Synovial Fluid Metabolome Can Differentiate between Healthy Joints and Joints Affected by Osteoarthritis in Horses
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Metabolome Observation by 1H-NMR
2.3. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Labens, R.; Schramme, M.C.; Barr, A.R. Orthopaedics 1: Diagnosis of Lameness. In Equine Medicine, Surgery and Reproduction; Saunders/Elsevier: Edinburgh, Scotland, 2013; pp. 309–328. [Google Scholar]
- Anderson, J.R.; Phelan, M.M.; Clegg, P.D.; Peffers, M.J.; Rubio-Martinez, L.M. Synovial Fluid Metabolites Differentiate between Septic and Nonseptic Joint Pathologies. J. Proteome Res. 2018, 17, 2735–2743. [Google Scholar] [CrossRef]
- Menarim, B.C.; Gillis, K.H.; Oliver, A.; Ngo, Y.; Werre, S.R.; Barrett, S.H.; Rodgerson, D.H.; Dahlgren, L.A. Macrophage Activation in the Synovium of Healthy and Osteoarthritic Equine Joints. Front. Vet. Sci. 2020, 7, 568756. [Google Scholar] [CrossRef]
- Sellam, J.; Berenbaum, F. The Role of Synovitis in Pathophysiology and Clinical Symptoms of Osteoarthritis. Nat. Rev. Rheumatol. 2010, 6, 625–635. [Google Scholar] [CrossRef] [PubMed]
- Goldring, M.B.; Goldring, S.R. Articular Cartilage and Subchondral Bone in the Pathogenesis of Osteoarthritis. Ann. N. Y. Acad. Sci. 2010, 1192, 230–237. [Google Scholar] [CrossRef] [PubMed]
- Ribitsch, I.; Oreff, G.L.; Jenner, F. Regenerative Medicine for Equine Musculoskeletal Diseases. Animals 2021, 11, 234. [Google Scholar] [CrossRef]
- Taylor, S.E.; Weaver, M.P.; Pitsillides, A.A.; Wheeler, B.T.; Wheeler-Jones, C.P.D.; Shaw, D.J.; Smith, R.K.W. Cartilage Oligomeric Matrix Protein and Hyaluronan Levels in Synovial Fluid from Horses with Osteoarthritis of the Tarsometatarsal Joint Compared to a Control Population. Equine Vet. J. 2006, 38, 502–507. [Google Scholar] [CrossRef] [PubMed]
- Zrimšek, P.; Kadunc Kos, V.; Mrkun, J.; Kosec, M. Diagnostic Value of MMP-2 and MMP-9 in Synovial Fluid for Identifying Osteoarthritis in the Distal Interphalangeal Joint in Horses. Acta Vet. Brno 2007, 76, 87–95. [Google Scholar] [CrossRef]
- Desjardin, C.; Riviere, J.; Vaiman, A.; Morgenthaler, C.; Diribarne, M.; Zivy, M.; Robert, C.; Le Moyec, L.; Wimel, L.; Lepage, O.; et al. Omics Technologies Provide New Insights into the Molecular Physiopathology of Equine Osteochondrosis. BMC Genom. 2014, 15, 947. [Google Scholar] [CrossRef]
- Nelson, B.B.; Goodrich, L.R. Treatment of Joint Disease. In Robinson’s Current Therapy in Equine Medicine, 7th ed.; Elsevier: Amsterdam, The Netherlands, 2015; pp. 798–804. [Google Scholar] [CrossRef]
- Billinghurst, R.C. Biomarkers of Joint Disease. In Current Therapy in Equine Medicine, 5th ed.; Elsevier: Amsterdam, The Netherlands, 2003; pp. 513–520. [Google Scholar] [CrossRef]
- Bujak, R.; Struck-Lewicka, W.; Markuszewski, M.J.; Kaliszan, R. Metabolomics for Laboratory Diagnostics. J. Pharm. Biomed. Anal. 2015, 113, 108–120. [Google Scholar] [CrossRef]
- Madsen, R.; Lundstedt, T.; Trygg, J. Chemometrics in Metabolomics—A Review in Human Disease Diagnosis. Anal. Chim. Acta 2010, 659, 23–33. [Google Scholar] [CrossRef]
- Ruiz-Romero, C.; Blanco, F.J. Proteomics Role in the Search for Improved Diagnosis, Prognosis and Treatment of Osteoarthritis. Osteoarthr. Cartil. 2010, 18, 500–509. [Google Scholar] [CrossRef] [PubMed]
- Mateos, J.; Lourido, L.; Fernández-Puente, P.; Calamia, V.; Fernández-López, C.; Oreiro, N.; Ruiz-Romero, C.; Blanco, F.J. Differential Protein Profiling of Synovial Fluid from Rheumatoid Arthritis and Osteoarthritis Patients Using LC-MALDI TOF/TOF. J. Proteom. 2012, 75, 2869–2878. [Google Scholar] [CrossRef]
- Haralambus, R.; Florczyk, A.; Sigl, E.; Gültekin, S.; Vogl, C.; Brandt, S.; Schnierer, M.; Gamerith, C.; Jenner, F. Detection of Synovial Sepsis in Horses Using Enzymes as Biomarkers. Equine Vet. J. 2022, 54, 513–522. [Google Scholar] [CrossRef]
- Lineham, B.; Altaie, A.; Harwood, P.; McGonagle, D.; Pandit, H.; Jones, E. A Systematic Review on the Potential Value of Synovial Fluid Biomarkers to Predict Clinical Outcomes in Cartilage Repair Treatments. Osteoarthr. Cartil. 2022, 30, 1035–1049. [Google Scholar] [CrossRef]
- Noordwijk, K.J.; Qin, R.; Diaz-Rubio, M.E.; Zhang, S.; Su, J.; Mahal, L.K.; Reesink, H.L. Metabolism and Global Protein Glycosylation Are Differentially Expressed in Healthy and Osteoarthritic Equine Carpal Synovial Fluid. Equine Vet. J. 2022, 54, 323–333. [Google Scholar] [CrossRef] [PubMed]
- Laghi, L.; Picone, G.; Capozzi, F. Nuclear Magnetic Resonance for Foodomics beyond Food Analysis. Trends Anal. Chem. 2014, 59, 93–102. [Google Scholar] [CrossRef]
- De Grauw, J.C.; Van De Lest, C.H.A.; Van Weeren, P.R. A Targeted Lipidomics Approach to the Study of Eicosanoid Release in Synovial Joints. Arthritis Res. Ther. 2011, 13, R123. [Google Scholar] [CrossRef]
- Kosinska, M.K.; Eichner, G.; Schmitz, G.; Liebisch, G.; Steinmeyer, J. A Comparative Study on the Lipidome of Normal Knee Synovial Fluid from Humans and Horses. PLoS ONE 2021, 16, e0250146. [Google Scholar] [CrossRef]
- Graham, R.J.T.Y.; Anderson, J.R.; Phelan, M.M.; Cillan-Garcia, E.; Bladon, B.M.; Taylor, S.E. Metabolomic Analysis of Synovial Fluid from Thoroughbred Racehorses Diagnosed with Palmar Osteochondral Disease Using Magnetic Resonance Imaging. Equine Vet. J. 2020, 52, 384–390. [Google Scholar] [CrossRef]
- Lacitignola, L.; Fanizzi, F.P.; Francioso, E.; Crovace, A. 1H NMR Investigation of Normal and Osteo-Arthritic Synovial Fluid in the Horse. Vet. Comp. Orthop. Traumatol. 2008, 21, 85–88. [Google Scholar] [CrossRef]
- Robinson, C.S.; Singer, E.R.; Piviani, M.; Rubio-Martinez, L.M. Are Serum Amyloid A or D-Lactate Useful to Diagnose Synovial Contamination or Sepsis in Horses? Vet. Rec. 2017, 181, 425. [Google Scholar] [CrossRef] [PubMed]
- Brugaletta, G.; De Cesare, A.; Laghi, L.; Manfreda, G.; Zampiga, M.; Oliveri, C.; Pérez-Calvo, E.; Litta, G.; Lolli, S.; Sirri, F. A Multi-Omics Approach to Elucidate the Mechanisms of Action of a Dietary Muramidase Administered to Broiler Chickens. Sci. Rep. 2022, 12, 5559. [Google Scholar] [CrossRef]
- R: The R Project for Statistical Computing. Available online: https://www.r-project.org/ (accessed on 22 June 2023).
- Wishart, D.S.; Tzur, D.; Knox, C.; Eisner, R.; Guo, A.C.; Young, N.; Cheng, D.; Jewell, K.; Arndt, D.; Sawhney, S.; et al. HMDB: The Human Metabolome Database. Nucleic Acids Res. 2007, 35, D521-6. [Google Scholar] [CrossRef] [PubMed]
- Box, G.E.P.; Cox, D.R. An Analysis of Transformations. J. R. Stat. Society. Ser. B 1964, 26, 211–252. [Google Scholar] [CrossRef]
- Parada-Turska, J.; Zgrajka, W.; Majdan, M. Kynurenic Acid in Synovial Fluid and Serum of Patients with Rheumatoid Arthritis, Spondyloarthropathy, and Osteoarthritis. J. Rheumatol. 2013, 40, 903–909. [Google Scholar] [CrossRef]
- Kang, K.Y.; Lee, S.H.; Jung, S.M.; Park, S.H.; Jung, B.H.; Ju, J.H. Downregulation of Tryptophan-Related Metabolomic Profile in Rheumatoid Arthritis Synovial Fluid. J. Rheumatol. 2015, 42, 2003–2011. [Google Scholar] [CrossRef]
- Nowicka-Stążka, P.; Langner, E.; Turski, W.; Rzeski, W.; Parada-Turska, J. Quinaldic Acid in Synovial Fluid of Patients with Rheumatoid Arthritis and Osteoarthritis and Its Effect on Synoviocytes in Vitro. Pharmacol. Rep. 2018, 70, 277–283. [Google Scholar] [CrossRef]
- Li, J.; Che, N.; Xu, L.; Zhang, Q.; Wang, Q.; Tan, W.; Zhang, M. LC-MS-Based Serum Metabolomics Reveals a Distinctive Signature in Patients with Rheumatoid Arthritis. Clin. Rheumatol. 2018, 37, 1493–1502. [Google Scholar] [CrossRef] [PubMed]
- Dai, M.; Sui, B.; Xue, Y.; Liu, X.; Sun, J. Cartilage Repair in Degenerative Osteoarthritis Mediated by Squid Type II Collagen via Immunomodulating Activation of M2 Macrophages, Inhibiting Apoptosis and Hypertrophy of Chondrocytes. Biomaterials 2018, 180, 91–103. [Google Scholar] [CrossRef]
- Deng, L.; Yao, P.; Li, L.; Ji, F.; Zhao, S.; Xu, C.; Lan, X.; Jiang, P. P53-Mediated Control of Aspartate-Asparagine Homeostasis Dictates LKB1 Activity and Modulates Cell Survival. Nat. Commun. 2020, 11, 1755. [Google Scholar] [CrossRef]
- Kim, H. Glutamine as an Immunonutrient. Yonsei Med. J. 2011, 52, 892–897. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, S.; Saegusa, J.; Sendo, S.; Okano, T.; Akashi, K.; Irino, Y.; Morinobu, A. Glutaminase 1 Plays a Key Role in the Cell Growth of Fibroblast-like Synoviocytes in Rheumatoid Arthritis. Arthritis Res. Ther. 2017, 19, 76. [Google Scholar] [CrossRef] [PubMed]
- McNeal, C.J.; Meininger, C.J.; Reddy, D.; Wilborn, C.D.; Wu, G. Safety and Effectiveness of Arginine in Adults. J. Nutr. 2016, 146, 2587S–2593S. [Google Scholar] [CrossRef] [PubMed]
- Carlson, A.K.; Rawle, R.A.; Wallace, C.W.; Adams, E.; Greenwood, M.C.; Bothner, B.; June, R.K. Global Metabolomic Profiling of Human Synovial Fluid for Rheumatoid Arthritis Biomarkers. Clin. Exp. Rheumatol. 2019, 37, 393–399. [Google Scholar] [PubMed]
- Aledo, J.C. Methionine in Proteins: The Cinderella of the Proteinogenic Amino Acids. Protein Sci. 2019, 28, 1785–1796. [Google Scholar] [CrossRef]
- Li, C.; Chen, B.; Fang, Z.; Leng, Y.F.; Wang, D.W.; Chen, F.Q.; Xu, X.; Sun, Z. ling Metabolomics in the Development and Progression of Rheumatoid Arthritis: A Systematic Review. Jt. Bone Spine 2020, 87, 425–430. [Google Scholar] [CrossRef]
- Liu, J.; Shikhman, A.R.; Lotz, M.K.; Wong, C.H. Hexosaminidase Inhibitors as New Drug Candidates for the Therapy of Osteoarthritis. Chem. Biol. 2001, 8, 701–711. [Google Scholar] [CrossRef]
- Shikhman, A.R.; Brinson, D.C.; Lotz, M. Profile of glycosaminoglycan-degrading glycosidases and glycoside sulfatases secreted by human articular chondrocytes in homeostasis and inflammation. Arthritis Rheum. 2000, 43, 1307–1314. [Google Scholar] [CrossRef]
- Narendra, S.C.; Chalise, J.P.; Magnusson, M.; Uppugunduri, S. Local but Not Systemic Administration of Uridine Prevents Development of Antigen-Induced Arthritis. PLoS ONE 2015, 10, e0141863. [Google Scholar] [CrossRef]
- Anderson, J.R.; Chokesuwattanaskul, S.; Phelan, M.M.; Welting, T.J.M.; Lian, L.Y.; Peffers, M.J.; Wright, H.L. 1H NMR Metabolomics Identifies Underlying Inflammatory Pathology in Osteoarthritis and Rheumatoid Arthritis Synovial Joints. J. Proteome Res. 2018, 17, 3780–3790. [Google Scholar] [CrossRef] [PubMed]
- Adams, S.B.; Setton, L.A.; Kensicki, E.; Bolognesi, M.P.; Toth, A.P.; Nettles, D.L. Global Metabolic Profiling of Human Osteoarthritic Synovium. Osteoarthr. Cartil. 2012, 20, 64–67. [Google Scholar] [CrossRef] [PubMed]
- Zhai, G.; Wang-Sattler, R.; Hart, D.J.; Arden, N.K.; Hakim, A.J.; Illig, T.; Spector, T.D. Serum Branched-Chain Amino Acid to Histidine Ratio: A Novel Metabolomic Biomarker of Knee Osteoarthritis. Ann. Rheum. Dis. 2010, 69, 1227–1231. [Google Scholar] [CrossRef] [PubMed]
- Blewett, H.J.H. Exploring the Mechanisms behind S-Adenosylmethionine (SAMe) in the Treatment of Osteoarthritis. Crit. Rev. Food Sci. Nutr. 2008, 48, 458–463. [Google Scholar] [CrossRef] [PubMed]
- Lauwers, M.; Courties, A.; Sellam, J.; Wen, C. The Cholinergic System in Joint Health and Osteoarthritis: A Narrative-Review. Osteoarthr. Cartil. 2021, 29, 643–653. [Google Scholar] [CrossRef] [PubMed]
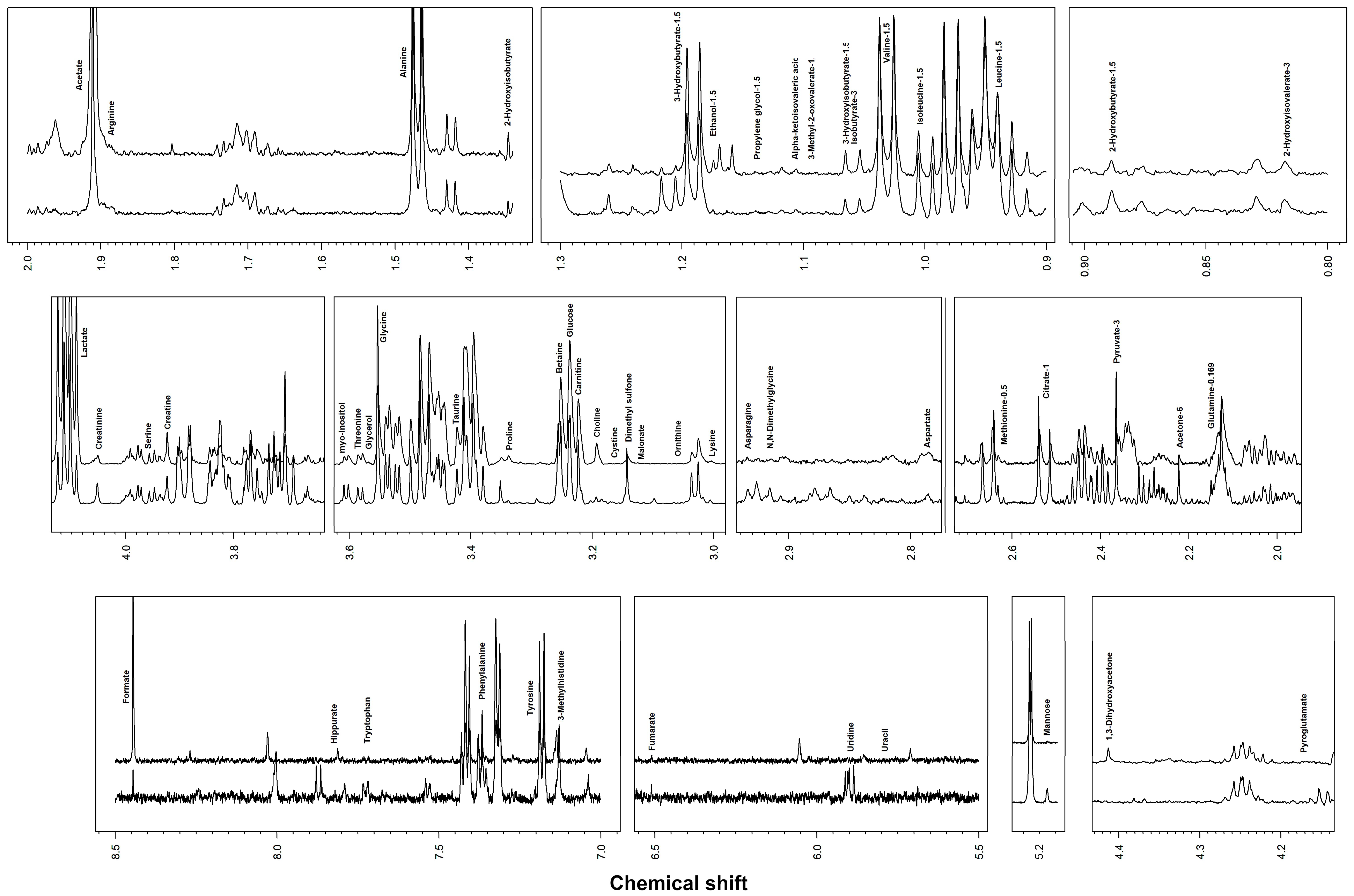
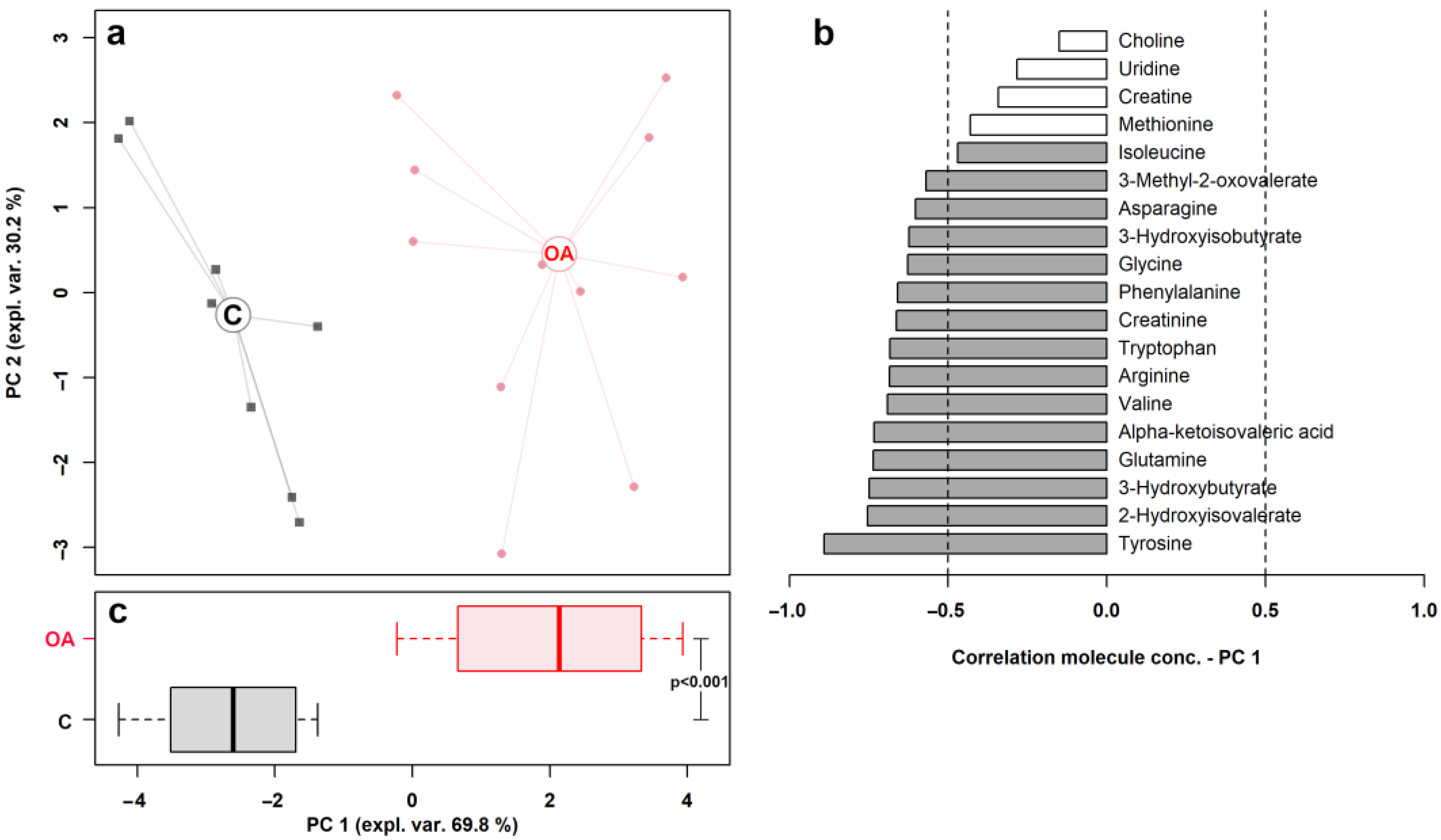
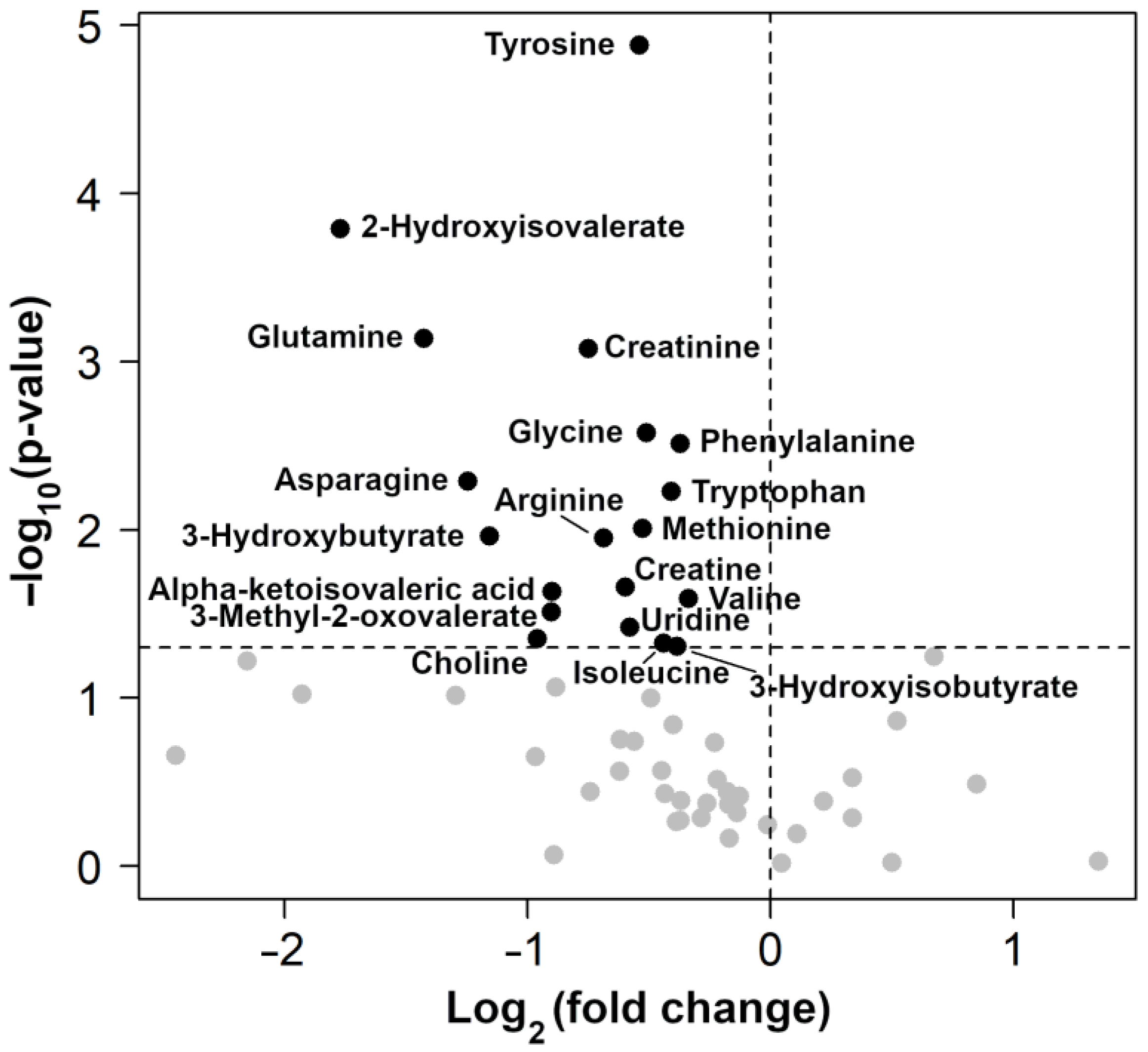
| Metabolite | Concentration (mmol/L) | p Value | |
|---|---|---|---|
| Control Group | OA Group | ||
| 1,3-Dihydroxyacetone | 0.003 ± 0.001 | 0.006 ± 0.004 | 0.03 |
| 2-Hydroxybutyrate | 0.01 ± 0.007 | 0.007 ± 0.004 | 0.18 |
| 2-Hydroxyisobutyrate | 0.033 ± 0.008 | 0.036 ± 0.008 | 0.03 |
| 2-Hydroxyisovalerate | 0.007 ± 0.002 | 0.003 ± 0.002 | 0.0001 |
| 3-Hydroxybutyrate | 0.317 ± 0.115 | 0.175 ± 0.121 | 0.01 |
| 3-Hydroxyisobutyrate | 0.017 ± 0.01 | 0.012 ± 0.012 | 0.05 |
| 3-Methyl-2-oxovalerate | 0.004 ± 0.002 | 0.002 ± 0.001 | 0.02 |
| 3-Methylhistidine | 0.154 ± 0.02 | 0.147 ± 0.05 | 0.34 |
| Acetate | 0.966 ± 0.731 | 0.721 ± 0.542 | 0.22 |
| Acetone | 0.014 ± 0.007 | 0.162 ± 0.49 | 0.17 |
| Alanine | 0.629 ± 0.043 | 0.59 ± 0.136 | 0.19 |
| Arginine | 0.226 ± 0.027 | 0.155 ± 0.075 | 0.006 |
| Asparagine | 0.079 ± 0.03 | 0.039 ± 0.024 | 0.004 |
| Aspartate | 0.051 ± 0.013 | 0.089 ± 0.07 | 0.06 |
| Betaine | 0.096 ± 0.033 | 0.071 ± 0.020 | 0.06 |
| Carnitine | 0.033 ± 0.009 | 0.032 ± 0.022 | 0.43 |
| Choline | 0.041 ± 0.019 | 0.025 ± 0.017 | 0.03 |
| Citrate | 0.602 ± 0.136 | 0.526 ± 0.251 | 0.12 |
| Creatine | 0.201 ± 0.038 | 0.145 ± 0.063 | 0.01 |
| Creatinine | 0.161 ± 0.025 | 0.101 ± 0.039 | 0.0004 |
| Cystine | 0.011 ± 0.008 | 0.021 ± 0.057 | 0.29 |
| Dimethyl sulfone | 0.069 ± 0.028 | 0.105 ± 0.126 | 0.19 |
| Ethanol | 0.0343 ± 0.872 | 0.025 ± 0.042 | 0.17 |
| Formate | 0.043 ± 0.006 | 0.072 ± 0.085 | 0.14 |
| Fumarate | 0.009 ± 0.001 | 0.009 ± 0.002 | 0.45 |
| Glucose | 7.296 ± 1.453 | 11.317 ± 7.441 | 0.06 |
| Glutamine | 0.450 ± 0.102 | 0.205 ± 0.169 | 0.001 |
| Glycerol | 0.047 ± 0.022 | 0.039 ± 0.028 | 0.25 |
| Glycine | 1.246 ± 0.088 | 0.906 ± 0.270 | 0.001 |
| Isobutyrate | 0.002 ± 0.002 | 0.001 ± 0.001 | 0.08 |
| Hippurate | 0.04 ± 0.018 | 0.028 ± 0.011 | 0.07 |
| Isoleucine | 0.128 ± 0.022 | 0.101 ± 0.047 | 0.05 |
| Lactate | 8.076 ± 3.231 | 5.733 ± 5.532 | 0.13 |
| Leucina | 0.322 ± 0.05 | 0.319 ± 0.164 | 0.48 |
| Lysine | 0.131 ± 0.014 | 0.128 ± 0.08 | 0.45 |
| Malonate | 0.003 ± 0.002 | 0.003 ± 0.003 | 0.47 |
| Mannose | 0.108 ± 0.033 | 0.093 ± 0.063 | 0.25 |
| Methionine | 0.048 ± 0.005 | 0.035 ± 0.015 | 0.01 |
| Myo-inositol | 0.069 ± 0.065 | 0.044 ± 0.027 | 0.17 |
| N,N-Dimethylglycine | 0.004 ± 0.001 | 0.003 ± 0.002 | 0.27 |
| Ornithine | 0.008 ± 0.005 | 0.004 ± 0.005 | 0.06 |
| Phenylalanine | 0.555 ± 0.047 | 0.437 ± 0.094 | 0.001 |
| Proline | 0.198 ± 0.058 | 0.276 ± 0.138 | 0.06 |
| Propylene glycol | 0.011 ± 0.004 | 0.061 ± 0.107 | 0.08 |
| Pyroglutamate | 0.028 ± 0.013 | 0.023 ± 0.013 | 0.24 |
| Pyruvate | 0.089 ± 0.04 | 0.069 ± 0.047 | 0.17 |
| Serine | 0.323 ± 0.039 | 0.296 ± 0.084 | 0.19 |
| Taurine | 0.006 ± 0.005 | 0.005 ± 0.037 | 0.31 |
| Threonine | 0.36 ± 0.08 | 0.306 ± 0.062 | 0.07 |
| Tryptophan | 0.059 ± 0.007 | 0.046 ± 0.012 | 0.003 |
| Tyrosine | 0.179 ± 0.013 | 0.125 ± 0.024 | 0.00001 |
| Uracil | 0.025 ± 0.003 | 0.027 ± 0.008 | 0.15 |
| Uridine | 0.045 ± 0.021 | 0.028 ± 0.008 | 0.03 |
| Valine | 0.437 ± 0.059 | 0.355 ± 0.087 | 0.01 |
| α-ketoisovaleric acid | 0.007 ± 0.003 | 0.004 ± 0.002 | 0.02 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Laus, F.; Gialletti, R.; Bazzano, M.; Laghi, L.; Dini, F.; Marchegiani, A. Synovial Fluid Metabolome Can Differentiate between Healthy Joints and Joints Affected by Osteoarthritis in Horses. Metabolites 2023, 13, 913. https://doi.org/10.3390/metabo13080913
Laus F, Gialletti R, Bazzano M, Laghi L, Dini F, Marchegiani A. Synovial Fluid Metabolome Can Differentiate between Healthy Joints and Joints Affected by Osteoarthritis in Horses. Metabolites. 2023; 13(8):913. https://doi.org/10.3390/metabo13080913
Chicago/Turabian StyleLaus, Fulvio, Rodolfo Gialletti, Marilena Bazzano, Luca Laghi, Fabrizio Dini, and Andrea Marchegiani. 2023. "Synovial Fluid Metabolome Can Differentiate between Healthy Joints and Joints Affected by Osteoarthritis in Horses" Metabolites 13, no. 8: 913. https://doi.org/10.3390/metabo13080913
APA StyleLaus, F., Gialletti, R., Bazzano, M., Laghi, L., Dini, F., & Marchegiani, A. (2023). Synovial Fluid Metabolome Can Differentiate between Healthy Joints and Joints Affected by Osteoarthritis in Horses. Metabolites, 13(8), 913. https://doi.org/10.3390/metabo13080913






