252, 184–190. [Google Scholar] [CrossRef]
- Xu, T.; Gu, L.; Schachtschneider, K.M.; Liu, X.; Huang, W.; Xie, M.; Hou, S. Identification of differentially expressed genes in breast muscle and skin fat of postnatal Pekin duck. PLoS ONE 2014, 9, e107574. [Google Scholar] [CrossRef]
- Yu, S.; Wang, G.; Liao, J.; Shen, X.; Chen, J.; Chen, X. Co-expression analysis of long non-coding RNAs and mRNAs involved in intramuscular fat deposition in Muchuan black-bone chicken. Br. Poult. Sci. 2023, 64, 289–298. [Google Scholar] [CrossRef]
- Bee, G.; Biolley, C.; Guex, G.; Herzog, W.; Lonergan, S.M.; Huff-Lonergan, E. Effects of available dietary carbohydrate and preslaughter treatment on glycolytic potential, protein degradation, and quality traits of pig muscles. J. Anim. Sci. 2006, 84, 191–203. [Google Scholar] [CrossRef]
- Schilling, M.W.; Suman, S.P.; Zhang, X.; Nair, M.N.; Desai, M.A.; Cai, K.; Ciaramella, M.A.; Allen, P.J. Proteomic approach to characterize biochemistry of meat quality defects. Meat Sci. 2017, 132, 131–138. [Google Scholar] [CrossRef]
- Lee, D.; Lee, H.J.; Jung, D.Y.; Kim, H.J.; Jang, A.; Jo, C. Effect of an animal-friendly raising environment on the quality, storage stability, and metabolomic profiles of chicken thigh meat. Food Res. Int. 2022, 155, 111046. [Google Scholar] [CrossRef] [PubMed]
- Mashek, D.G.; Coleman, R.A. Cellular fatty acid uptake: The contribution of metabolism. Curr. Opin. Lipidol. 2006, 17, 274–278. [Google Scholar] [CrossRef] [PubMed]
- Cui, H.; Liu, L.; Liu, X.; Wang, Y.; Luo, N.; Tan, X.; Zhu, Y.; Liu, R.; Zhao, G.; Wen, J. A selected population study reveals the biochemical mechanism of intramuscular fat deposition in chicken meat. J. Anim. Sci. Biotechnol. 2022, 13, 54. [Google Scholar] [CrossRef] [PubMed]


| Ingredient (%) | Rearing Stage (%) | Laying Stage (%) |
|---|---|---|
| Corn | 63.00 | 59.80 |
| Soybean meal (sol.) | 11.50 | 12.80 |
| Maize gluten meal | 3.00 | 5.00 |
| Wheat bran | 7.00 | 4.40 |
| Rapeseed meal (sol.) | 8.00 | 7.00 |
| Soybean oil | 2.80 | 3.30 |
| Dicalcium phosphate | 1.20 | 1.00 |
| Limestone | 1.00 | 4.20 |
| Premixes a | 2.50 | 2.50 |
| Nutrition content | ||
| ME (MJ/Kg) | 12.15 | 12.14 |
| CP (%) | 16.22 | 17.01 |
| Calcium (%) | 0.85 | 2.00 |
| Available P (%) | 0.4 | 0.34 |
| Lys (%) | 0.64 | 0.66 |
| Met (%) | 0.29 | 0.31 |
| Classfication | Percent (%) |
|---|---|
| Lipids and lipid-like molecules | 31.16 |
| Organic acids and derivatives | 25.98 |
| Undefined | 10.57 |
| Organoheterocyclic compounds | 8.10 |
| Organic oxygen compounds | 7.76 |
| Benzenoids | 5.62 |
| Nucleosides, nucleotides, and analogues | 4.50 |
| Organic nitrogen compounds | 3.04 |
| Phenylpropanoids and polyketides | 2.36 |
| Alkaloids and derivatives | 0.68 |
| Homogeneous non-metal compounds | 0.112 |
| Organosulfur compounds | 0.112 |
| Name | SuperClass | Fold Change | FC Type | p-Value | VIP |
|---|---|---|---|---|---|
| Negative ion mode | |||||
| Pi 38:5 | Lipids and lipid-like molecules | 3.07 | up | <0.05 | 2.57 |
| 1-palmitoyl-2-oleoyl-phosphatidylglycerol | Lipids and lipid-like molecules | 2.99 | up | <0.05 | 4.66 |
| Gln-his | Organic acids and derivatives | 2.15 | up | <0.05 | 1.01 |
| Pi(16:0e/15-hete) | Lipids and lipid-like molecules | 2.15 | up | <0.05 | 1.17 |
| 2-linoleoyl-1-palmitoyl-sn-glycero-3-phosphoethanolamine | Lipids and lipid-like molecules | 2.15 | up | <0.05 | 1.73 |
| Pc(16:1e/9-hode) | Lipids and lipid-like molecules | 2.09 | up | <0.05 | 5.19 |
| 1-stearoyl-2-linoleoyl-sn-glycero-3-phospho-l-serine | Lipids and lipid-like molecules | 2.08 | up | <0.05 | 1.57 |
| 1-stearoyl-2-linoleoyl-sn-glycero-3-phosphoethanolamine | Lipids and lipid-like molecules | 1.87 | up | <0.05 | 1.56 |
| 2-arachidonoyl-1-palmitoyl-sn-glycero-3-phosphoethanolamine | Lipids and lipid-like molecules | 1.86 | up | <0.05 | 3.09 |
| Histidine | Organic acids and derivatives | 1.74 | up | <0.05 | 4.42 |
| Histamine | Organic nitrogen compounds | 1.72 | up | <0.05 | 1.79 |
| Pe(16:1e/14-hdohe) | Lipids and lipid-like molecules | 1.68 | up | <0.05 | 1.16 |
| L-Carnosine | Organic acids and derivatives | 1.65 | up | <0.05 | 23.99 |
| O-acetylserine | Organic acids and derivatives | 1.65 | up | <0.05 | 1.02 |
| Pi 36:4 | Lipids and lipid-like molecules | 1.55 | up | <0.05 | 1.16 |
| Uridine | Nucleosides, nucleotides, and analogues | 1.48 | up | <0.05 | 2.52 |
| 1-palmitoyl-2-linoleoyl-sn-glycero-3-phospho-(1′-rac-glycerol) | Lipids and lipid-like molecules | 1.47 | up | <0.05 | 1.11 |
| L-homocysteic acid | Organic acids and derivatives | 0.86 | down | <0.05 | 3.44 |
| Palmitic acid | Lipids and lipid-like molecules | 0.82 | down | <0.05 | 6.56 |
| 4,6-diamino-5-formamidopyrimidine | Organoheterocyclic compounds | 0.80 | down | <0.05 | 1.24 |
| Myristic acid | Lipids and lipid-like molecules | 0.77 | down | <0.05 | 1.06 |
| Taurine | Organic acids and derivatives | 0.73 | down | <0.05 | 5.10 |
| Pc 38:4 | Undefined | 0.68 | down | <0.05 | 1.11 |
| 1-oleoyl-2-hydroxy-sn-glycero-3-phospho-(1′-rac-glycerol) | Lipids and lipid-like molecules | 0.67 | down | <0.05 | 1.07 |
| Malate | Organic acids and derivatives | 0.61 | down | <0.05 | 2.14 |
| Trifluoroacetic acid | Organic acids and derivatives | 0.55 | down | <0.05 | 1.32 |
| Pyruvate | Organic acids and derivatives | 0.55 | down | <0.05 | 7.65 |
| Glyceric acid | Organic oxygen compounds | 0.55 | down | <0.05 | 1.02 |
| 3,4-dihydroxyhydrocinnamic acid | Undefined | 0.54 | down | <0.05 | 1.10 |
| Cis,cis-muconic acid | Lipids and lipid-like molecules | 0.51 | down | <0.05 | 5.76 |
| Positive ion mode | |||||
| Ala-Lys | Organic acids and derivatives | 4.65 | up | <0.05 | 1.96 |
| Carbidopa | Phenylpropanoids and polyketides | 2.65 | up | <0.05 | 3.14 |
| β-nicotinamide adenine dinucleotide(NAD) | Undefined | 2.44 | up | <0.05 | 1.08 |
| Adenine | Organoheterocyclic compounds | 2.30 | up | <0.05 | 1.21 |
| 1-palmitoyl-2-oleoyl-sn-glycerol | Lipids and lipid-like molecules | 2.28 | up | <0.05 | 1.15 |
| 2-epahpa [dmed-fahfa] | Undefined | 2.19 | up | <0.05 | 1.58 |
| 1,2-dipalmitoleoyl-sn-glycero-3-phosphocholine | Lipids and lipid-like molecules | 2.18 | up | <0.05 | 2.46 |
| 3-pyridinepropionic acid | Undefined | 2.16 | up | <0.05 | 1.28 |
| Arg-Ala | Organic acids and derivatives | 2.11 | up | <0.05 | 2.26 |
| 1,2-dilinoleoylglycerol | Lipids and lipid-like molecules | 2.09 | up | <0.05 | 1.12 |
| Isopropyl 4-hydroxybenzoate | Benzenoids | 2.07 | up | <0.05 | 1.71 |
| Anthranilic acid | Benzenoids | 2.06 | up | <0.05 | 1.54 |
| Lpc 18:2 | Lipids and lipid-like molecules | 2.00 | up | <0.05 | 3.70 |
| DL-arginine | Organic acids and derivatives | 1.99 | up | <0.05 | 1.29 |
| 1,2-diamino-2-methylpropane | Organic nitrogen compounds | 1.97 | up | <0.05 | 1.09 |
| Methylergonovine | Alkaloids and derivatives | 1.95 | up | <0.05 | 1.06 |
| N-acetylhistidine | Organic acids and derivatives | 1.94 | up | <0.05 | 2.69 |
| Thioetheramide-PC | Undefined | 1.87 | up | <0.05 | 17.77 |
| N-Acetyl-L-Histidine | Undefined | 1.74 | up | <0.05 | 3.00 |
| 1-hexadecanoyl-2-octadecadienoyl-sn-glycero-3-phosphocholine | Lipids and lipid-like molecules | 1.74 | up | <0.05 | 24.45 |
| N-α-(tert-butoxycarbonyl)-l-histidine | Organic acids and derivatives | 1.72 | up | <0.05 | 3.84 |
| 3-dehydrocarnitine | Organic acids and derivatives | 1.67 | up | <0.05 | 1.14 |
| PC(16:0/16:0) | Lipids and lipid-like molecules | 1.60 | up | <0.05 | 2.74 |
| 1,2-dilinoleoyl-sn-glycero-3-phosphoethanolamine | Lipids and lipid-like molecules | 1.57 | up | <0.05 | 1.81 |
| N-α-acetyl-l-ornithine | Organic acids and derivatives | 1.53 | up | <0.05 | 1.32 |
| L-Histidine | Organic acids and derivatives | 1.45 | up | <0.05 | 2.22 |
| Pantothenic acid | Organic acids and derivatives | 1.41 | up | <0.05 | 1.45 |
| 1-Stearoyl-2-arachidonoyl-sn-glycerol | Lipids and lipid-like molecules | 1.36 | up | <0.05 | 4.39 |
| 1-Palmitoylglycerol | Undefined | 1.27 | up | <0.05 | 1.12 |
| Inosine 5′-monophosphate | Nucleosides, nucleotides, and analogues | 1.25 | up | <0.05 | 5.66 |
| 1-hexadecyl-2-(8z,11z,14z-eicosatrienoyl)-sn-glycero-3-phosphocholine | Lipids and lipid-like molecules | 0.79 | down | <0.05 | 6.09 |
| 3-hydroxyanthranilic acid | Benzenoids | 0.77 | down | <0.05 | 5.70 |
| 2-oleoyl-1-stearoyl-sn-glycero-3-phosphoserine | Lipids and lipid-like molecules | 0.76 | down | <0.05 | 4.50 |
| Adenosine | Nucleosides, nucleotides, and analogues | 0.75 | down | <0.05 | 3.31 |
| 1-hexadecyl-2-(5z,8z,11z,14z-eicosatetraenoyl)-sn-glycero-3-phosphocholine | Lipids and lipid-like molecules | 0.73 | down | <0.05 | 3.05 |
| Betaine | Organic acids and derivatives | 0.72 | down | <0.05 | 15.85 |
| 1-(1z-octadecenyl)-2-(4z,7z,10z,13z,16z,19z-docosahexaenoyl)-sn-glycero-3-phosphocholine | Lipids and lipid-like molecules | 0.66 | down | <0.05 | 1.71 |
| Pro-leu | Organic acids and derivatives | 0.66 | down | <0.05 | 13.01 |
| Pheniramine | Organoheterocyclic compounds | 0.61 | down | <0.05 | 1.71 |
| Carnitine | Organic nitrogen compounds | 0.60 | down | <0.05 | 12.08 |
| Methyl (1-(cyclohexylmethyl)-1h-indole-3-carbonyl)-l-valinate | Organic acids and derivatives | 0.57 | down | <0.05 | 1.12 |
| Palmitoyl sphingomyelin | Lipids and lipid-like molecules | 0.53 | down | <0.05 | 8.81 |
| L-Alanine | Organic acids and derivatives | 0.48 | down | <0.05 | 1.99 |
| Fenbuconazole | Undefined | 0.41 | down | <0.05 | 1.92 |
| Group | Classification | KEGG Pathway | Metabolite |
|---|---|---|---|
| Group H | Unsaturated fatty acids metabolism | Arachidonic acid metabolism | Pc(16:0/16:1) |
| Linoleic acid metabolism | Pc(16:0/16:0) | ||
| Alpha-linolenic acid metabolism | Pc(16:0/16:1) | ||
| Amino acid metabolism | Phenylalanine, tyrosine and tryptophan biosynthesis | Anthranilic acid | |
| D-arginine and d-ornithine metabolism | Dl-arginine | ||
| Arginine biosynthesis | N-alpha-acetyl-l-ornithine | ||
| Histidine metabolism | L-histidine, Histamine, L-carnosine | ||
| Beta-alanine metabolism | Histidine, L-carnosine, L-histidine, Pantothenic acid | ||
| Reproduction pathway | GnRH signaling pathway | Diacylglycerol(18:0/20:4) | |
| Group G | Energy metabolism | Glycolysis/gluconeogenesis | Pyruvate |
| Citrate cycle (TCA cycle) | Pyruvate, Malate | ||
| Pentose phosphate pathway | Pyruvate, Glyceric acid | ||
| Pentose and glucuronate interconversions | Pyruvate | ||
| Pyruvate metabolism | Pyruvate, Malate | ||
| Carbon metabolism | Pyruvate, Malate, Glyceric acid, O-acetylserine, L-alanine | ||
| Fatty acid synthesis and metabolism | Fatty acid biosynthesis | Myristic acid, Palmitic acid | |
| Fatty acid elongation | Palmitic acid | ||
| Fatty acid degradation | Palmitic acid | ||
| Biosynthesis of unsaturated fatty acids | Palmitic acid | ||
| Fatty acid metabolism | Palmitic acid | ||
| Glycerolipid metabolism | Glyceric acid |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yin, L.; Liu, L.; Tang, Y.; Chen, Q.; Zhang, D.; Lin, Z.; Wang, Y.; Liu, Y. The Implications in Meat Quality and Nutrition by Comparing the Metabolites of Pectoral Muscle between Adult Indigenous Chickens and Commercial Laying Hens. Metabolites 2023, 13, 840. https://doi.org/10.3390/metabo13070840
Yin L, Liu L, Tang Y, Chen Q, Zhang D, Lin Z, Wang Y, Liu Y. The Implications in Meat Quality and Nutrition by Comparing the Metabolites of Pectoral Muscle between Adult Indigenous Chickens and Commercial Laying Hens. Metabolites. 2023; 13(7):840. https://doi.org/10.3390/metabo13070840
Chicago/Turabian StyleYin, Lingqian, Li Liu, Yuan Tang, Qian Chen, Donghao Zhang, Zhongzhen Lin, Yan Wang, and Yiping Liu. 2023. "The Implications in Meat Quality and Nutrition by Comparing the Metabolites of Pectoral Muscle between Adult Indigenous Chickens and Commercial Laying Hens" Metabolites 13, no. 7: 840. https://doi.org/10.3390/metabo13070840
APA StyleYin, L., Liu, L., Tang, Y., Chen, Q., Zhang, D., Lin, Z., Wang, Y., & Liu, Y. (2023). The Implications in Meat Quality and Nutrition by Comparing the Metabolites of Pectoral Muscle between Adult Indigenous Chickens and Commercial Laying Hens. Metabolites, 13(7), 840. https://doi.org/10.3390/metabo13070840





