Aqueous Extracts of Fermented Macrofungi Cultivated in Oilseed Cakes as a Carbon Source for Probiotic Bacteria and Potential Antibacterial Activity
Abstract
:1. Introduction
2. Materials and Methods
2.1. Obtaining Supernatants and Extracts
2.2. Determination of Prebiotic Activity
2.3. Determination of Antimicrobial Activity
2.4. Statistical Analysis
3. Results
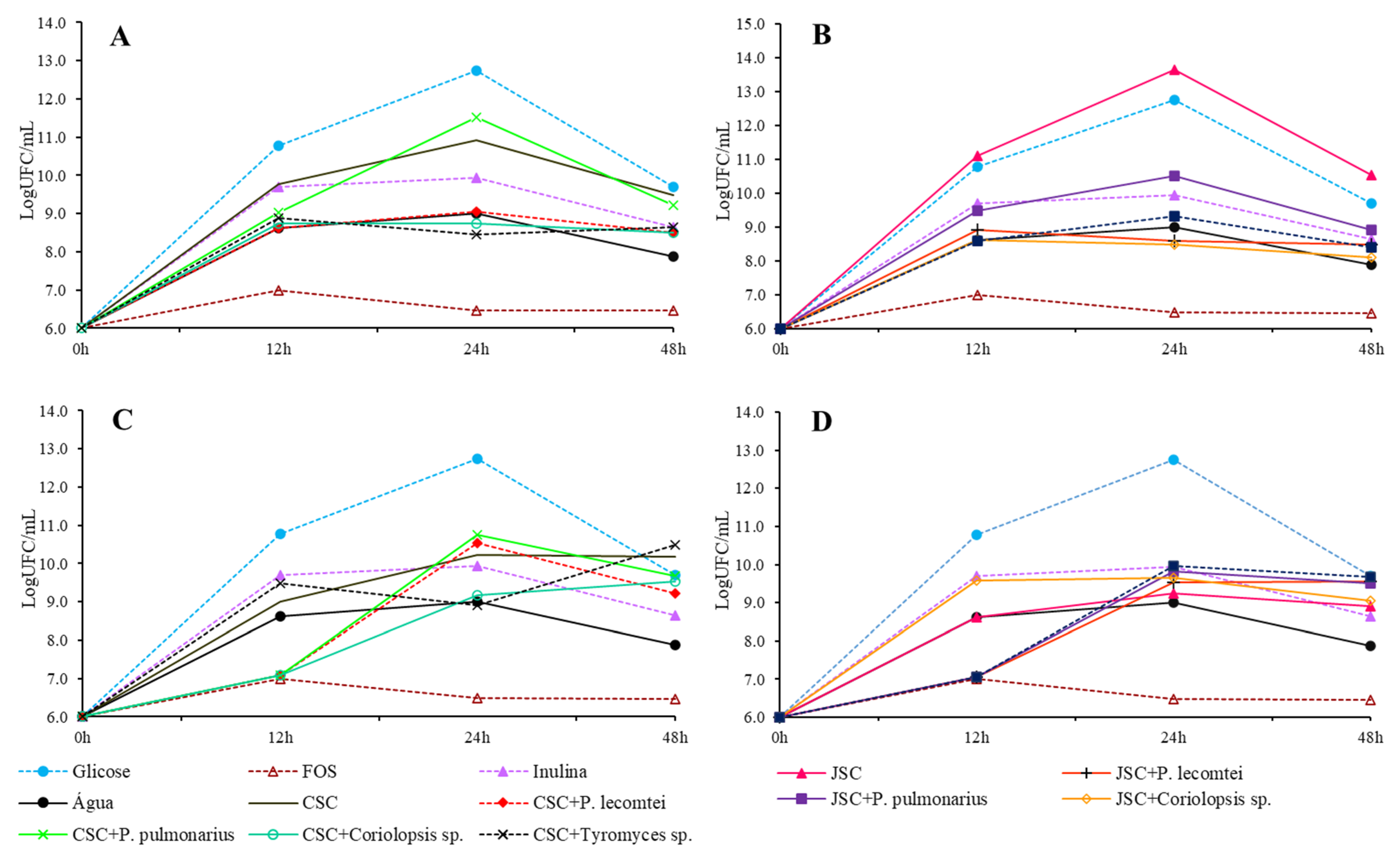
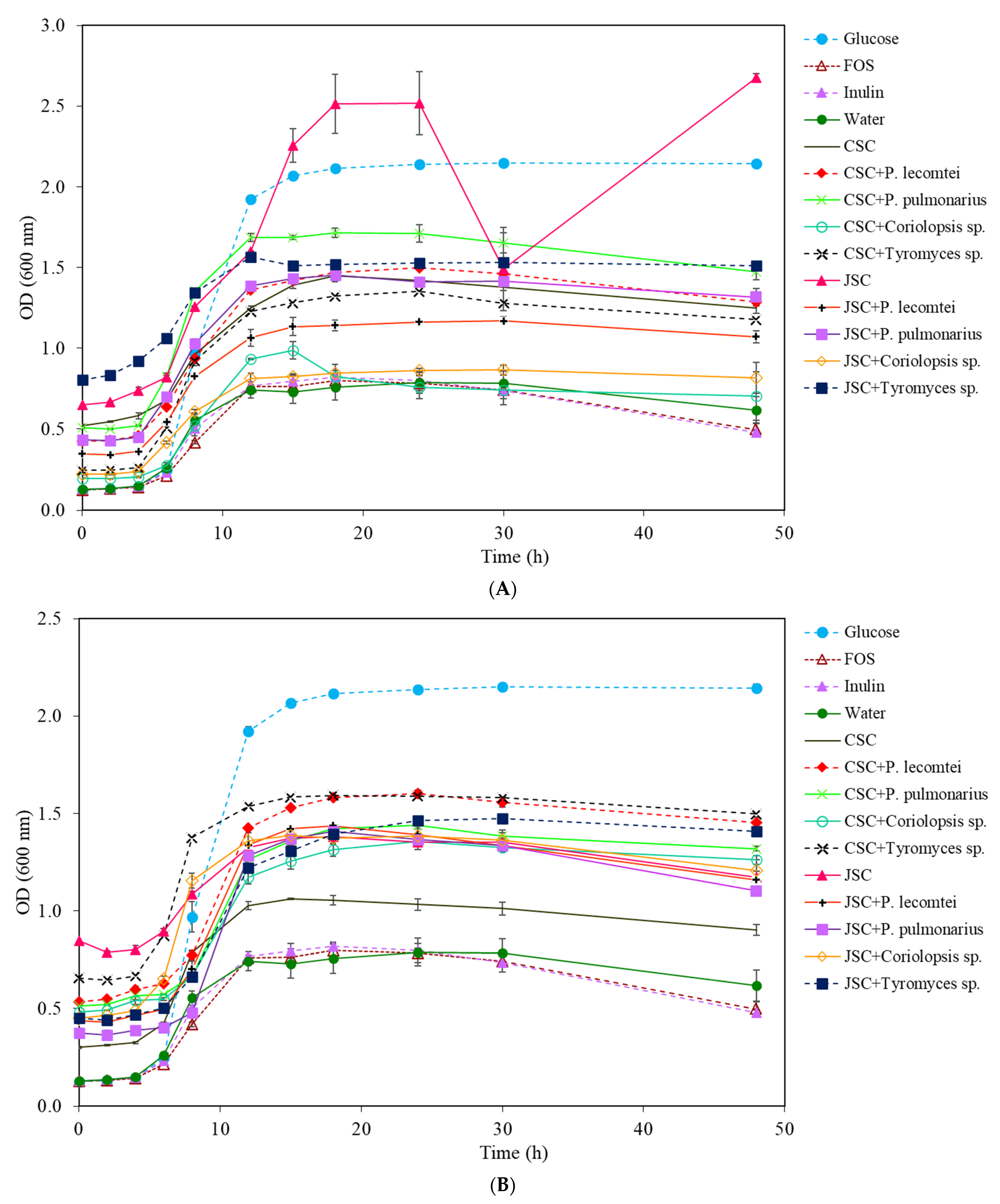
3.1. Prebiotic Activity
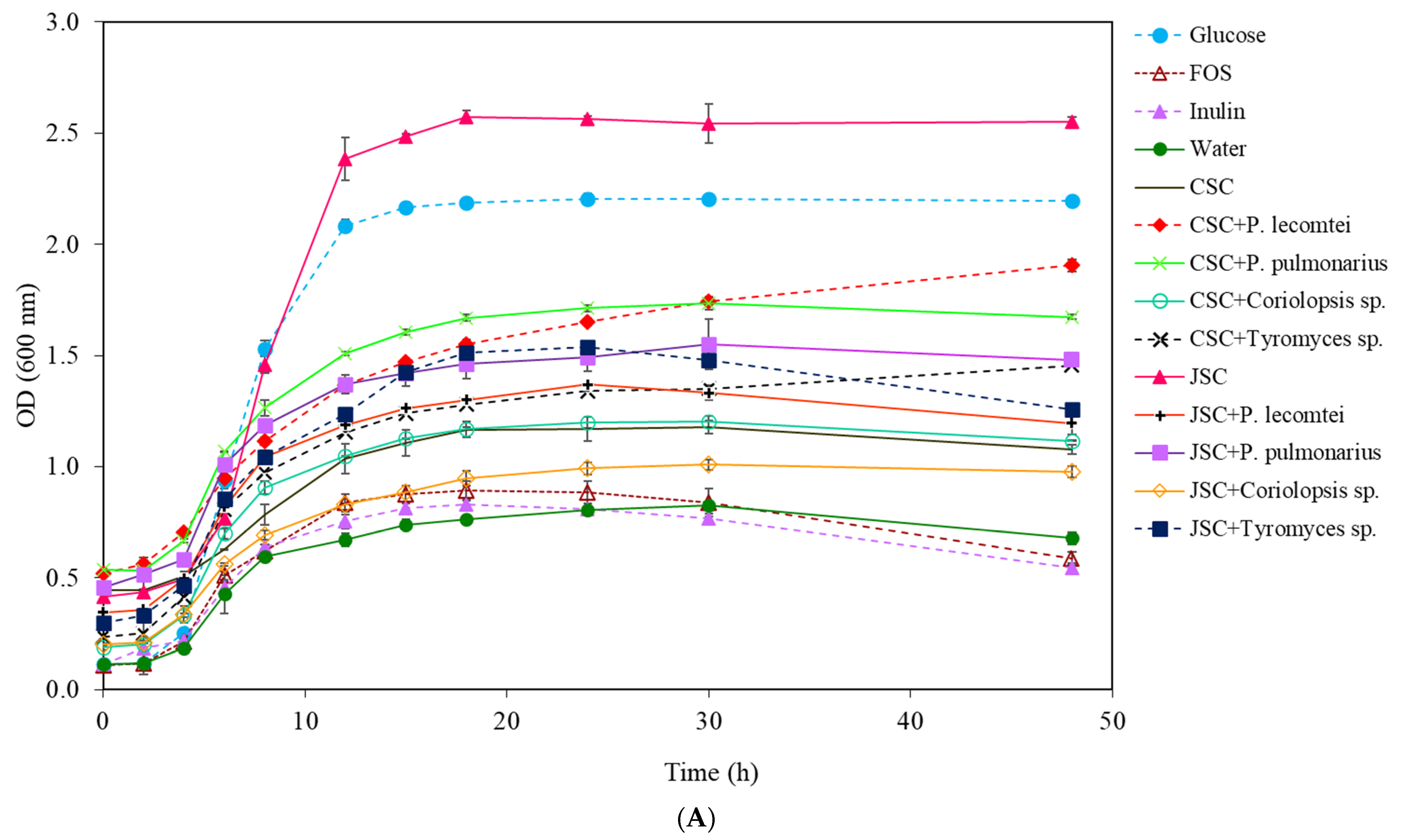
3.1.1. Lactobacillus acidophilus
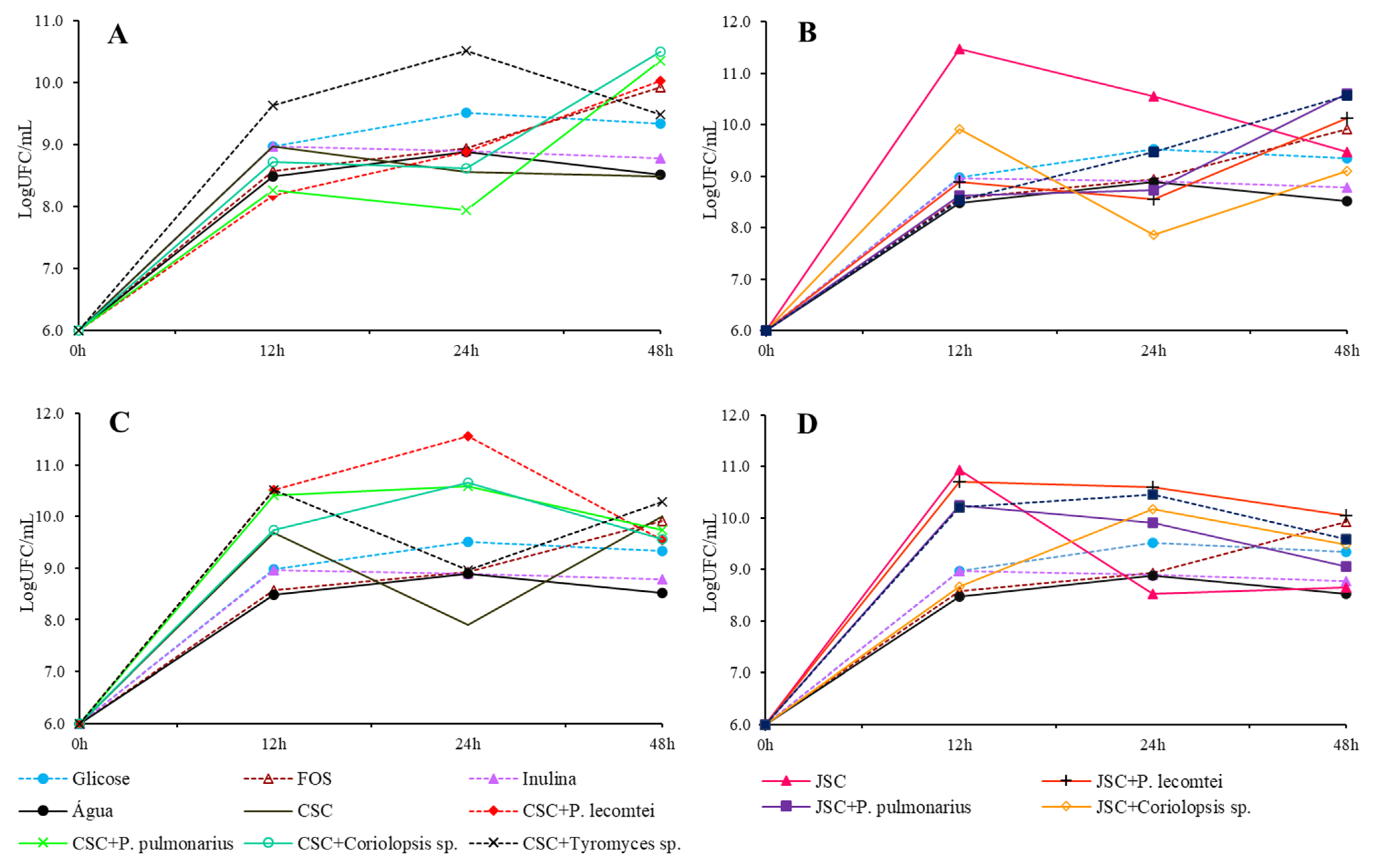
3.1.2. Bifidobacterium lactis
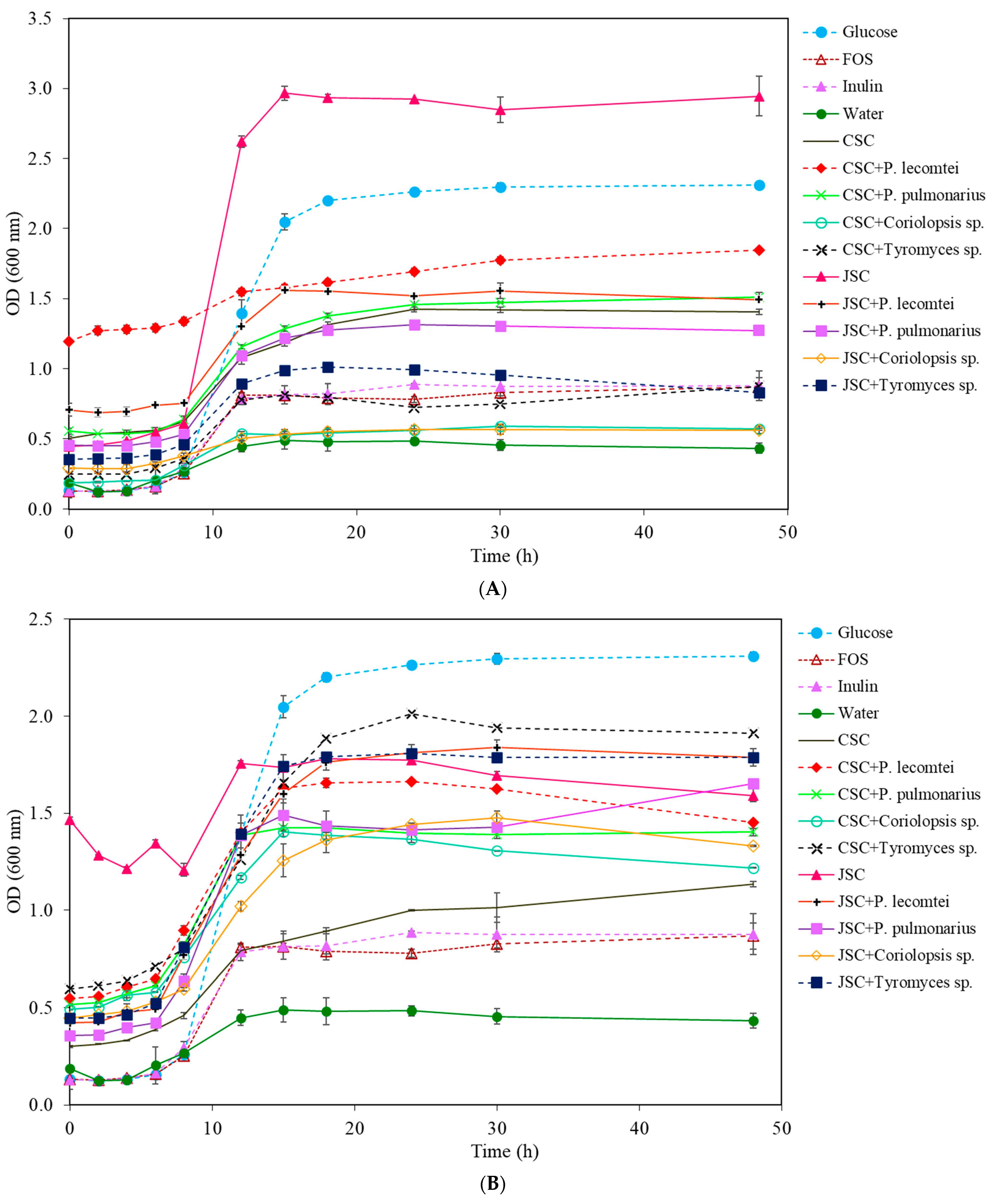
3.1.3. Lactobacillus rhamnosus
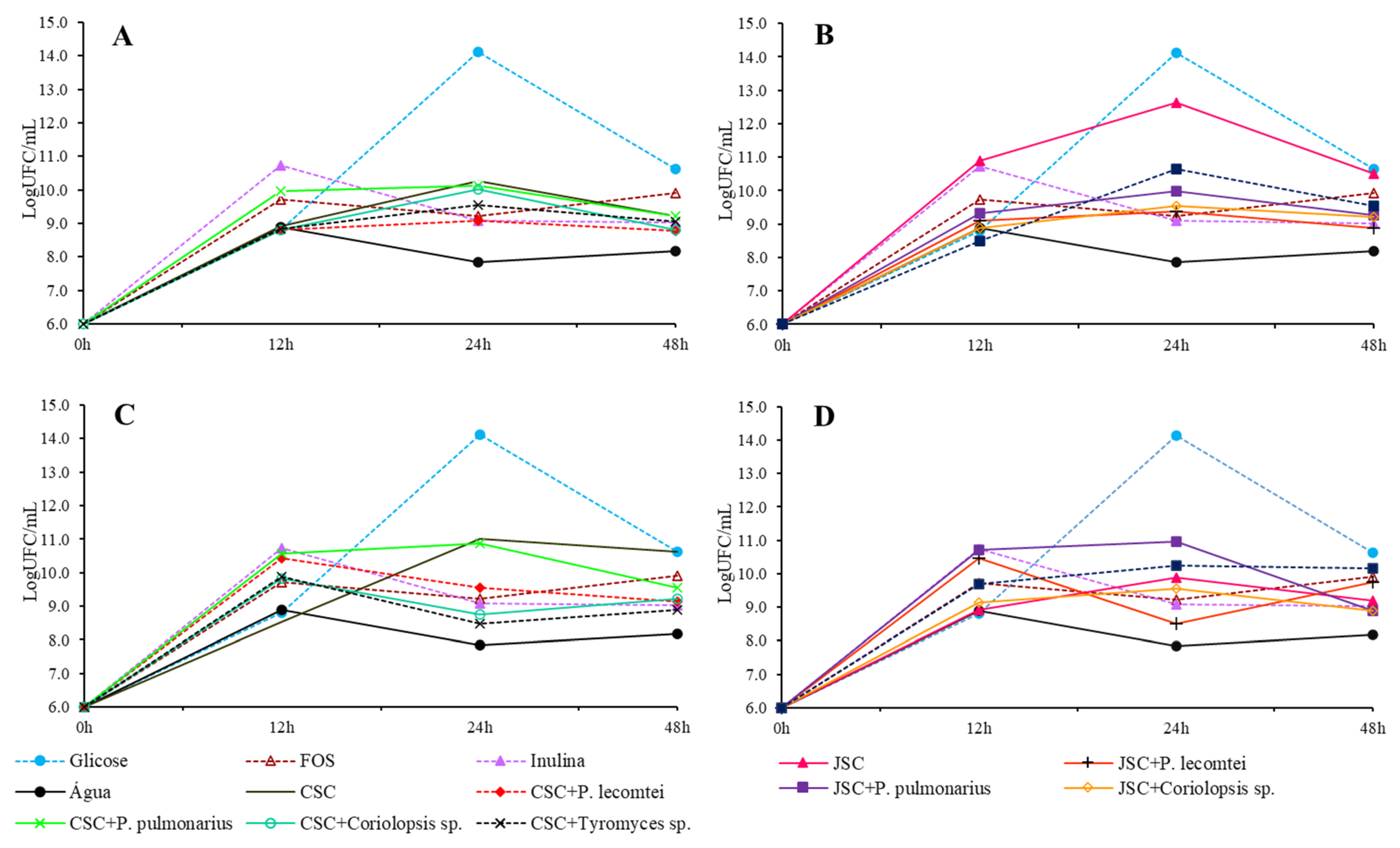
3.1.4. Lactobacillus plantarum
3.2. Antimicrobial Activity
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Adebayo, E.A.; Oloke, J.K.; Ayandele, A.A.; Adegunlola, C.O. Phytochemical, antioxidant and antimicrobial assay of mushroom metabolite from Pleurotus pulmonarius–LAU 09 (JF736658). J. Microbiol. Biotechnol. Res. 2012, 2, 366–374. [Google Scholar]
- Aida, F.M.N.A.; Shuhaimi, M.; Yazid, M.; Maaruf, A.G. Mushroom as a potential source of prebiotics: A review. Trends Food Sci. Technol. 2009, 20, 567–575. [Google Scholar] [CrossRef]
- Ruthes, A.C.; Smiderle, F.R.; Iacomini, M. Mushroom heteropolysaccharides: A review on their sources, structure and biological effects. Carbohydr. Polym. 2016, 136, 358–375. [Google Scholar] [CrossRef]
- Cheung, P.C. Mini-review on edible mushrooms as source of dietary fiber: Preparation and health benefits. Food Sci. Hum. Wellness 2013, 2, 162–166. [Google Scholar] [CrossRef] [Green Version]
- El Enshasy, H.A.; Hattikaul, R. Mushroom immunomodulators: Unique molecules with unlimited applications. Trends Biotechnol. 2013, 31, 668–677. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Bao, L.; Liu, D.; Yang, X.; Li, S.; Gao, H.; Yao, X.; Wen, H.; Liu, H. Two new sesquiterpenes and six norsesquiterpenes from the solid culture of the edible mushroom Flammulina velutipes. Tetrahedron 2012, 68, 3012–3018. [Google Scholar] [CrossRef]
- Lee, I.; Ahn, B.; Choi, J.; Hattori, M.; Min, B.; Bae, K. Selective cholinesterase inhibition by lanostane triterpenes from fruiting bodies of Ganoderma lucidum. Bioorg. Med. Chem. Lett. 2011, 21, 6603–6607. [Google Scholar] [CrossRef]
- Tel-Cayan, G.; Ozturk, M.; Duru, M.E.; Turkoglu, A. Fatty acid profiles in wild mushroom species from Anatolia. Chem. Nat. Compd. 2017, 53, 351–353. [Google Scholar] [CrossRef]
- Heleno, S.A.; Martins, A.; Queiroz, M.J.R.P.; Ferreira, I.C.F.R. Bioactivity of phenolic acids: Metabolites versus parent compounds: A review. Food Chem. 2015, 173, 501–513. [Google Scholar] [CrossRef] [Green Version]
- Ma, G.; Yang, W.; Zhao, L.; Pei, F.; Fang, D.; Hu, Q. A critical review on the health promoting effects of mushrooms nutraceuticals. Food Sci. Hum. Wellness 2018, 7, 125–133. [Google Scholar] [CrossRef]
- Rathore, H.; Prasad, S.; Sharm, S. Mushroom nutraceuticals for improved nutrition and better human health: A review. PharmaNutrition 2017, 5, 35–46. [Google Scholar] [CrossRef]
- Azmi, A.F.M.N.; Mustafa, S.; Hashim, D.M.; Manap, Y.A. Prebiotic activity of polysaccharides extracted from Gigantochloa levis (Buluh beting) shoots. Molecules 2012, 17, 1635–1651. [Google Scholar] [CrossRef] [PubMed]
- Kleerebezem, M.; Vaughan, E.E. Probiotic and gut lactobacilli and bifidobacteria: Molecular approaches to study diversity and activity. Annu. Rev. Microb. 2009, 63, 269–290. [Google Scholar] [CrossRef] [PubMed]
- Anjum, N.; Maqsood, S.; Masud, T.; Ahmad, A.; Sohail, A.; Momin, A. Lactobacillus acidophilus: Characterization of the species and application in food production. Crit. Rev. Food Sci. Nutr. 2014, 54, 1241–1251. [Google Scholar] [CrossRef]
- Tran, C.; Cock, I.E.; Chen, X.; Feng, Y. Antimicrobial Bacillus: Metabolites and Their Mode of Action. Antibiotics 2022, 11, 88. [Google Scholar] [CrossRef]
- Mitsuoka, T. Intestinal flora and human health. Asia Pac. J. Clin. Nutr. 1996, 5, 2–9. [Google Scholar] [CrossRef] [Green Version]
- Jayachandran, M.; Xiao, J.; Xu, B. A Critical Review on Health Promoting Benefits of Edible Mushrooms through Gut Microbiota. Int. J. Mol. Sci. 2017, 18, 1934. [Google Scholar] [CrossRef] [Green Version]
- Alves, M.J.; Ferreira, I.C.F.R.; Dias, J.; Teixeira, V.; Martins, A.; Pintado, M. A review on antimicrobial activity of mushroom (Basidiomycetes) extracts and isolated compounds. Planta Med. 2012, 78, 1707–1718. [Google Scholar] [CrossRef]
- Escobar, C.G.; Silva, T.; Costa, B.; Oliveira, M.; Correia, P.; Ferreira, G.C.; Costa, I.; Júlio, C.; Rodrigues, J.; Marques, J.M.A.; et al. Gastroenterite aguda em crianças internadas na área de Lisboa. Acta Pediatr. Port. 2013, 44, 155–162. [Google Scholar]
- Angelini, P.; Girometta, C.; Tirillini, B.; Moretti, S.; Covino, S.; Cipriani, M.; D’Ellena, E.; Angeles, G.; Federici, E.; Savino, E.; et al. A comparative study of the antimicrobial and antioxidant activities of Inonotus hispidus fruit and their mycelia extracts. Int. J. Food Prop. 2019, 22, 768–783. [Google Scholar] [CrossRef] [Green Version]
- WHO. World Health Organization—Antibiotic Resistance. Available online: http://www.who.int/news-room/fact-sheets/detail/antibiotic-resistance (accessed on 17 July 2021).
- Modarresi-Chahardehi, A.; Ibrahim, D.; Fariza-Sulaiman, S.; Mousavi, L. Screening antimicrobial activity of various extracts of Urtica dioica. Rev. Biol. Trop. 2012, 60, 1567–1576. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferreira, D.F. Análise estatística por meio do SISVAR (Sistema para Análise de Variancia) para Windows versão 4.0. In Proceedings of the 45th Reunião Anual da Região Brasileira da Sociedade Internacional de Biometria, São Carlos, SP, Brazil, July 2000; pp. 255–258. [Google Scholar]
- Rodrigues, D.; Walton, G.; Sousa, S.; Rocha-Santos, T.A.P.; Duarte, A.C.; Freitas, A.C.; Gomes, A.M.P. In vitro fermentation and prebiotic potential of selected extracts from seaweeds and mushrooms. LWT-Food Sci. Technol. 2016, 73, 131–139. [Google Scholar] [CrossRef]
- Silva, J.G.; Souza, I.A.; Higino, J.S.; Siqueira-Junior, J.P.; Pereira, J.V.; Pereira, M.S.V. Atividade antimicrobiana do extrato de Anacardium occidentale Linn. em amostras multiresistentes de Staphylococcus aureus. Rev. Bras. Farmacogn. 2007, 17, 572–577. [Google Scholar] [CrossRef]
- Oliveira, I.S.; Lima, J.C.S.; Silva, R.M.; Martins, D.T.O. Triagem da atividade antibacteriana in vitro do látex e extratos de Croton urucurana Baillon. Rev. Bras. Farmacogn. 2008, 18, 587–593. [Google Scholar] [CrossRef]
- Aguiar, J.S.; Costa, M.C.C.D.; Nascimento, S.C.; Sena, K.X.F.R. Antimicrobial activity of Lippia alba (Mill.) NE Brown (Verbenaceae). Rev. Bras. Farmacogn. 2008, 18, 436–440. [Google Scholar] [CrossRef] [Green Version]
- Douglas, L.C.; Sanders, M.E. Probiotics and prebiotics in dietetics practice. J. Am. Diet. Assoc. 2008, 108, 510–521. [Google Scholar] [CrossRef]
- Candela, M.; Centanni, M.; Fiori, J.; Biagi, E.; Turroni, S.; Orrico, C.; Bergmann, S.; Hammerschmidt, S.; Brigidi, P. DnaK from Bifidobacterium animalis subsp. lactis is a surfaceexposed human plasminogen receptor upregulated in response to bile salts. Microbiology 2010, 146, 1609–1618. [Google Scholar] [CrossRef] [Green Version]
- Meng, X.; Liang, H.; Luo, L. Antitumor polysaccharides from mushrooms: A review on the structural characteristics, antitumor mechanisms and immunomodulating activities. Carbohydr. Res. 2016, 424, 30–41. [Google Scholar] [CrossRef]
- Synytsya, A.; Mickova, K.; Synytsya, A.; Jablonsky, I.; Spevacek, J.; Erban, V.; Kováříkovád, E.; Čopíková, J. Glucans from fruit bodies of cultivated mushrooms Pleurotus ostreatus and Pleurotus eryngii: Structure and potential prebiotic activity. Carbohydr. Polym. 2008, 76, 548–556. [Google Scholar] [CrossRef]
- Kunjadia, P.D.; Nagee, A.; Pandya, P.Y.; Mukhopadhyaya, P.N.; Sanghvi, G.V.; Dave, G.S. Medicinal and Antimicrobial Role of the Oyster Culinary-Medicinal Mushroom Pleurotus ostreatus (Higher Basidiomycetes) Cultivated on Banana Agrowastes in India. Int. J. Med. Mushrooms 2014, 16, 227–238. [Google Scholar] [CrossRef]
- Venturini, M.E.; Rivera, C.S.; Gonzalez, C.; Blanco, D. Antimicrobial activity of extracts of edible wild and cultivated mushrooms against foodborne bacterial strains. J. Food Prot. 2008, 71, 1701–1706. [Google Scholar] [CrossRef] [PubMed]
- Shen, H.-S.; Shao, S.; Chen, J.-C.; Zhou, T. Antimicrobials from Mushrooms for Assuring Food Safety. CRFSFS 2017, 16, 316–329. [Google Scholar] [CrossRef] [PubMed]
- Clericuzio, M.; Bivona, M.; Gamalero, E.; Bona, E.; Novello, G.; Massa, N.; Dovana, F.; Marengo, E.; Robotti, E. A Systematic Study of the Antibacterial Activity of Basidiomycota Crude Extracts. Antibiotics 2021, 10, 1424. [Google Scholar] [CrossRef] [PubMed]
- Osińska-Jaroszuk, M.; Jarosz-Wilkołazka, A.; Jaroszuk-Ściseł, J.; Szałapata, K.; Nowak, A.; Jaszek, M.; Ozimek, E.; Majewska, M. Extracellular polysaccharides from Ascomycota and Basidiomycota: Production conditions, biochemical characteristics, and biological properties. World J. Microbiol. Biotechnol. 2015, 31, 1823–1844. [Google Scholar] [CrossRef] [Green Version]
- Demir, M.S.; Yamaç, M. Antimicrobial activities of basidiocarp, submerged mycelium and exopolysaccharide of some native basidiomycetes strains. J. Appl. Biol. Sci. 2008, 2, 89–93. [Google Scholar]
- Duvnjak, D.; Pantić, M.; Pavlović, V.; Nedović, V.; Lević, S.; Matijašević, D.; Sknepnek, A.; Nikšić, M. Advances in batch culture fermented Coriolus versicolor medicinal mushroom for the production of antibacterial compounds. IFSET 2016, 34, 1–8. [Google Scholar] [CrossRef]
- Chepkirui, C.; Cheng, T.; Matasyoh, J.; Decock, C.; Stadler, M. An unprecedented spiro [furan-2,1′-indene]-3-one derivative and other nematicidal and antimicrobial metabolites from Sanghuangporus sp. (Hymenochaetaceae, Basidiomycota) collected in Kenya. Phytochem. Lett. 2018, 25, 141–146. [Google Scholar] [CrossRef]
- Mahamat, O.; André-Ledoux, N.; Chrisopher, T.; Mbifu, A.A.; Albert, K. Assessment of antimicrobial and immunomodulatory activities of termite associated fungi, Termitomyces clypeatus R. Heim (Lyophyllaceae, Basidiomycota). Clin. Phytosci. 2018, 4, 28. [Google Scholar] [CrossRef] [Green Version]



| Type of Fermentation | Fermented Biomass | Variation of OD (Tf-Ti *) |
|---|---|---|
| Control (C−) | Water | 0.564 ± 0.028 j |
| Controls (C+) | Glucose | 2.081 ± 0.017 b |
| FOS | 0.476 ± 0.031 k | |
| Inulin | 0.432 ± 0.026 k | |
| Supernatants of submerged fermentations | CSC | 0.630 ± 0.026 i |
| CSC + P. lecomtei | 1.383 ± 0.028 c | |
| CSC + P. pulmonarius | 1.139 ± 0.018 d | |
| CSC + Coriolopsis sp. | 0.922 ± 0.009 e | |
| CSC + Tyromyces sp. | 1.217 ± 0.005 d | |
| Aqueous extracts of solid-state fermentations | CSC | 0.720 ± 0.056 h |
| CSC + P. lecomtei | 0.991 ± 0.026 e | |
| CSC + P. pulmonarius | 0.865 ± 0.026 f | |
| CSC + Coriolopsis sp. | 0.851 ± 0.007 f | |
| CSC + Tyromyces sp. | 1.203 ± 0.058 d | |
| Supernatants of submerged fermentations | JSC | 2.139 ± 0.022 a |
| JSC + P. lecomtei | 0.849 ± 0.053 f | |
| JSC + P. pulmonarius | 1.022 ± 0.015 e | |
| JSC + Coriolopsis sp. | 0.774 ± 0.028 h | |
| JSC + Tyromyces sp. | 0.960 ± 0.058 e | |
| Aqueous extracts of solid-state fermentations | JSC | 0.107 ± 0.046 l |
| JSC + P. lecomtei | 0.816 ± 0.001 g | |
| JSC + P. pulmonarius | 0.748 ± 0.062 g | |
| JSC + Coriolopsis sp. | 0.764 ± 0.008 g | |
| JSC + Tyromyces sp. | 0.861 ± 0.019 f |
| Type of Fermentation | Fermented Biomass | Variation of OD (Tf-Ti *) |
|---|---|---|
| Control (C−) | Water | 0.303 ± 0.041 i |
| Controls (C+) | Glucose | 2.122 ± 0.086 b |
| FOS | 0.735 ± 0.077 e | |
| Inulin | 0.745 ± 0.120 e | |
| Supernatants of submerged fermentation | CSC | 0.907 ± 0.012 d |
| CSC + P. lecomtei | 0.654 ± 0.003 e | |
| CSC + P. pulmonarius | 0.949 ± 0.059 d | |
| CSC + Coriolopsis sp. | 0.379 ± 0.007 h | |
| CSC + Tyromyces sp. | 0.627 ± 0.021 f | |
| Aqueous extracts of solid-state fermentation | CSC | 0.833 ± 0.020 e |
| CSC + P. lecomtei | 0.906 ± 0.001 d | |
| CSC + P. pulmonarius | 0.888 ± 0.015 d | |
| CSC + Coriolopsis sp. | 0.728 ± 0.005 e | |
| CSC + Tyromyces sp. | 1.318 ± 0.087 c | |
| Supernatants of submerged fermentation | JSC | 2.501 ± 0.140 a |
| JSC + P. lecomtei | 0.845 ± 0.052 d | |
| JSC + P. pulmonarius | 0.817 ± 0.020 d | |
| JSC + Coriolopsis sp. | 0.272 ± 0.027 i | |
| JSC + Tyromyces sp. | 0.478 ± 0.067 g | |
| Aqueous extracts of solid-state fermentation | JSC | 0.126 ± 0.020 j |
| JSC + P. lecomtei | 1.366 ± 0.034 c | |
| JSC + P. pulmonarius | 1.295 ± 0.017 c | |
| JSC + Coriolopsis sp. | 0.906 ± 0.140 d | |
| JSC + Tyromyces sp. | 1.342 ± 0.022 c |
| Type of Fermentation | Fermented Biomass | Variation of OD (Tf-Ti *) |
|---|---|---|
| Control (C−) | Water | 0.438 ± 0.113 h |
| Controls (C+) | Glucose | 2.279 ± 0.025 b |
| FOS | 0.137 ± 0.126 i | |
| Inulin | 0.677 ± 0.046 f | |
| Supernatants of submerged fermentation | CSC | 0.881 ± 0.059 ef |
| CSC + P. lecomtei | 0.481 ± 0.003 fg | |
| CSC + P. pulmonarius | 0.825 ± 0.002 e | |
| CSC + Coriolopsis sp. | 0.404 ± 0.081 fg | |
| CSC + Tyromyces sp. | 0.636 ± 0.038 f | |
| Aqueous extracts of solid-state fermentation | CSC | 0.913 ± 0.089 cd |
| CSC + P. lecomtei | 0.802 ± 0.003 f | |
| CSC + P. pulmonarius | 0.965 ± 0.104 de | |
| CSC + Coriolopsis sp. | 0.825 ± 0.137 ef | |
| CSC + Tyromyces sp. | 1.199 ± 0.091 c | |
| Supernatants of submerged fermentation | JSC | 2.570 ± 0.057 a |
| JSC + P. lecomtei | 0.641 ± 0.025 f | |
| JSC + P. pulmonarius | 0.766 ± 0.005 e | |
| JSC + Coriolopsis sp. | 0.302 ± 0.032 h | |
| JSC + Tyromyces sp. | 0.512 ± 0.042 g | |
| Aqueous extracts of solid-state fermentation | JSC | 0.397 ± 0.091 h |
| JSC + P. lecomtei | 0.725 ± 0.029 f | |
| JSC + P. pulmonarius | 1.042 ± 0.049 d | |
| JSC + Coriolopsis sp. | 0.922 ± 0.051 e | |
| JSC + Tyromyces sp. | 0.920 ± 0.026 e |
| Type of Fermentation | Fermented Biomass | Variation of OD (Tf-Ti *) |
|---|---|---|
| Control (C−) | Water | 0.462 ± 0.105 f |
| Controls (C+) | Glucose | 2.014 ± 0.018 a |
| FOS | 0.370 ± 0.041 f | |
| Inulin | 0.351 ± 0.017 f | |
| Supernatants of submerged fermentation | CSC | 0.728 ± 0.023 d |
| CSC + P. lecomtei | 0.862 ± 0.018 c | |
| CSC + P. pulmonarius | 0.965 ± 0.048 b | |
| CSC + Coriolopsis sp. | 0.578 ± 0.107 e | |
| CSC + Tyromyces sp. | 0.931 ± 0.011 b | |
| Aqueous extracts of solid-state fermentation | CSC | 0.601 ± 0.033 e |
| CSC + P. lecomtei | 0.922 ± 0.026 b | |
| CSC + P. pulmonarius | 0.805 ± 0.016 d | |
| CSC + Coriolopsis sp. | 0.779 ± 0.022 d | |
| CSC + Tyromyces sp. | 0.776 ± 0.058 d | |
| Supernatants of submerged fermentation | JSC | 2.026 ± 0.025 a |
| JSC + P. lecomtei | 0.723 ± 0.039 d | |
| JSC + P. pulmonarius | 0.881 ± 0.054 bc | |
| JSC + Coriolopsis sp. | 0.593 ± 0.095 e | |
| JSC + Tyromyces sp. | 0.706 ± 0.033 d | |
| Aqueous extracts of solid-state fermentation | JSC | 0.329 ± 0.030 f |
| JSC + P. lecomtei | 0.724 ± 0.017 d | |
| JSC + P. pulmonarius | 0.727 ± 0.019 d | |
| JSC + Coriolopsis sp. | 0.729 ± 0.044 d | |
| JSC + Tyromyces sp. | 0.954 ± 0.001 b |
| Treatment | Inhibition Halo Diameter | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| E. coli | S. aureus | S. enterica | |||||||
| 100 mg/mL | 10 mg/mL | 1 mg/mL | 100 mg/mL | 10 mg/mL | 1 mg/mL | 100 mg/mL | 10 mg/mL | 1 mg/mL | |
| Water | - | - | - | - | - | - | - | - | - |
| CSC (SmF) | - | - | - | - | - | - | - | - | - |
| CSC + BRM 044603 (SmF) | - | - | - | - | - | - | - | - | - |
| CSC + BRM 055674 (SmF) | - | - | - | - | - | - | - | - | - |
| CSC + INPA1646 (SmF) | - | - | - | - | - | - | - | - | - |
| CSC + INPA1696 (SmF) | - | - | - | - | - | - | - | - | - |
| JSC (SmF) | - | - | - | - | - | - | - | - | - |
| JSC + BRM 044603 (SmF) | - | - | - | - | - | - | - | - | - |
| JSC + BRM 055674 (SmF) | - | - | - | - | - | - | - | - | - |
| JSC + INPA1646 (SmF) | - | - | - | - | - | - | - | - | - |
| JSC + INPA1696 (SmF) | - | - | - | - | - | - | - | - | - |
| CSC (SSF) | - | - | - | - | - | - | - | - | - |
| CSC + BRM 044603 (SSF) | - | - | - | - | - | - | 12 mm | - | - |
| CSC + BRM 055674 (SSF) | - | - | - | - | - | - | - | - | - |
| CSC + INPA1646 (SSF) | - | - | - | - | - | - | 7.3 mm | - | - |
| CSC + INPA1696 (SSF) | - | - | - | - | - | - | - | - | |
| JSC (SSF) | - | - | - | - | - | - | - | - | - |
| JSC + BRM 044603 (SSF) | - | - | - | - | - | - | - | - | - |
| JSC + BRM 055674 (SSF) | - | - | - | - | - | - | - | - | - |
| JSC + INPA1646 (SSF) | - | - | - | - | - | - | - | - | - |
| JSC + INPA1696 (SSF) | - | - | - | - | - | - | - | - | - |
| Antibiotic (C+) | 21 mm | 37.5 mm | 36 mm | ||||||
| L. acidophilus Supernatants | B. lactis Supernatants | L. rhamnosus Supernatants | |||||||
|---|---|---|---|---|---|---|---|---|---|
| E. coli | S. aureus | S. enterica | E. coli | S. aureus | S. enterica | E. coli | S. aureus | S. enterica | |
| Glucose | - | 9.5 mm | - | - | - | - | - | - | - |
| FOS | - | 9 mm | - | - | - | - | - | - | - |
| Inulin | - | 7 mm | - | - | - | - | - | - | - |
| Water | - | - | - | - | - | - | - | - | - |
| CSC (SmF) | - | - | - | - | - | - | - | - | - |
| CSC + BRM 044603 (SmF) | - | - | - | - | - | - | - | - | - |
| CSC + BRM 055674 (SmF) | - | - | - | - | - | - | - | - | - |
| CSC + INPA1646 (SmF) | - | 8 mm | - | - | - | - | - | 13 mm | - |
| CSC + INPA1696 (SmF) | - | 7 mm | - | - | - | - | - | - | - |
| JSC (SmF) | - | 10 mm | - | - | - | - | - | 10 mm | - |
| JSC + BRM 044603 (SmF) | - | 8 mm | - | - | 7 mm | - | - | - | - |
| JSC + BRM 055674 (SmF) | - | 10 mm | - | - | - | - | - | - | - |
| JSC + INPA1646 (SmF) | - | 10 mm | - | - | 10.5 mm | - | - | - | - |
| JSC + INPA1696 (SmF) | - | - | - | - | - | - | - | - | - |
| CSC (SSF) | - | 11 mm | - | - | 9.5 mm | - | - | - | - |
| CSC + BRM 044603 (SSF) | - | - | - | - | - | - | - | - | - |
| CSC + BRM 055674 (SSF) | - | - | - | - | - | - | - | - | - |
| CSC + INPA1646 (SSF) | - | - | - | - | - | - | - | - | - |
| CSC + INPA1696 (SSF) | - | - | 14 mm | - | 11.5 mm | 14 mm | - | - | - |
| JSC (SSF) | - | 9 mm | 13 mm | - | 8 mm | 13 mm | - | - | - |
| JSC + BRM 044603 (SSF) | - | - | - | - | - | - | - | - | - |
| JSC + BRM 055674 (SSF) | - | - | - | - | - | - | - | - | - |
| JSC + INPA1646 (SSF) | - | 10 mm | - | - | 12 mm | - | - | - | - |
| JSS + INPA1696 (SSF) | - | - | - | - | - | - | - | - | - |
| Antibiotic (C+) | 21 mm | 37.5 mm | 36 mm | 21 mm | 37.5 mm | 36 mm | 21 mm | 37.5 mm | 36 mm |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cunha, J.R.B.; Wischral, D.; Peláez, R.D.R.; De Oliveira Magalhães, P.; Guimarães, M.B.; de Jesus, M.A.; Sales-Campos, C.; Mendes, T.D.; Dias, E.S.; Mendonça, S.; et al. Aqueous Extracts of Fermented Macrofungi Cultivated in Oilseed Cakes as a Carbon Source for Probiotic Bacteria and Potential Antibacterial Activity. Metabolites 2023, 13, 854. https://doi.org/10.3390/metabo13070854
Cunha JRB, Wischral D, Peláez RDR, De Oliveira Magalhães P, Guimarães MB, de Jesus MA, Sales-Campos C, Mendes TD, Dias ES, Mendonça S, et al. Aqueous Extracts of Fermented Macrofungi Cultivated in Oilseed Cakes as a Carbon Source for Probiotic Bacteria and Potential Antibacterial Activity. Metabolites. 2023; 13(7):854. https://doi.org/10.3390/metabo13070854
Chicago/Turabian StyleCunha, Joice Raísa Barbosa, Daiana Wischral, Rubén Darío Romero Peláez, Pérola De Oliveira Magalhães, Marina Borges Guimarães, Maria Aparecida de Jesus, Ceci Sales-Campos, Thais Demarchi Mendes, Eustáquio Souza Dias, Simone Mendonça, and et al. 2023. "Aqueous Extracts of Fermented Macrofungi Cultivated in Oilseed Cakes as a Carbon Source for Probiotic Bacteria and Potential Antibacterial Activity" Metabolites 13, no. 7: 854. https://doi.org/10.3390/metabo13070854
APA StyleCunha, J. R. B., Wischral, D., Peláez, R. D. R., De Oliveira Magalhães, P., Guimarães, M. B., de Jesus, M. A., Sales-Campos, C., Mendes, T. D., Dias, E. S., Mendonça, S., & de Siqueira, F. G. (2023). Aqueous Extracts of Fermented Macrofungi Cultivated in Oilseed Cakes as a Carbon Source for Probiotic Bacteria and Potential Antibacterial Activity. Metabolites, 13(7), 854. https://doi.org/10.3390/metabo13070854







