Crosstalk between Breast Milk N-Acetylneuraminic Acid and Infant Growth in a Gut Microbiota-Dependent Manner
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Cohorts and Sample Collection
2.2. Assessment of Infant Growth
2.3. Quantification of Neu5Ac and Sialylated Oligosaccharides in Breast Milk
2.4. Fecal Microbiota Transplantation to Germ-Free Mice
2.5. Metagenomic Analysis of the Newborn Gut Microbiota
2.6. Bile Acid Analysis
2.7. Statistical Analysis
3. Results
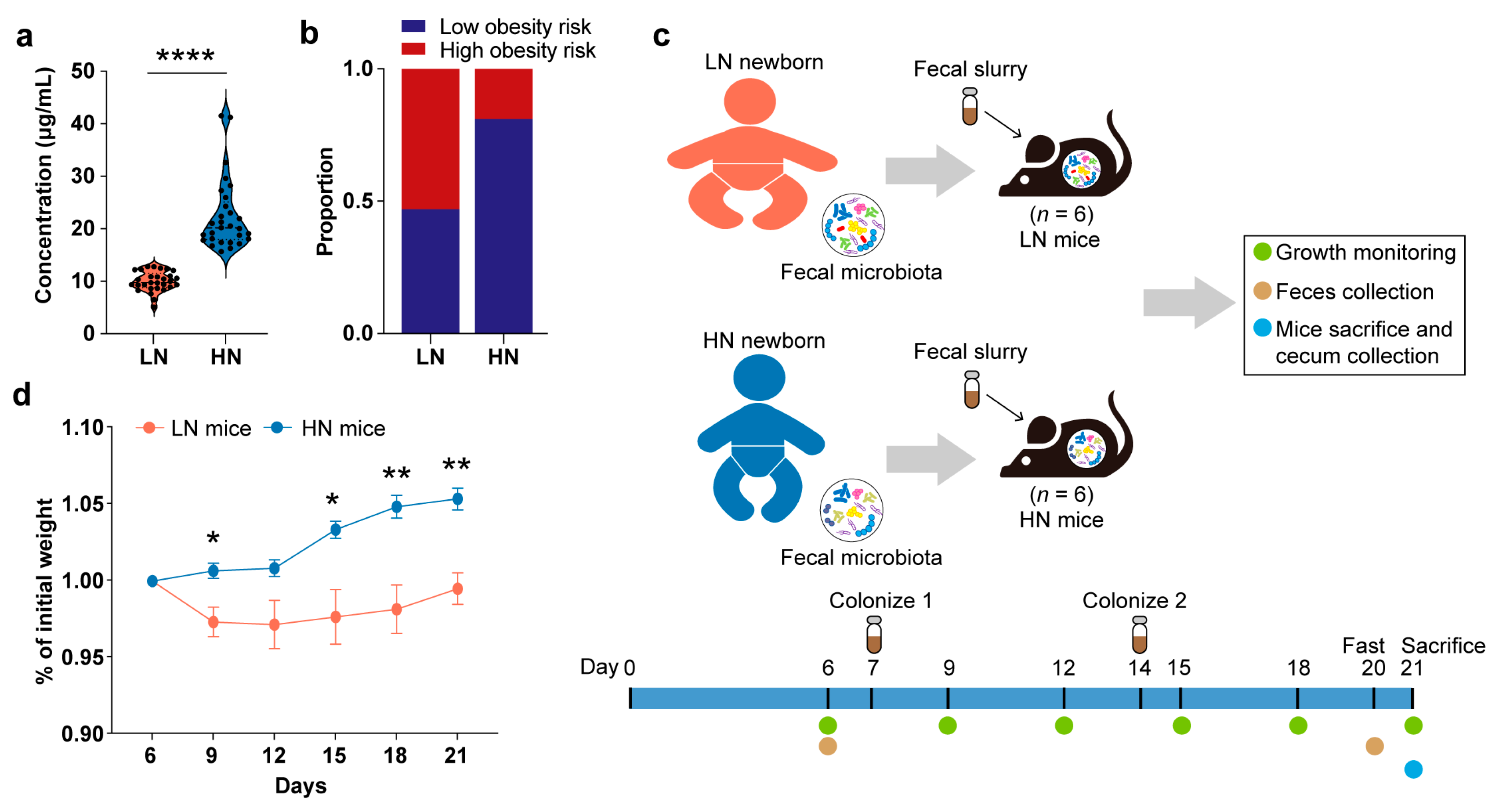
3.1. Correlation between Breast Milk Neu5Ac Concentrations and Obesity Risk in Chinese Infants
3.2. Gut Microbiota-Dependent Association between Breast Milk Neu5Ac and Growth
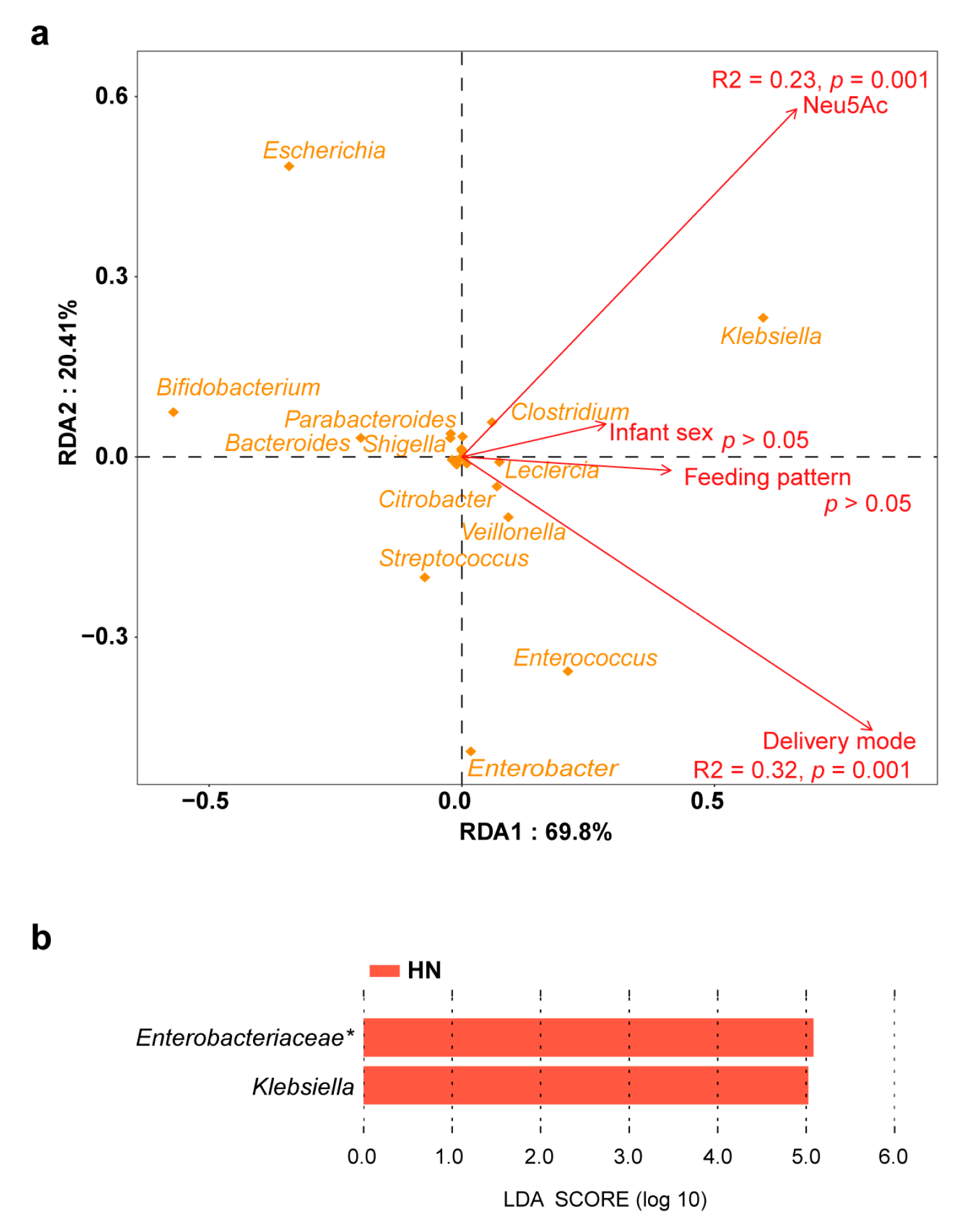
3.3. Effect of Breast Milk Neu5Ac on the Composition and Function of Gut Microbiota
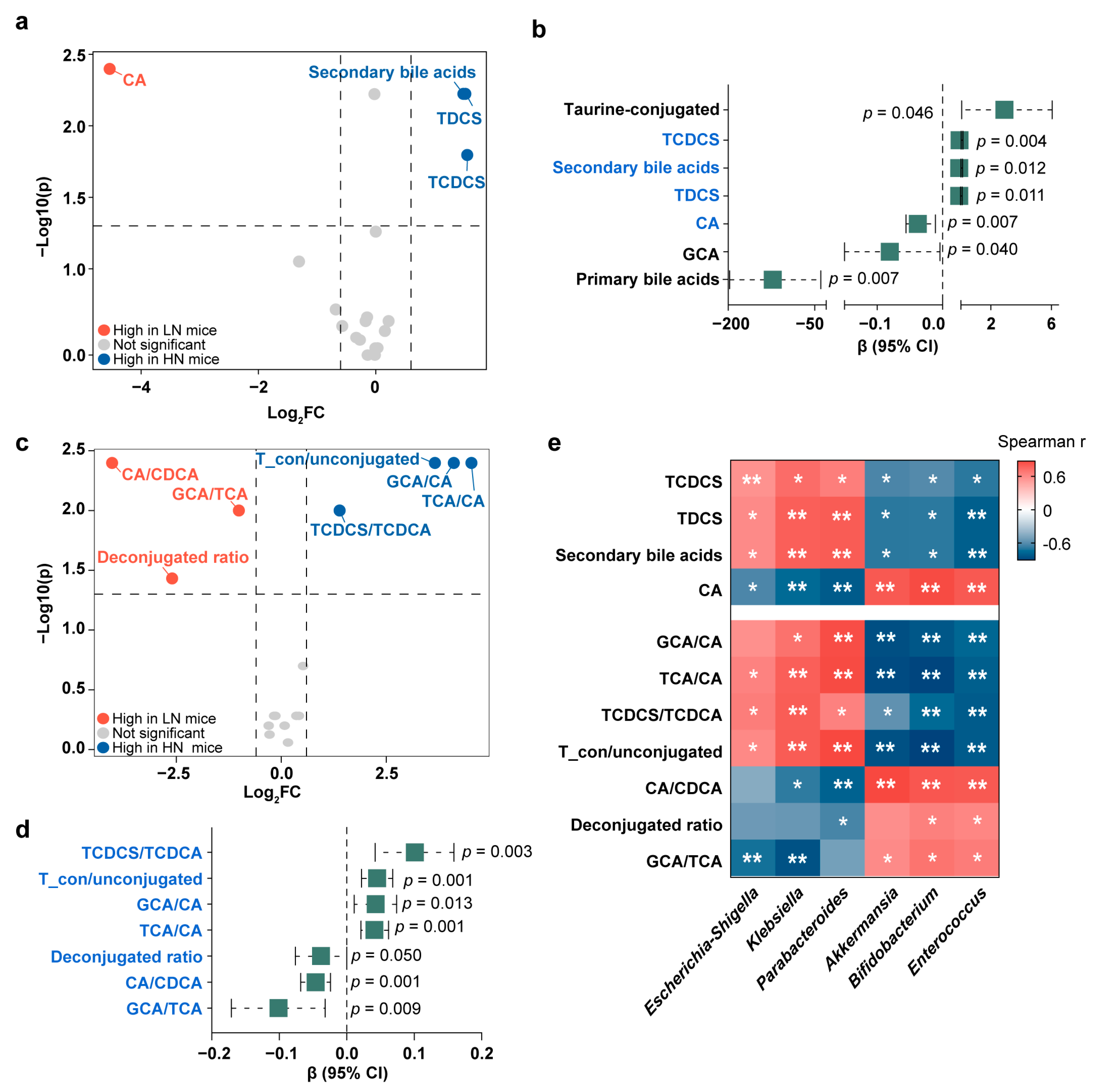
3.4. Conjugated Bile Acids in the Correlations between Breast Milk Neu5Ac and Infant Obesity Risk
3.5. Validation of the Interactions among Neu5Ac, Gut Microbiota, Bile Acid Metabolism, and Healthy Growth in Gnotobiotic Mice
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zucker, I.; Zloof, Y.; Bardugo, A.; Tsur, A.M.; Lutski, M.; Cohen, Y.; Cukierman-Yaffe, T.; Minsky, N.; Derazne, E.; Tzur, D.; et al. Obesity in late adolescence and incident type 1 diabetes in young adulthood. Diabetologia 2022, 65, 1473–1482. [Google Scholar] [CrossRef] [PubMed]
- Umer, A.; Kelley, G.A.; Cottrell, L.E.; Giacobbi, P., Jr.; Innes, K.E.; Lilly, C.L. Childhood obesity and adult cardiovascular disease risk factors: A systematic review with meta-analysis. BMC Public Health 2017, 17, 683. [Google Scholar] [CrossRef]
- World Health Organization. WHO Child Growth Standards; World Health Organization: Geneva, Switzerland, 2006. [Google Scholar]
- Forbes, J.D.; Azad, M.B.; Vehling, L.; Tun, H.M.; Konya, T.B.; Guttman, D.S.; Field, C.J.; Lefebvre, D.; Sears, M.R.; Becker, A.B.; et al. Association of exposure to formula in the hospital and subsequent infant feeding practices with gut microbiota and risk of overweight in the first year of life. JAMA Pediatr. 2018, 172, e181161. [Google Scholar] [CrossRef] [PubMed]
- NCD Risk Factor Collaboration (NCD-RisC). Worldwide trends in body-mass index, underweight, overweight, and obesity from 1975 to 2016: A pooled analysis of 2416 population-based measurement studies in 128·9 million children, adolescents, and adults. Lancet 2017, 390, 2627–2642. [Google Scholar] [CrossRef]
- Stanislawski, M.A.; Dabelea, D.; Wagner, B.D.; Iszatt, N.; Dahl, C.; Sontag, M.K.; Knight, R.; Lozupone, C.A.; Eggesbo, M. Gut microbiota in the first 2 years of life and the association with body mass index at age 12 in a norwegian birth cohort. mBio 2018, 9, e01751-18. [Google Scholar] [CrossRef] [PubMed]
- Alderete, T.L.; Jones, R.B.; Shaffer, J.P.; Holzhausen, E.A.; Patterson, W.B.; Kazemian, E.; Chatzi, L.; Knight, R.; Plows, J.F.; Berger, P.K.; et al. Early life gut microbiota is associated with rapid infant growth in Hispanics from Southern California. Gut Microbes 2021, 13, 1961203. [Google Scholar] [CrossRef]
- Fehr, K.; Moossavi, S.; Sbihi, H.; Boutin, R.C.T.; Bode, L.; Robertson, B.; Yonemitsu, C.; Field, C.J.; Becker, A.B.; Mandhane, P.J.; et al. Breastmilk feeding practices are associated with the co-occurrence of bacteria in mothers’ milk and the infant gut: The CHILD cohort study. Cell Host Microbe 2020, 28, 285–297.e284. [Google Scholar] [CrossRef]
- Lis-Kuberka, J.; Orczyk-Pawilowicz, M. Sialylated oligosaccharides and glycoconjugates of human milk. The impact on infant and newborn protection, development and well-being. Nutrients 2019, 11, 306. [Google Scholar] [CrossRef]
- Charbonneau, M.R.; O’Donnell, D.; Blanton, L.V.; Totten, S.M.; Davis, J.C.; Barratt, M.J.; Cheng, J.; Guruge, J.; Talcott, M.; Bain, J.R.; et al. Sialylated milk oligosaccharides promote microbiota-dependent growth in models of infant undernutrition. Cell 2016, 164, 859–871. [Google Scholar] [CrossRef]
- Harris, J.E.; Pinckard, K.M.; Wright, K.R.; Baer, L.A.; Arts, P.J.; Abay, E.; Shettigar, V.K.; Lehnig, A.C.; Robertson, B.; Madaris, K.; et al. Exercise-induced 3′-sialyllactose in breast milk is a critical mediator to improve metabolic health and cardiac function in mouse offspring. Nat. Metab. 2020, 2, 678–687. [Google Scholar] [CrossRef]
- Juge, N.; Tailford, L.; Owen, C.D. Sialidases from gut bacteria: A mini-review. Biochem. Soc. Trans. 2016, 44, 166–175. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Brand-Miller, J.; McVeagh, P.; Petocz, P. Concentration and distribution of sialic acid in human milk and infant formulas. Am. J. Clin. Nutr. 2001, 74, 510–515. [Google Scholar] [CrossRef] [PubMed]
- Claumarchirant, L.; Sanchez-Siles, L.M.; Matencio, E.; Alegria, A.; Lagarda, M.J. Evaluation of sialic acid in infant feeding: Contents and bioavailability. J. Agric. Food Chem. 2016, 64, 8333–8342. [Google Scholar] [CrossRef] [PubMed]
- Varki, A. Sialic acids in human health and disease. Trends Mol. Med. 2008, 14, 351–360. [Google Scholar] [CrossRef] [PubMed]
- Ding, J.; Ouyang, R.; Zheng, S.; Wang, Y.; Huang, Y.; Ma, X.; Zou, Y.; Chen, R.; Zhuo, Z.; Li, Z.; et al. Effect of breastmilk microbiota and sialylated oligosaccharides on the colonization of infant gut microbial community and fecal metabolome. Metabolites 2022, 12, 1136. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Zhou, Y.; Chen, Y.; Gu, J. Fastp: An ultra-fast all-in-one fastq preprocessor. Bioinformatics 2018, 34, i884–i890. [Google Scholar] [CrossRef]
- Li, H.; Durbin, R. Fast and accurate short read alignment with burrows-wheeler transform. Bioinformatics 2009, 25, 1754–1760. [Google Scholar] [CrossRef]
- Li, D.; Liu, C.-M.; Luo, R.; Sadakane, K.; Lam, T.-W. Megahit: An ultra-fast single-node solution for large and complex metagenomics assembly via succinct de bruijn graph. Bioinformatics 2015, 31, 1674–1676. [Google Scholar] [CrossRef]
- Noguchi, H.; Park, J.; Takagi, T. Metagene: Prokaryotic gene finding from environmental genome shotgun sequences. Nucleic Acids Res. 2006, 34, 5623–5630. [Google Scholar] [CrossRef]
- Fu, L.; Niu, B.; Zhu, Z.; Wu, S.; Li, W. CD-HIT: Accelerated for clustering the next-generation sequencing data. Bioinformatics 2012, 28, 3150–3152. [Google Scholar] [CrossRef]
- Li, R.; Li, Y.; Kristiansen, K.; Wang, J. SOAP: Short oligonucleotide alignment program. Bioinformatics 2008, 24, 713–714. [Google Scholar] [CrossRef]
- Qin, J.; Li, Y.; Cai, Z.; Li, S.; Zhu, J.; Zhang, F.; Liang, S.; Zhang, W.; Guan, Y.; Shen, D.; et al. A metagenome-wide association study of gut microbiota in type 2 diabetes. Nature 2012, 490, 55–60. [Google Scholar] [CrossRef]
- Buchfink, B.; Xie, C.; Huson, D.H. Fast and sensitive protein alignment using diamond. Nat. Methods 2015, 12, 59–60. [Google Scholar] [CrossRef]
- Bäckhed, F.; Roswall, J.; Peng, Y.; Feng, Q.; Jia, H.; Kovatcheva-Datchary, P.; Li, Y.; Xia, Y.; Xie, H.; Zhong, H.; et al. Dynamics and stabilization of the human gut microbiome during the first year of life. Cell Host Microbe 2015, 17, 690–703. [Google Scholar] [CrossRef]
- Segata, N.; Izard, J.; Waldron, L.; Gevers, D.; Miropolsky, L.; Garrett, W.S.; Huttenhower, C. Metagenomic biomarker discovery and explanation. Genome Biol. 2011, 12, R60. [Google Scholar] [CrossRef]
- Parks, D.H.; Tyson, G.W.; Hugenholtz, P.; Beiko, R.G. STAMP: Statistical analysis of taxonomic and functional profiles. Bioinformatics 2014, 30, 3123–3124. [Google Scholar] [CrossRef]
- Saben, J.L.; Sims, C.R.; Abraham, A.; Bode, L.; Andres, A. Human milk oligosaccharide concentrations and infant intakes are associated with maternal overweight and obesity and predict infant growth. Nutrients 2021, 13, 446. [Google Scholar] [CrossRef]
- Chen, Z.; Chang, Y.; Liu, H.; You, Y.; Liu, Y.; Yu, X.; Dou, Y.; Ma, D.; Chen, L.; Tong, X.; et al. Distribution and influencing factors of the sialic acid content in the breast milk of preterm mothers at different stages. Front. Nutr. 2022, 9, 753919. [Google Scholar] [CrossRef]
- Han, S.M.; Derraik, J.G.B.; Binia, A.; Sprenger, N.; Vickers, M.H.; Cutfield, W.S. Maternal and infant factors influencing human milk oligosaccharide composition: Beyond maternal genetics. J. Nutr. 2021, 151, 1383–1393. [Google Scholar] [CrossRef]
- Armstrong, J.; Reilly, J.J. Breastfeeding and lowering the risk of childhood obesity. Lancet 2002, 359, 2003–2004. [Google Scholar] [CrossRef]
- Quecke, B.; Graf, Y.; Epure, A.-M.; Santschi, V.; Chiolero, A.; Carmeli, C.; Cullati, S. Caesarean section and obesity in young adult offspring: Update of a systematic review with meta-analysis. Obes. Rev. 2022, 23, e13368. [Google Scholar] [CrossRef] [PubMed]
- Gal, B.; Ruano, M.J.; Puente, R.; García-Pardo, L.A.; Rueda, R.; Gil, A.; Hueso, P. Developmental changes in UDP-N-acetylglucosamine 2-epimerase activity of rat and guinea-pig liver. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 1997, 118, 13–15. [Google Scholar] [CrossRef] [PubMed]
- Kiyohara, M.; Tanigawa, K.; Chaiwangsri, T.; Katayama, T.; Ashida, H.; Yamamoto, K. An exo-alpha-sialidase from bifidobacteria involved in the degradation of sialyloligosaccharides in human milk and intestinal glycoconjugates. Glycobiology 2011, 21, 437–447. [Google Scholar] [CrossRef] [PubMed]
- McDonald, N.D.; Lubin, J.B.; Chowdhury, N.; Boyd, E.F. Host-derived sialic acids are an important nutrient source required for optimal bacterial fitness in vivo. mBio 2016, 7, e02237-15. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, J.P.; Xie, G.; Raufman, J.-P.; Hogan, S.; Griffin, T.L.; Packard, C.A.; Chatfield, D.A.; Hagey, L.R.; Steinbach, J.H.; Hofmann, A.F. Human cecal bile acids: Concentration and spectrum. Am. J. Physiol. Gastrointest. Liver Physiol. 2007, 293, G256–G263. [Google Scholar] [CrossRef]
- Guzior, D.V.; Quinn, R.A. Review: Microbial transformations of human bile acids. Microbiome 2021, 9, 140. [Google Scholar] [CrossRef]
- Alnouti, Y. Bile acid sulfation: A pathway of bile acid elimination and detoxification. Toxicol. Sci. 2009, 108, 225–246. [Google Scholar] [CrossRef]
- Sillner, N.; Walker, A.; Lucio, M.; Maier, T.V.; Bazanella, M.; Rychlik, M.; Haller, D.; Schmitt-Kopplin, P. Longitudinal profiles of dietary and microbial metabolites in formula- and breastfed infants. Front. Mol. Biosci. 2021, 8, 660456. [Google Scholar] [CrossRef]
- Franzosa, E.A.; Sirota-Madi, A.; Avila-Pacheco, J.; Fornelos, N.; Haiser, H.J.; Reinker, S.; Vatanen, T.; Hall, A.B.; Mallick, H.; McIver, L.J.; et al. Gut microbiome structure and metabolic activity in inflammatory bowel disease. Nat. Microbiol. 2019, 4, 293–305. [Google Scholar] [CrossRef]
- Wang, Y.; Gao, X.; Zhang, X.; Xiao, Y.; Huang, J.; Yu, D.; Li, X.; Hu, H.; Ge, T.; Li, D.; et al. Gut microbiota dysbiosis is associated with altered bile acid metabolism in infantile cholestasis. mSystems 2019, 4, e00463-19. [Google Scholar] [CrossRef]
- Jia, W.; Xie, G.; Jia, W. Bile acid-microbiota crosstalk in gastrointestinal inflammation and carcinogenesis. Nat. Rev. Gastroenterol. Hepatol. 2017, 15, 111–128. [Google Scholar] [CrossRef]
- Perino, A.; Schoonjans, K. Metabolic messengers: Bile acids. Nat. Metab. 2022, 4, 416–423. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Setchell, K.D.R.; Huang, R.; Mallawaarachchi, I.; Ehsan, L.; Dobrzykowski, E., III; Zhao, J.; Syed, S.; Ma, J.Z.; Iqbal, N.T.; et al. Bile acid profiling reveals distinct signatures in undernourished children with environmental enteric dysfunction. J. Nutr. 2021, 151, 3689–3700. [Google Scholar] [CrossRef] [PubMed]
- So, S.S.Y.; Yeung, C.H.C.; Schooling, C.M.; El-Nezami, H. Targeting bile acid metabolism in obesity reduction: A systematic review and meta-analysis. Obes. Rev. 2020, 21, e13017. [Google Scholar] [CrossRef]
- Roberts, R.E.; Glicksman, C.; Alaghband-Zadeh, J.; Sherwood, R.A.; Akuji, N.; le Roux, C.W. The relationship between postprandial bile acid concentration, GLP-1, PYY and ghrelin. Clin. Endocrinol. 2011, 74, 67–72. [Google Scholar] [CrossRef]
- de Aguiar Vallim, T.Q.; Tarling, E.J.; Edwards, P.A. Pleiotropic roles of bile acids in metabolism. Cell Metab. 2013, 17, 657–669. [Google Scholar] [CrossRef]
- Fang, S.; Suh, J.M.; Reilly, S.M.; Yu, E.; Osborn, O.; Lackey, D.; Yoshihara, E.; Perino, A.; Jacinto, S.; Lukasheva, Y.; et al. Intestinal FXR agonism promotes adipose tissue browning and reduces obesity and insulin resistance. Nat. Med. 2015, 21, 159–165. [Google Scholar] [CrossRef] [PubMed]
- Varki, A. Uniquely human evolution of sialic acid genetics and biology. Proc. Natl. Acad. Sci. USA 2010, 107 (Suppl. 2), 8939–8946. [Google Scholar] [CrossRef]
- Moretti, C.H.; Schiffer, T.A.; Li, X.; Weitzberg, E.; Carlstrom, M.; Lundberg, J.O. Germ-free mice are not protected against diet-induced obesity and metabolic dysfunction. Acta Physiol. 2021, 231, e13581. [Google Scholar] [CrossRef]
- Hiltunen, H.; Hanani, H.; Luoto, R.; Turjeman, S.; Ziv, O.; Isolauri, E.; Salminen, S.; Koren, O.; Rautava, S. Preterm infant meconium microbiota transplant induces growth failure, inflammatory activation, and metabolic disturbances in germ-free mice. Cell Rep. Med. 2021, 2, 100447. [Google Scholar] [CrossRef]
- Honda, A.; Miyazaki, T.; Iwamoto, J.; Hirayama, T.; Morishita, Y.; Monma, T.; Ueda, H.; Mizuno, S.; Sugiyama, F.; Takahashi, S.; et al. Regulation of bile acid metabolism in mouse models with hydrophobic bile acid composition. J. Lipid Res. 2020, 61, 54–69. [Google Scholar] [CrossRef]
- Collins, S.L.; Stine, J.G.; Bisanz, J.E.; Okafor, C.D.; Patterson, A.D. Bile acids and the gut microbiota: Metabolic interactions and impacts on disease. Nat. Rev. Microbiol. 2023, 21, 236–247. [Google Scholar] [CrossRef]
- Bäckhed, F.; Ding, H.; Wang, T.; Hooper, L.V.; Koh, G.Y.; Nagy, A.; Semenkovich, C.F.; Gordon, J.I. The gut microbiota as an environmental factor that regulates fat storage. Proc. Natl. Acad. Sci. USA 2004, 101, 15718–15723. [Google Scholar] [CrossRef]
- Li, T.; Chiang, J.Y.L. Bile acid signaling in metabolic disease and drug therapy. Pharmacol. Rev. 2014, 66, 948–983. [Google Scholar] [CrossRef]
- Li, M.; Wang, S.; Li, Y.; Zhao, M.; Kuang, J.; Liang, D.; Wang, J.; Wei, M.; Rajani, C.; Ma, X.; et al. Gut microbiota-bile acid crosstalk contributes to the rebound weight gain after calorie restriction in mice. Nat. Commun. 2022, 13, 2060. [Google Scholar] [CrossRef]
- Wang, K.; Liao, M.; Zhou, N.; Bao, L.; Ma, K.; Zheng, Z.; Wang, Y.; Liu, C.; Wang, W.; Wang, J.; et al. Parabacteroides distasonis alleviates obesity and metabolic dysfunctions via production of succinate and secondary bile acids. Cell Rep. 2019, 26, 222–235. [Google Scholar] [CrossRef]



| Zhengzhou Cohort (n = 58) | Wuhan Cohort (n = 201) | |
|---|---|---|
| Sampling age of infant (days) | ||
| Breast milk | 4 (3–7) | 41 (33–43) |
| Newborn feces | 5 (3–7) | Not collected |
| Maternal characteristics | ||
| Gestational age (day) | 277 (272–281) | 273 (266–280) |
| Delivery mode | ||
| Natural delivery | 41 | 70 |
| C-section | 17 | 131 |
| Maternal antibiotic usage 1 | 23 | No information |
| Maternal BMI (kg/m2) 2 | ||
| Pre-pregnancy | 20.26 (18.81–22.63) | 20.06 (18.99–21.64) |
| Pre-delivery | 27.07 (24.57–29.55) | 27.32 (24.80–29.24) |
| Neonatal characteristics | ||
| Infant sex 3 | ||
| Male | 25 | 111 |
| Female | 33 | 83 |
| Feeding pattern 4 | ||
| Mostly breastmilk feeding | 18 | 102 |
| Mixed feeding | 40 | 98 |
| Growth indicator 5 | ||
| Infant age at time of BMI recording (months) | 12 (12–13) | 30 (24–36) |
| Infant BMI | 16.98 (16.01–18.41) | 16.78 (15.52–17.72) |
| Low obesity risk (No.) | 22 | 118 |
| High obesity risk (No.) | 13 | 83 |
| LN Group (n = 29) | HN Group (n = 29) | |
|---|---|---|
| Sampling age of infant (days) | ||
| Breast milk | 6 (4–13) | 4 (3–5) |
| Newborn feces | 5 (4–13) | 4 (3–5) |
| Maternal characteristics | ||
| Gestational age (day) | 277 (274–280) | 273 (270–284) |
| Delivery mode | ||
| Natural delivery | 25 | 16 |
| C-section | 4 | 13 |
| Maternal antibiotic usage 1 | 8 | 15 |
| Maternal BMI (kg/m2) 2 | ||
| Pre-pregnancy | 19.53 (18.78–22.03) | 21.57 (19.53–23.42) |
| Pre-delivery | 26.30 (24.45–28.40) | 27.55 (26.02–30.20) |
| Neonatal characteristics | ||
| Infant sex | ||
| Male | 13 | 12 |
| Female | 16 | 17 |
| Feeding pattern 3 | ||
| Mostly breastmilk feeding | 10 | 8 |
| Mixed feeding | 19 | 21 |
| Growth indicator 4 | ||
| Infant age at time of BMI recording (months) | 13 (12–13) | 12 (12–13) |
| Infant BMI | 17.97 (16.01–18.66) | 16.64 (16.00–17.12) |
| Low obesity risk (No.) | 9 | 13 |
| High obesity risk (No.) | 10 | 3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ouyang, R.; Zheng, S.; Wang, X.; Li, Q.; Ding, J.; Ma, X.; Zhuo, Z.; Li, Z.; Xin, Q.; Lu, X.; et al. Crosstalk between Breast Milk N-Acetylneuraminic Acid and Infant Growth in a Gut Microbiota-Dependent Manner. Metabolites 2023, 13, 846. https://doi.org/10.3390/metabo13070846
Ouyang R, Zheng S, Wang X, Li Q, Ding J, Ma X, Zhuo Z, Li Z, Xin Q, Lu X, et al. Crosstalk between Breast Milk N-Acetylneuraminic Acid and Infant Growth in a Gut Microbiota-Dependent Manner. Metabolites. 2023; 13(7):846. https://doi.org/10.3390/metabo13070846
Chicago/Turabian StyleOuyang, Runze, Sijia Zheng, Xiaolin Wang, Qi Li, Juan Ding, Xiao Ma, Zhihong Zhuo, Zhen Li, Qi Xin, Xin Lu, and et al. 2023. "Crosstalk between Breast Milk N-Acetylneuraminic Acid and Infant Growth in a Gut Microbiota-Dependent Manner" Metabolites 13, no. 7: 846. https://doi.org/10.3390/metabo13070846
APA StyleOuyang, R., Zheng, S., Wang, X., Li, Q., Ding, J., Ma, X., Zhuo, Z., Li, Z., Xin, Q., Lu, X., Zhou, L., Ren, Z., Mei, S., Liu, X., & Xu, G. (2023). Crosstalk between Breast Milk N-Acetylneuraminic Acid and Infant Growth in a Gut Microbiota-Dependent Manner. Metabolites, 13(7), 846. https://doi.org/10.3390/metabo13070846








