Rapid Detection of Volatile Organic Metabolites in Urine by High-Pressure Photoionization Mass Spectrometry for Breast Cancer Screening: A Pilot Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Instrumentation
2.2. Chemicals and Reagents
2.3. Urine Sample Collection, Preparation, and Detection
2.4. Statistical Analysis
3. Results
3.1. Influence of Acid and Salt Addition
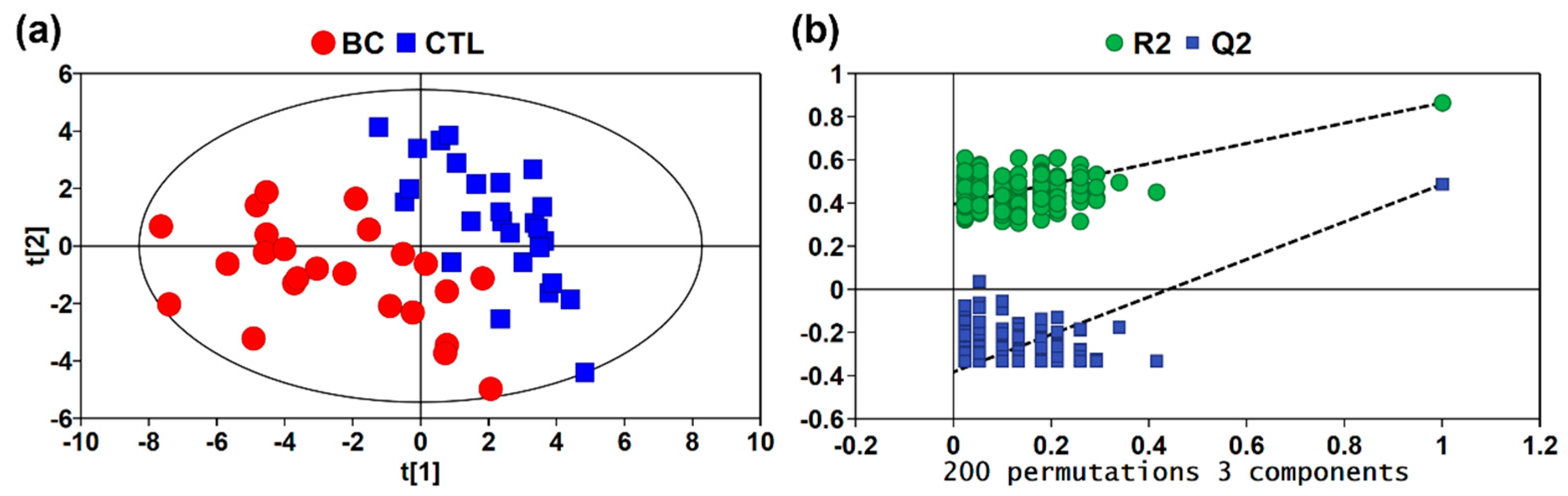
3.2. Multivariate Statistical Analysis
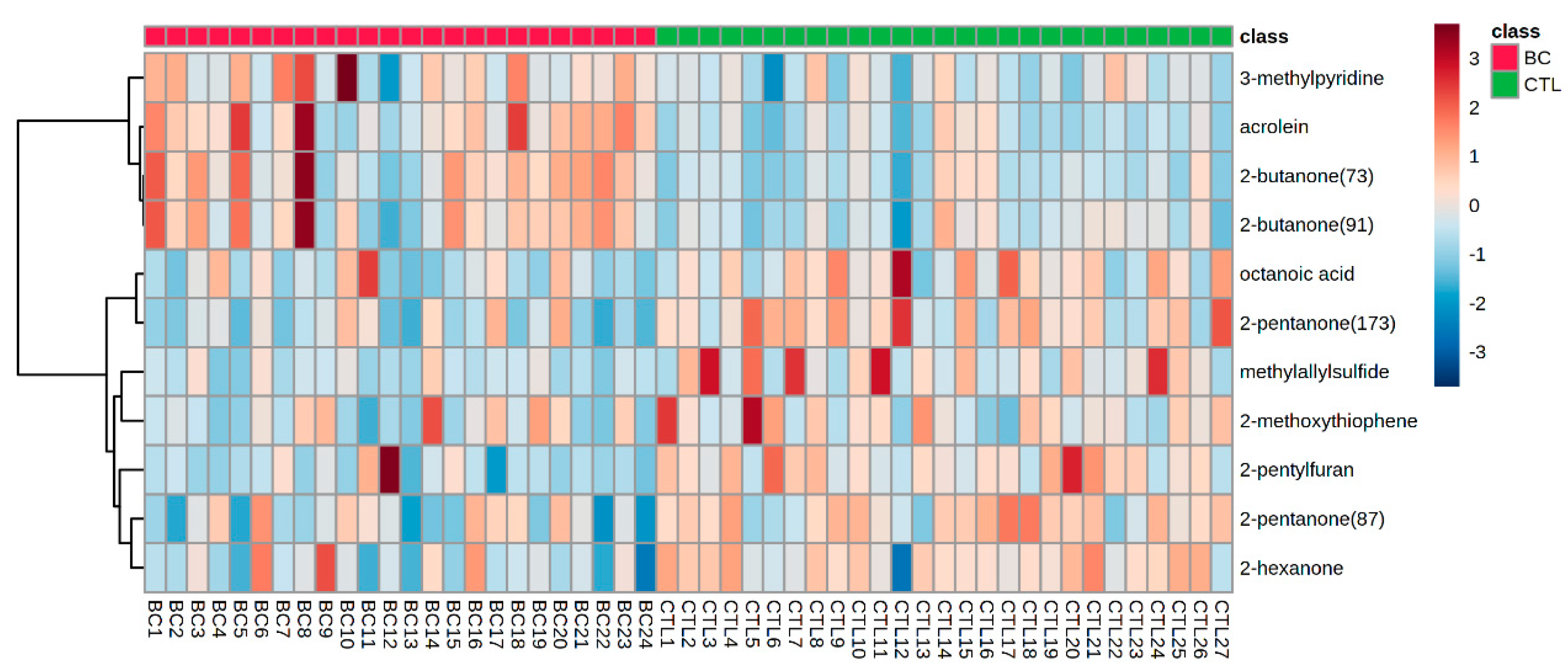
3.3. Differential Metabolites in Urine of BC Patients
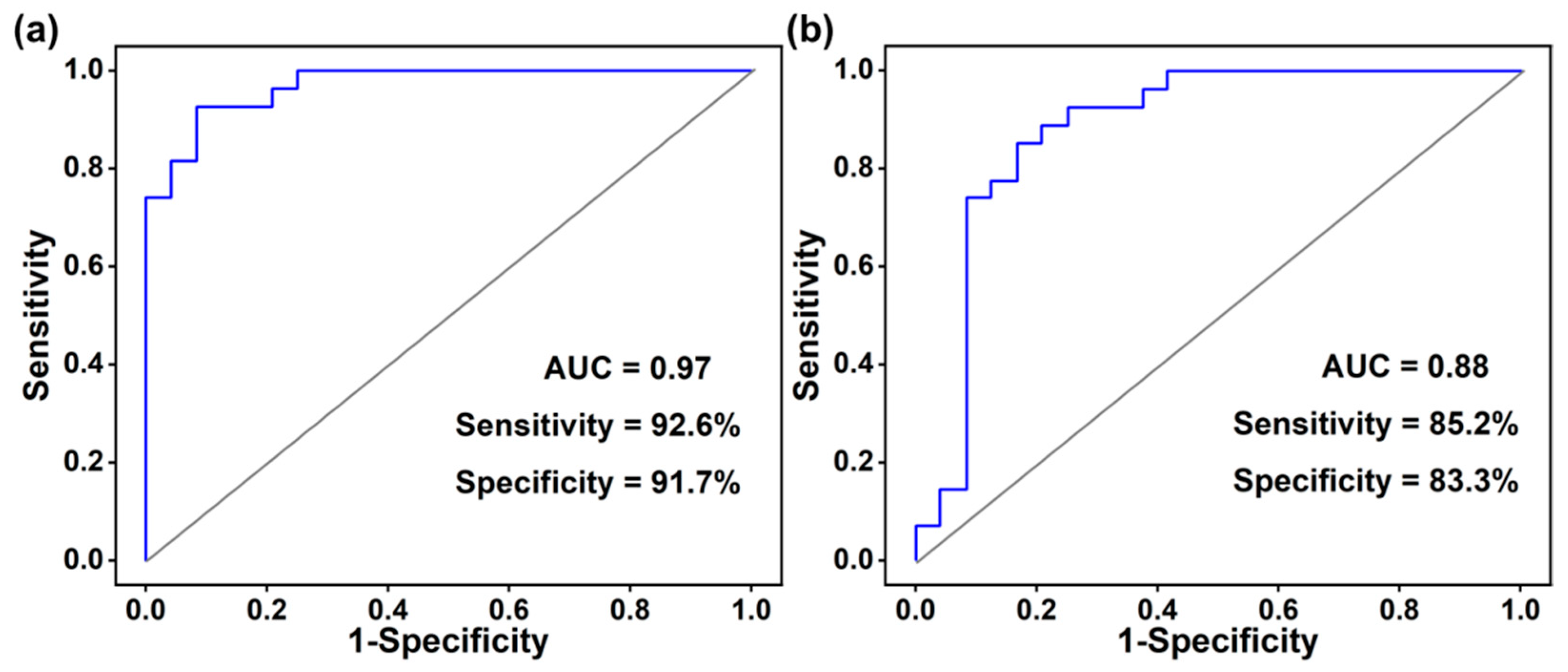
3.4. Receiver Operating Characteristic Curve Analysis
4. Discussion
4.1. Potential Metabolic Pathway Analysis
4.2. Methods Comparison and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- McCormack, V.; McKenzie, F.; Foerster, M.; Zietsman, A.; Galukande, M.; Adisa, C.; Anele, A.; Parham, G.; Pinder, L.F.; Cubasch, H.; et al. Breast cancer survival and survival gap apportionment in sub-Saharan Africa (ABC-DO): A prospective cohort study. Lancet Glob. Health 2020, 8, e1203–e1212. [Google Scholar] [CrossRef] [PubMed]
- Silva, C.L.; Perestrelo, R.; Silva, P.; Tomas, H.; Camara, J.S. Implementing a central composite design for the optimization of solid phase microextraction to establish the urinary volatomic expression: A first approach for breast cancer. Metabolomics 2019, 15, 64. [Google Scholar] [CrossRef]
- Silva, C.L.; Passos, M.; Camara, J.S. Solid phase microextraction, mass spectrometry and metabolomic approaches for detection of potential urinary cancer biomarkers—A powerful strategy for breast cancer diagnosis. Talanta 2012, 89, 360–368. [Google Scholar] [CrossRef]
- Hellquist, B.N.; Czene, K.; Hjälm, A.; Nyström, L.; Jonsson, H. Effectiveness of population-based service screening with mammography for women ages 40 to 49 years with a high or low risk of breast cancer: Socioeconomic status, parity, and age at birth of first child. Cancer 2015, 121, 251–258. [Google Scholar] [CrossRef] [PubMed]
- Onega, T.; Goldman, L.E.; Walker, R.L.; Miglioretti, D.L.; Buist, D.S.; Taplin, S.; Geller, B.M.; Hill, D.A.; Smith-Bindman, R. Facility Mammography Volume in Relation to Breast Cancer Screening Outcomes. J. Med. Screen. 2016, 23, 31–37. [Google Scholar] [CrossRef]
- Ozmen, N.; Dapp, R.; Zapf, M.; Gemmeke, H.; Ruiter, N.V.; van Dongen, K.W. Comparing different ultrasound imaging methods for breast cancer detection. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2015, 62, 637–646. [Google Scholar] [CrossRef]
- Roganovic, D.; Djilas, D.; Vujnovic, S.; Pavic, D.; Stojanov, D. Breast MRI, digital mammography and breast tomosynthesis: Comparison of three methods for early detection of breast cancer. Bosn. J. Basic. Med. Sci. 2015, 15, 64–68. [Google Scholar] [CrossRef]
- Hassan, A.M.; El-Shenawee, M. Review of electromagnetic techniques for breast cancer detection. IEEE Rev. Biomed. Eng. 2011, 4, 103–118. [Google Scholar] [CrossRef]
- Broza, Y.Y.; Mochalski, P.; Ruzsanyi, V.; Amann, A.; Haick, H. Hybrid volatolomics and disease detection. Angew. Chem. Int. Ed. Engl. 2015, 54, 11036–11048. [Google Scholar] [CrossRef]
- Hu, J.; Liu, F.; Chen, Y.; Shangguan, G.; Ju, H. Mass Spectrometric Biosensing: A Powerful Approach for Multiplexed Analysis of Clinical Biomolecules. ACS Sens. 2021, 6, 3517–3535. [Google Scholar] [CrossRef]
- Hasin, Y.; Seldin, M.; Lusis, A. Multi-omics approaches to disease. Genome Biol. 2017, 18, 83. [Google Scholar] [CrossRef] [PubMed]
- Hanai, Y.; Shimono, K.; Matsumura, K.; Vachani, A.; Albelda, S.; Yamazaki, K.; Beauchamp, G.K.; Oka, H. Urinary volatile compounds as biomarkers for lung cancer. Biosci. Biotechnol. Biochem. 2012, 76, 679–684. [Google Scholar] [CrossRef]
- da Costa, B.R.B.; De Martinis, B.S. Analysis of urinary VOCs using mass spectrometric methods to diagnose cancer: A review. Clin. Mass. Spectrom. 2020, 18, 27–37. [Google Scholar] [CrossRef]
- Taunk, K.; Taware, R.; More, T.H.; Porto-Figueira, P.; Pereira, J.A.M.; Mohapatra, R.; Soneji, D.; Câmara, J.S.; Nagarajaram, H.A.; Rapole, S. A non-invasive approach to explore the discriminatory potential of the urinary volatilome of invasive ductal carcinoma of the breast. RSC Adv. 2018, 8, 25040–25050. [Google Scholar] [CrossRef]
- Filipiak, W.; Filipiak, A.; Sponring, A.; Schmid, T.; Zelger, B.; Ager, C.; Klodzinska, E.; Denz, H.; Pizzini, A.; Lucciarini, P.; et al. Comparative analyses of volatile organic compounds (VOCs) from patients, tumors and transformed cell lines for the validation of lung cancer-derived breath markers. J. Breath Res. 2014, 8, 027111. [Google Scholar] [CrossRef] [PubMed]
- Abaffy, T.; Moller, M.G.; Riemer, D.D.; Milikowski, C.; DeFazio, R.A. Comparative analysis of volatile metabolomics signals from melanoma and benign skin: A pilot study. Metabolomics 2013, 9, 998–1008. [Google Scholar] [CrossRef]
- Di Lena, M.; Porcelli, F.; Altomare, D.F. Volatile organic compounds as new biomarkers for colorectal cancer: A review. Color. Dis. 2016, 18, 654–663. [Google Scholar] [CrossRef]
- Jimenez-Pacheco, A.; Salinero-Bachiller, M.; Iribar, M.C.; Lopez-Luque, A.; Mijan-Ortiz, J.L.; Peinado, J.M. Furan and p-xylene as candidate biomarkers for prostate cancer. Urol. Oncol. 2018, 36, 243.e21–243.e27. [Google Scholar] [CrossRef] [PubMed]
- Mochalski, P.; Leja, M.; Gasenko, E.; Skapars, R.; Santare, D.; Sivins, A.; Aronsson, D.E.; Ager, C.; Jaeschke, C.; Shani, G.; et al. Ex vivo emission of volatile organic compounds from gastric cancer and non-cancerous tissue. J. Breath Res. 2018, 12, 046005. [Google Scholar] [CrossRef]
- Portillo-Estrada, M.; Van Moorleghem, C.; Janssenswillen, S.; Cooper, R.J.; Birkemeyer, C.; Roelants, K.; Van Damme, R.; Durand, P. Proton-transfer-reaction time-of-flight mass spectrometry (PTR-TOF-MS) as a tool for studying animal volatile organic compound (VOC) emissions. Methods Ecol. Evol. 2021, 12, 748–766. [Google Scholar] [CrossRef]
- Wu, C.; Wen, Y.; Hua, L.; Jiang, J.; Xie, Y.; Cao, Y.; Chai, S.; Hou, K.; Li, H. Rapid and highly sensitive measurement of trimethylamine in seawater using dynamic purge-release and dopant-assisted atmospheric pressure photoionization mass spectrometry. Anal. Chim. Acta 2020, 1137, 56–63. [Google Scholar] [CrossRef]
- Wang, Y.; Hua, L.; Li, Q.; Jiang, J.; Hou, K.; Wu, C.; Li, H. Direct Detection of Small n-Alkanes at Sub-ppbv Level by Photoelectron-Induced O2+ Cation Chemical Ionization Mass Spectrometry at kPa Pressure. Anal. Chem. 2018, 90, 5398–5404. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Hua, L.; Jiang, J.; Xie, Y.; Hou, K.; Li, Q.; Wu, C.; Li, H. High-pressure photon ionization time-of-flight mass spectrometry combined with dynamic purge-injection for rapid analysis of volatile metabolites in urine. Anal. Chim. Acta 2018, 1008, 74–81. [Google Scholar] [CrossRef]
- Wang, Y.; Jiang, J.; Hua, L.; Hou, K.; Xie, Y.; Chen, P.; Liu, W.; Li, Q.; Wang, S.; Li, H. High-Pressure Photon Ionization Source for TOFMS and Its Application for Online Breath Analysis. Anal. Chem. 2016, 88, 9047–9055. [Google Scholar] [CrossRef] [PubMed]
- Meng, S.; Li, Q.; Zhou, Z.; Li, H.; Liu, X.; Pan, S.; Li, M.; Wang, L.; Guo, Y.; Qiu, M.; et al. Assessment of an Exhaled Breath Test Using High-Pressure Photon Ionization Time-of-Flight Mass Spectrometry to Detect Lung Cancer. JAMA Netw. Open. 2021, 4, e213486. [Google Scholar] [CrossRef]
- Huang, Q.; Wang, S.; Li, Q.; Wang, P.; Li, J.; Meng, S.; Li, H.; Wu, H.; Qi, Y.; Li, X.; et al. Assessment of Breathomics Testing Using High-Pressure Photon Ionization Time-of-Flight Mass Spectrometry to Detect Esophageal Cancer. JAMA Netw. Open. 2021, 4, e2127042. [Google Scholar] [CrossRef]
- Gao, Y.; Zhang, J.; Chen, H.; Wang, Z.; Hou, J.; Wang, L. Dimethylamine enhances platelet hyperactivity in chronic kidney disease model. J. Bioenerg. Biomembr. 2021, 53, 585–595. [Google Scholar] [CrossRef] [PubMed]
- Jankowski, J.; Westhof, T.; Vaziri, N.D.; Ingrosso, D.; Perna, A.F. Gases as uremic toxins: Is there something in the air? Semin. Nephrol. 2014, 34, 135–150. [Google Scholar] [CrossRef]
- Smith, S.; Burden, H.; Persad, R.; Whittington, K.; de Lacy Costello, B.; Ratcliffe, N.M.; Probert, C.S. A comparative study of the analysis of human urine headspace using gas chromatography-mass spectrometry. J. Breath Res. 2008, 2, 037022. [Google Scholar] [CrossRef]
- Aggio, R.B.; Mayor, A.; Coyle, S.; Reade, S.; Khalid, T.; Ratcliffe, N.M.; Probert, C.S. Freeze-drying: An alternative method for the analysis of volatile organic compounds in the headspace of urine samples using solid phase micro-extraction coupled to gas chromatography—Mass spectrometry. Chem. Cent. J. 2016, 10, 9. [Google Scholar] [CrossRef] [PubMed]
- Sumner, L.W.; Amberg, A.; Barrett, D.; Beale, M.H.; Beger, R.; Daykin, C.A.; Fan, T.W.; Fiehn, O.; Goodacre, R.; Griffin, J.L.; et al. Proposed minimum reporting standards for chemical analysis Chemical Analysis Working Group (CAWG) Metabolomics Standards Initiative (MSI). Metabolomics 2007, 3, 211–221. [Google Scholar] [CrossRef]
- Kato, S.; Post, G.C.; Bierbaum, V.M.; Koch, T.H. Chemical ionization mass spectrometric determination of acrolein in human breast cancer cells. Anal. Biochem. 2002, 305, 251–259. [Google Scholar] [CrossRef] [PubMed]
- Amal, H.; Leja, M.; Funka, K.; Skapars, R.; Sivins, A.; Ancans, G.; Liepniece-Karele, I.; Kikuste, I.; Lasina, I.; Haick, H. Detection of precancerous gastric lesions and gastric cancer through exhaled breath. Gut 2016, 65, 400–407. [Google Scholar] [CrossRef]
- Alonso, M.; Castellanos, M.; Besalu, E.; Sanchez, J.M. A headspace needle-trap method for the analysis of volatile organic compounds in whole blood. J. Chromatogr. A 2012, 1252, 23–30. [Google Scholar] [CrossRef]
- Mochalski, P.; Unterkofler, K. Quantification of selected volatile organic compounds in human urine by gas chromatography selective reagent ionization time of flight mass spectrometry (GC-SRI-TOF-MS) coupled with head-space solid-phase microextraction (HS-SPME). Analyst 2016, 141, 4796–4803. [Google Scholar] [CrossRef]
- Porto-Figueira, P.; Pereira, J.; Miekisch, W.; Camara, J.S. Exploring the potential of NTME/GC-MS, in the establishment of urinary volatomic profiles. Lung cancer patients as case study. Sci. Rep. 2018, 8, 13113. [Google Scholar] [CrossRef] [PubMed]
- Tangerman, A. Measurement and biological significance of the volatile sulfur compounds hydrogen sulfide, methanethiol and dimethyl sulfide in various biological matrices. J. Chromatogr. B 2009, 877, 3366–3377. [Google Scholar] [CrossRef] [PubMed]
- Blom, H.J.; Boers, G.H.J.; Vandenelzen, J.; Gahl, W.A.; Tangerman, A. Transamination of methionine in humans. Clin. Sci. 1989, 76, 43–49. [Google Scholar] [CrossRef]
- Scholler, C.; Molin, S.; Wilkins, K. Volatile metabolites from some gram-negative bacteria. Chemosphere 1997, 35, 1487–1495. [Google Scholar] [CrossRef]
- Bakhiya, N.; Appel, K.E. Toxicity and carcinogenicity of furan in human diet. Arch. Toxicol. 2010, 84, 563–578. [Google Scholar] [CrossRef] [PubMed]
- Silva, C.L.; Passos, M.; Camara, J.S. Investigation of urinary volatile organic metabolites as potential cancer biomarkers by solid-phase microextraction in combination with gas chromatography-mass spectrometry. Br. J. Cancer 2011, 105, 1894–1904. [Google Scholar] [CrossRef] [PubMed]
- Mochalski, P.; Diem, E.; Unterkofler, K.; Mundlein, A.; Drexel, H.; Mayhew, C.A.; Leiherer, A. In vitro profiling of volatile organic compounds released by Simpson-Golabi-Behmel syndrome adipocytes. J. Chromatogr. B 2019, 1104, 256–261. [Google Scholar] [CrossRef]
- Silva, C.; Perestrelo, R.; Silva, P.; Tomas, H.; Camara, J.S. Breast Cancer Metabolomics: From Analytical Platforms to Multivariate Data Analysis. A Review. Metabolites 2019, 9, 102. [Google Scholar] [CrossRef] [PubMed]
- Herman-Saffar, O.; Boger, Z.; Libson, S.; Lieberman, D.; Gonen, R.; Zeiri, Y. Early non-invasive detection of breast cancer using exhaled breath and urine analysis. Comput. Biol. Med. 2018, 96, 227–232. [Google Scholar] [CrossRef]
- Giro Benet, J.; Seo, M.; Khine, M.; Guma Padro, J.; Pardo Martnez, A.; Kurdahi, F. Breast cancer detection by analyzing the volatile organic compound (VOC) signature in human urine. Sci. Rep. 2022, 12, 14873. [Google Scholar] [CrossRef]
- Cala, M.; Aldana, J.; Sanchez, J.; Guio, J.; Meesters, R.J.W. Urinary metabolite and lipid alterations in Colombian Hispanic women with breast cancer: A pilot study. J. Pharm. Biomed. Anal. 2018, 152, 234–241. [Google Scholar] [CrossRef]
- Zou, X.; Lu, Y.; Xia, L.; Zhang, Y.; Li, A.; Wang, H.; Huang, C.; Shen, C.; Chu, Y. Detection of volatile organic compounds in a drop of urine by ultrasonic nebulization extraction Proton Transfer Reaction Mass Spectrometry. Anal. Chem. 2018, 90, 2210–2215. [Google Scholar] [CrossRef]
- Xu, W.; Zou, X.; Ding, H.W.; Ding, Y.T.; Zhang, J.; Liu, W.T.; Gong, T.T.; Nie, Z.C.; Yang, M.; Zhou, Q.; et al. Rapid and non-invasive diagnosis of type 2 diabetes through sniffing urinary acetone by a proton transfer reaction mass spectrometry. Talanta 2023, 256, 124265. [Google Scholar] [CrossRef]
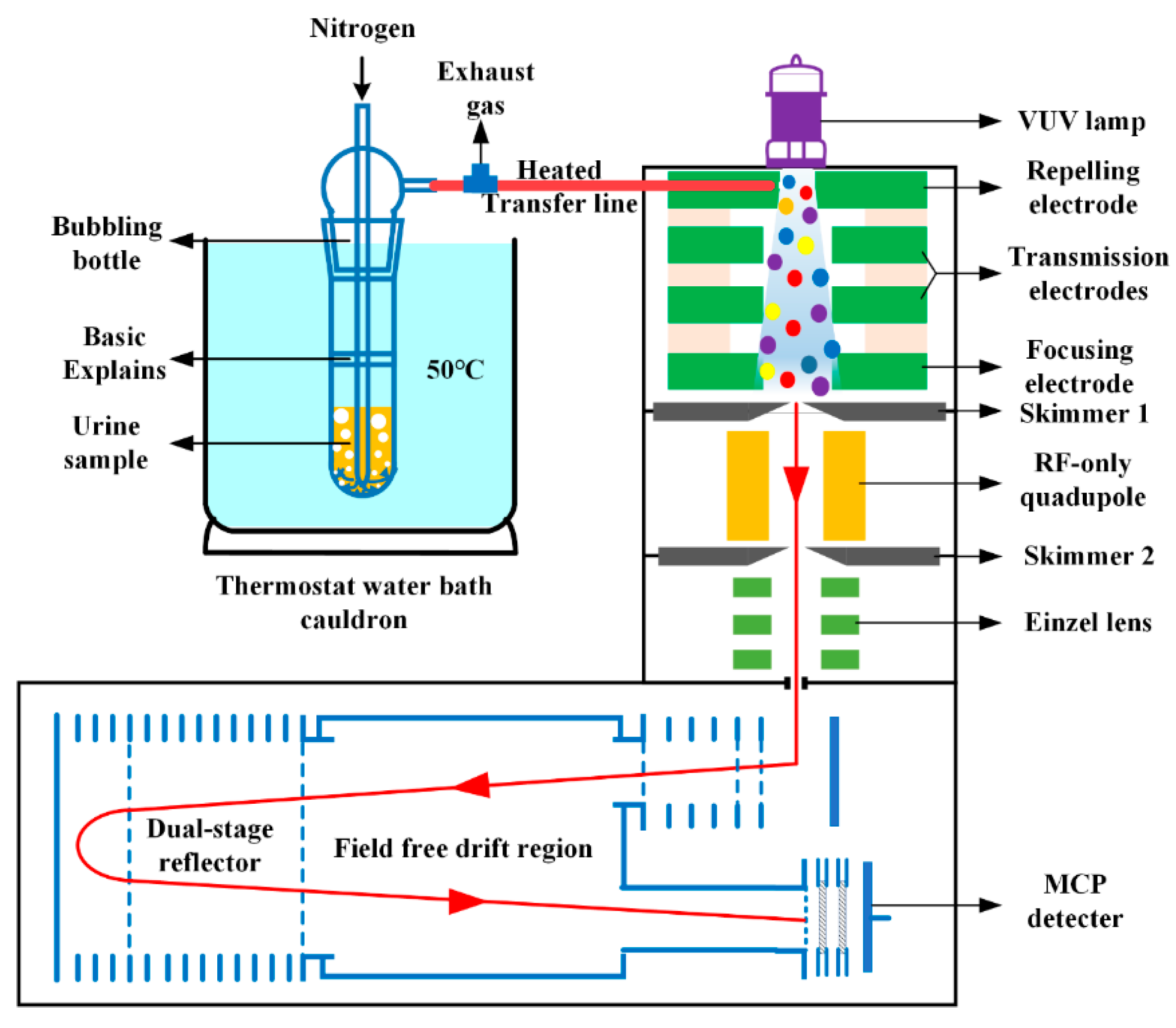
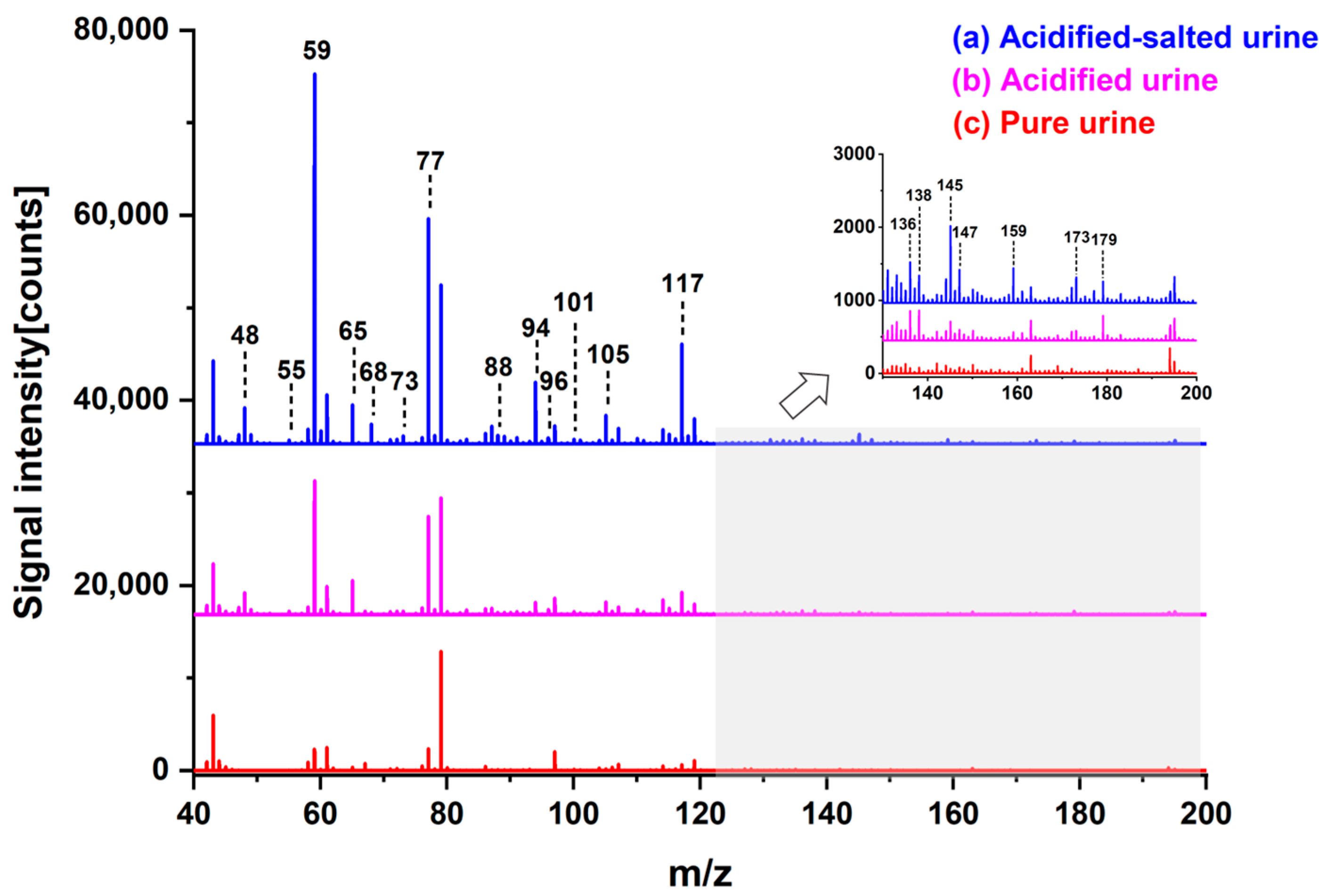
| Measured Mass (Th) | Theoretical Mass (Th) | Mass Error (ppm) | Characteristic Peaks | Chemicals |
|---|---|---|---|---|
| 47.0495 | 47.0496 | −2 | C2H6O·H+ | ethanol |
| 45.0328 | 45.0340 | −27 | C2H4O·H+ | acetaldehyde |
| 48.0031 | 48.0033 | −4 | CH4S+ | methanethiol |
| 49.0106 | 49.0112 | −12 | CH4S·H+ | |
| 57.0338 | 57.0341 | −4 | C3H4O·H+ | acrolein |
| 59.0498 | 59.0496 | 3 | C3H6O·H+ | acetone |
| 77.0603 | 77.0602 | 1 | C3H6O·H3O+ | |
| 117.0915 | 117.0915 | 0 | (C3H6O)2·H+ | |
| 61.0280 | 61.0289 | −15 | C2H4O2·H+ | acetic acid |
| 79.0398 | 79.0395 | 4 | C2H4O2·H3O+ | |
| 73.0652 | 73.0653 | −1 | C4H8O·H+ | 2-butanone |
| 91.0754 | 91.0759 | −5 | C4H8O·H3O+ | |
| 144.1131 | 144.1150 | −13 | C8H16O2+ | octanoic acid |
| 145.1225 | 145.1228 | −2 | C8H16O2·H+ | |
| 83.0715 | 83.0735 | −24 | C5H9N+ | pentanenitrile |
| 87.0808 | 87.0810 | −2 | C5H10O·H+ | 2-pentanone |
| 105.0915 | 105.0915 | 0 | C5H10O·H3O+ | |
| 173.1519 | 173.1542 | −13 | (C5H10O)2·H+ | |
| 88.0346 | 88.0346 | 0 | C4H8S+ | methyl allyl sulfide |
| 92.0629 | 92.0626 | 3 | C7H8+ | toluene |
| 93.0581 | 93.0578 | 2 | C6H7N+ | 3-methylpyridine |
| 93.9908 | 93.9910 | −2 | C2H6S2+ | disulfide, dimethyl |
| 96.0576 | 96.0575 | 1 | C6H8O+ | 2,5-dimethylfuran |
| 97.0507 | 97.0527 | −21 | C5H7NO+ | 2,5-dimethyloxazole |
| 101.0599 | 101.0602 | −3 | C5H9O2+ | 2,3-pentanedione |
| 101.0955 | 101.0966 | −11 | C6H12O·H+ | 2-hexanone |
| 107.0713 | 107.0735 | −21 | C7H9N+ | 2,6-lutidine |
| 110.0725 | 110.0731 | −5 | C7H10O+ | 2-propylfuran |
| 114.0135 | 114.0139 | −4 | C5H6OS+ | 2-methoxythiophene |
| 115.1112 | 115.1123 | −10 | C7H14O·H+ | 2-heptanal |
| 136.1240 | 136.1252 | −9 | C10H16+ | limonene |
| 139.1120 | 139.1123 | −2 | C9H14O·H+ | 2-pentylfuran |
| VOMs | Chemical Formula | Characteristic Peaks | Ratio | p-Value | VIP | Ref. |
|---|---|---|---|---|---|---|
| acrolein | C3H4O | C3H4O·H+ | 2.00 | 2.57 × 10−5 | 1.63 | [33] |
| 2-butanone | C4H8O | C4H8O·H+ | 1.92 | 8.66 × 10−5 | 1.54 | [34,35] |
| C4H8O·H3O+ | 1.58 | 0.0016 | 1.32 | |||
| 2-pentanone | C5H10O | C5H10O·H+ | 0.53 | 0.0062 | 1.17 | [34,35] |
| (C5H10O)2·H+ | 0.38 | 2.51 × 10−4 | 1.47 | |||
| methyl allyl sulfide | C4H8S | C4H8S+ | 0.16 | 0.0012 | 1.37 | [30,36] |
| 3-methylpyridine | C6H7N | C6H7N+ | 2.16 | 0.0043 | 1.14 | [20] |
| 2-hexanone | C6H12O | C6H12O·H+ | 0.56 | 7.30 × 10−4 | 1.12 | [3,37] |
| 2-methoxythiophene | C5H6OS | C5H6OS+ | 0.48 | 0.0097 | 1.11 | [4,30] |
| 2-pentylfuran | C9H14O | C9H14O·H+ | 0.46 | 2.18 × 10−5 | 1.38 | [3,37] |
| octanoic acid | C8H17O2 | C8H16O2·H+ | 0.45 | 0.0108 | 1.21 | [4] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, M.; Jiang, J.; Hua, L.; Jiang, D.; Wang, Y.; Li, D.; Wang, R.; Zhang, X.; Li, H. Rapid Detection of Volatile Organic Metabolites in Urine by High-Pressure Photoionization Mass Spectrometry for Breast Cancer Screening: A Pilot Study. Metabolites 2023, 13, 870. https://doi.org/10.3390/metabo13070870
Yang M, Jiang J, Hua L, Jiang D, Wang Y, Li D, Wang R, Zhang X, Li H. Rapid Detection of Volatile Organic Metabolites in Urine by High-Pressure Photoionization Mass Spectrometry for Breast Cancer Screening: A Pilot Study. Metabolites. 2023; 13(7):870. https://doi.org/10.3390/metabo13070870
Chicago/Turabian StyleYang, Ming, Jichun Jiang, Lei Hua, Dandan Jiang, Yadong Wang, Depeng Li, Ruoyu Wang, Xiaohui Zhang, and Haiyang Li. 2023. "Rapid Detection of Volatile Organic Metabolites in Urine by High-Pressure Photoionization Mass Spectrometry for Breast Cancer Screening: A Pilot Study" Metabolites 13, no. 7: 870. https://doi.org/10.3390/metabo13070870
APA StyleYang, M., Jiang, J., Hua, L., Jiang, D., Wang, Y., Li, D., Wang, R., Zhang, X., & Li, H. (2023). Rapid Detection of Volatile Organic Metabolites in Urine by High-Pressure Photoionization Mass Spectrometry for Breast Cancer Screening: A Pilot Study. Metabolites, 13(7), 870. https://doi.org/10.3390/metabo13070870






