Metabolomics Approaches for the Diagnosis, Treatment, and Better Disease Management of Viral Infections
Abstract
:1. Introduction
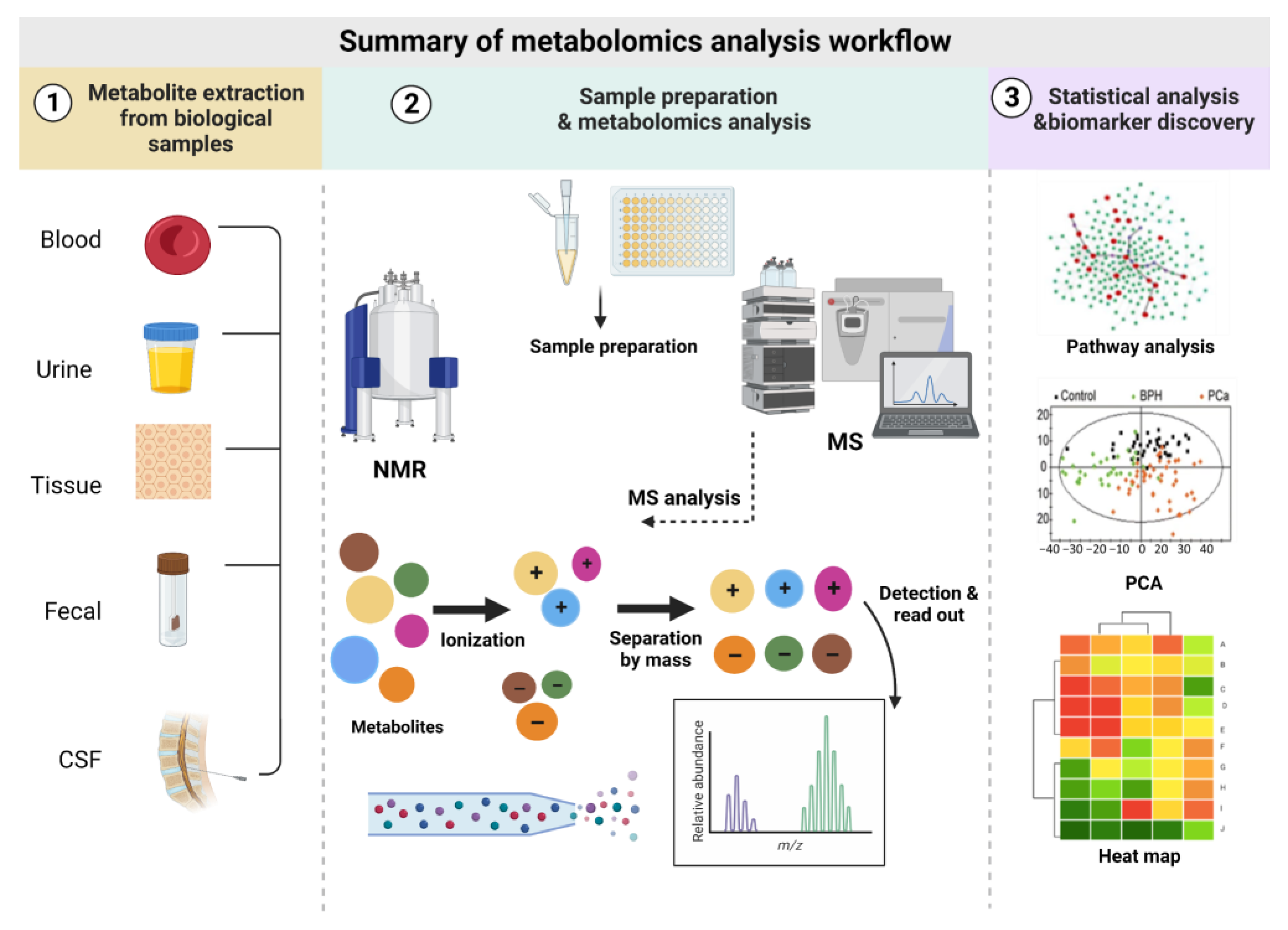
2. Metabolomics Analysis Workflow
2.1. Biofluids Used in Metabolomics and Sample Preparation
2.2. Metabolomics Analytical Tools
2.3. Metabolomics Approaches and Their Application
2.4. Statistical Analysis and Data Visualization
3. Metabolomics Challenges
4. Metabolomics Potential to Characterize Viral Infections
4.1. Metabolomics Study of Respiratory Pathogens
4.1.1. Coronaviridae
4.1.2. Orthomyxoviridae
4.2. Metabolomics in Chronic Viral Infections
4.2.1. Human Immunodeficiency Virus (HIV)
4.2.2. Hepatitis B Virus (HBV)
4.2.3. Hepatitis C Virus (HCV)
4.2.4. Human Cytomegalovirus (HCMV)
5. Metabolomics in Viral Neurological Infections
6. Conclusions and Future Perspectives
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Rinschen, M.M.; Ivanisevic, J.; Giera, M.; Siuzdak, G. Identification of bioactive metabolites using activity metabolomics. Nat. Rev. Mol. Cell Biol. 2019, 20, 353–367. [Google Scholar] [CrossRef] [PubMed]
- Patti, G.J.; Yanes, O.; Siuzdak, G. Innovation: Metabolomics: The apogee of the omics trilogy. Nat. Rev. Mol. Cell. Biol. 2012, 13, 263–269. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nordström, A.; Lewensohn, R. Metabolomics: Moving to the Clinic. J. Neuroimmune Pharmacol. 2009, 5, 4–17. [Google Scholar] [CrossRef]
- Hyötyläinen, T. Novel methodologies in metabolic profiling with a focus on molecular diagnostic applications. Expert Rev. Mol. Diagn. 2012, 12, 527–538. [Google Scholar] [CrossRef]
- Manchester, M.; Anand, A. Chapter Two—Metabolomics: Strategies to Define the Role of Metabolism in Virus Infection and Pathogenesis. In Advances in Virus Research; Kielian, M., Mettenleiter, T.C., Roossinck, M.J., Eds.; Academic Press: Cambridge, MA, USA, 2017; pp. 57–81. [Google Scholar]
- Lanpher, B.; Brunetti-Pierri, N.; Lee, B. Inborn errors of metabolism: The flux from Mendelian to complex diseases. Nat. Rev. Genet. 2006, 7, 449–459. [Google Scholar] [CrossRef] [PubMed]
- Cuperlovic-Culf, M.; Culf, A.S. Applied metabolomics in drug discovery. Expert Opin. Drug Discov. 2016, 11, 759–770. [Google Scholar] [CrossRef]
- Hanash, S.M.; Baik, C.S.; Kallioniemi, O. Emerging molecular biomarkers—Blood-based strategies to detect and monitor cancer. Nat. Rev. Clin. Oncol. 2011, 8, 142–150. [Google Scholar] [CrossRef]
- Munn, Z.; Peters, M.D.J.; Stern, C.; Tufanaru, C.; McArthur, A.; Aromataris, E. Systematic Review or Scoping Review? Guidance for Authors When Choosing between a Systematic or Scoping Review Approach. BMC Med. Res. Methodol. 2018, 18, 143. [Google Scholar] [CrossRef]
- BMJ. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, 105906. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Moher, D. Updating guidance for reporting systematic reviews: Development of the PRISMA 2020 statement. J. Clin. Epidemiol. 2021, 134, 103–112. [Google Scholar] [CrossRef]
- Want, E.J.; O’Maille, G.; Smith, C.A.; Brandon, T.R.; Uritboonthai, W.; Qin, C.; Trauger, S.A.; Siuzdak, G. Solvent-Dependent Metabolite Distribution, Clustering, and Protein Extraction for Serum Profiling with Mass Spectrometry. Anal. Chem. 2005, 78, 743–752. [Google Scholar] [CrossRef] [PubMed]
- Yanes, O.; Tautenhahn, R.; Patti, G.J.; Siuzdak, G. Expanding Coverage of the Metabolome for Global Metabolite Profiling. Anal. Chem. 2011, 83, 2152–2161. [Google Scholar] [CrossRef] [Green Version]
- Mushtaq, M.Y.; Choi, Y.H.; Verpoorte, R.; Wilson, E.G. Extraction for Metabolomics: Access to The Metabolome. Phytochem. Anal. 2014, 25, 291–306. [Google Scholar] [CrossRef]
- Ryan, D.; Robards, K.; Prenzler, P.; Kendall, M. Recent and potential developments in the analysis of urine: A review. Anal. Chim. Acta 2011, 684, 17–29. [Google Scholar] [CrossRef] [PubMed]
- Smith, L.; Villaret-Cazadamont, J.; Claus, S.P.; Canlet, C.; Guillou, H.; Cabaton, N.J.; Ellero-Simatos, S. Important Considera-tions for Sample Collection in Metabolomics Studies with a Special Focus on Applications to Liver Functions. Metabolites 2020, 10, 104. [Google Scholar] [CrossRef] [Green Version]
- Schoonenboom, N.S.M.; Pijnenburg, Y.A.L.; Mulder, C.; Rosso, S.M.; Van Elk, E.J.; Van Kamp, G.J.; Van Swieten, J.C.; Scheltens, P. Amyloid beta(1-42) and phosphorylated tau in CSF as markers for early-onset Alzheimer disease. Neurology 2004, 62, 1580–1584. [Google Scholar] [CrossRef]
- Lewis, G.D.; Asnani, A.; Gerszten, R.E. Application of metabolomics to cardiovascular biomarker and pathway discovery. J. Am. Coll. Cardiol. 2008, 52, 117–123. [Google Scholar] [CrossRef] [Green Version]
- Hocher, B.; Adamski, J. Metabolomics for clinical use and research in chronic kidney disease. Nat. Rev. Nephrol. 2017, 13, 269–284. [Google Scholar] [CrossRef]
- Hopfgartner, G.; Varesio, E.; Tschäppät, V.; Grivet, C.; Bourgogne, E.; Leuthold, L.A. Triple quadrupole linear ion trap mass spectrometer for the analysis of small molecules and macromolecules. J. Mass Spectrom. 2004, 39, 845–855. [Google Scholar] [CrossRef] [PubMed]
- Campbell, J.L.; Le Blanc, J.Y. Using high-resolution quadrupole TOF technology in DMPK analyses. Bioanalysis 2012, 4, 487–500. [Google Scholar] [CrossRef]
- Evans, A.M.; DeHaven, C.D.; Barrett, T.; Mitchell, M.; Milgram, E. Integrated, Nontargeted Ultrahigh Performance Liquid Chromatography/Electrospray Ionization Tandem Mass Spectrometry Platform for the Identification and Relative Quantification of the Small-Molecule Complement of Biological Systems. Anal. Chem. 2009, 81, 6656–6667. [Google Scholar] [CrossRef]
- Lu, W.; Bennett, B.D.; Rabinowitz, J.D. Analytical strategies for LC–MS-based targeted metabolomics. J. Chromatogr. B 2008, 871, 236–242. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, H.; Tennessen, J.M. Methods for studying the metabolic basis of Drosophila development. Wiley Interdiscip. Rev. Dev. Biol. 2017, 6, e280. [Google Scholar] [CrossRef] [Green Version]
- Tounta, V.; Liu, Y.; Cheyne, A.; Larrouy-Maumus, G. Metabolomics in infectious diseases and drug discovery. Mol. Omics 2021, 17, 376–393. [Google Scholar] [CrossRef]
- Wawrzyniak, R.; Ruperez, F.J.; Godzień, J.B. Editorial: Advances and challenges in untargeted metabolomics. Front. Mol. Biosci. 2023, 10, 1097443. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, D.R.; Patel, R.; Kirsch, D.G.; Lewis, C.A.; Vander Heiden, M.G.; Locasale, J.W. Metabolomics in cancer research and emerging applications in clinical oncology. CA Cancer J. Clin. 2021, 71, 333–358. [Google Scholar] [CrossRef] [PubMed]
- Cambiaghi, A.; Ferrario, M.; Masseroli, M. Analysis of metabolomic data: Tools, current strategies and future challenges for omics data integration. Brief. Bioinform. 2016, 18, 498–510. [Google Scholar] [CrossRef] [Green Version]
- Nicholson, J.K.; Connelly, J.; Lindon, J.C.; Holmes, E. Metabonomics: A platform for studying drug toxicity and gene function. Nat. Rev. Drug Discov. 2002, 1, 153–161. [Google Scholar] [CrossRef]
- Alonso, A.; Marsal, S.; Juliã, A. Analytical Methods in Untargeted Metabolomics: State of the Art in 2015. Front. Bioeng. Biotechnol. 2015, 3, 23. [Google Scholar] [CrossRef] [Green Version]
- Considine, E.C.; Thomas, G.; Boulesteix, A.L.; Khashan, A.S.; Kenny, L.C. Critical review of reporting of the data analysis step in metabolomics. Metabolomics 2017, 14, 7. [Google Scholar] [CrossRef]
- Al-Sulaiti, H.; Diboun, I.; Agha, M.V.; Mohamed, F.F.S.; Atkin, S.; Dömling, A.S.; Elrayess, M.A.; Mazloum, N.A. Metabolic signature of obesity-associated insulin resistance and type 2 diabetes. J. Transl. Med. 2019, 17, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jolliffe, I.T.; Cadima, J. Principal component analysis: A review and recent developments. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 2016, 374, 20150202. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fernandez-Garcia, M.; Rojo, D.; Rey-Stolle, F.; Garcia, A.; Barbas, C. Metabolomic-Based Methods in Diagnosis and Monitoring Infection Progression. Exp. Suppl. 2018, 109, 283–315. [Google Scholar] [CrossRef] [PubMed]
- Johnson, C.H.; Gonzalez, F.J. Challenges and opportunities of metabolomics. J. Cell. Physiol. 2011, 227, 2975–2981. [Google Scholar] [CrossRef]
- Herrington, C.; Coates, P.; Duprex, W. Viruses and disease: Emerging concepts for prevention, diagnosis and treatment. J. Pathol. 2014, 235, 149–152. [Google Scholar] [CrossRef]
- Rai, K.R.; Shrestha, P.; Yang, B.; Chen, Y.; Liu, S.; Maarouf, M.; Chen, J.-L. Acute Infection of Viral Pathogens and Their Innate Immune Escape. Front. Microbiol. 2021, 12, 672026. [Google Scholar] [CrossRef]
- Salomon, R.; Webster, R.G. The Influenza Virus Enigma. Cell 2009, 136, 402–410. [Google Scholar] [CrossRef] [Green Version]
- Worldwide World Health Organization, Summary of Probable SARS Cases with Onset of Illness from 1 November 2002 to 31 July 2003. Available online: https://www.who.int/publications/m/item/summary-of-probable-sars-cases-with-onset-of-illness-from-1-november-2002-to-31-july-2003 (accessed on 31 July 2003).
- Centre for Disease Control and Prevention (CDC), First Global Estimates of 2009 H1N1 Pandemic Mortality Released by CDC-Led Collaboration. Available online: https://www.cdc.gov/flu/pandemic-resources/2009-h1n1-pandemic.html (accessed on 10 April 2010).
- Shen, M.; Xiao, Y.; Rong, L. Modeling the effect of comprehensive interventions on Ebola virus transmission. Sci. Rep. 2015, 5, 15818. [Google Scholar] [CrossRef] [Green Version]
- Shuaib, W.; Stanazai, H.; Abazid, A.G.; Mattar, A.A. Re-Emergence of Zika Virus: A Review on Pathogenesis, Clinical Man-ifestations, Diagnosis, Treatment, and Prevention. Am. J. Med. 2016, 129, 879.e7–879.e12. [Google Scholar] [CrossRef] [Green Version]
- WHO. Issues Best Practices for Naming New Human Infectious Diseases. Available online: https://www.who.int/news/item/08-05-2015-who-issues-best-practices-for-naming-new-human-infectious-diseases (accessed on 10 April 2010).
- Hasan, M.R.; Suleiman, M.; Pérez-López, A. Metabolomics in the Diagnosis and Prognosis of COVID-19. Front. Genet. 2021, 12, 721556. [Google Scholar] [CrossRef]
- Manchester, M.; Anand, A. Metabolomics: Strategies to Define the Role of Metabolism in Virus Infection and Pathogenesis. Adv. Virus Res. 2017, 98, 57–81. [Google Scholar] [PubMed]
- Kleinehr, J.; Wilden, J.J.; Boergeling, Y.; Ludwig, S.; Hrincius, E.R. Metabolic Modifications by Common Respiratory Viruses and Their Potential as New Antiviral Targets. Viruses 2021, 13, 2068. [Google Scholar] [CrossRef] [PubMed]
- Galeas-Pena, M.; McLaughlin, N.; Pociask, D. The role of the innate immune system on pulmonary infections. Biol. Chem. 2018, 400, 443–456. [Google Scholar] [CrossRef] [PubMed]
- Shi, T.; Arnott, A.; Semogas, I.; Falsey, A.R.; Openshaw, P.; Wedzicha, J.A.; Campbell, H.; Nair, H.; Zhang, S.; Li, Y.; et al. The Etiological Role of Common Respiratory Viruses in Acute Respiratory Infections in Older Adults: A Systematic Review and Meta-analysis. J. Infect. Dis. 2019, 222, S563–S569. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mishra, S.K.; Tripathi, T. One year update on the COVID-19 pandemic: Where are we now? Acta Trop. 2021, 214, 105778. [Google Scholar] [CrossRef]
- Sharma, A.; Ahmad Farouk, I.; Lal, S.K. COVID-19: A Review on the Novel Coronavirus Disease Evolution, Transmission, Detection, Control and Prevention. Viruses 2021, 13, 202. [Google Scholar] [CrossRef]
- Cascella, M.; Rajnik, M.; Aleem, A.; Dulebohn, S.; Di Napoli, R. Features, Evaluation, and Treatment of Coronavirus (COVID-19); StatPearls: Treasure Island, FL, USA, 2022. [Google Scholar]
- Li, X.; Liu, Y.; Xu, G.; Xie, Y.; Wang, X.; Wu, J.; Chen, H. Plasma metabolomic characterization of SARS-CoV-2 Omicron infection. Cell Death Dis. 2023, 14, 276. [Google Scholar] [CrossRef]
- Taleb, S.; Yassine, H.M.; Benslimane, F.M.; Smatti, M.K.; Schuchardt, S.; Albagha, O.; Al-Thani, A.A.; Hssain, A.A.; Diboun, I.; Elrayess, M.A. Predictive Biomarkers of Intensive Care Unit and Mechanical Ventilation Duration in Critically-Ill Coronavirus Disease 2019 Patients. Front. Med. 2021, 8, 733657. [Google Scholar] [CrossRef]
- Meoni, G.; Ghini, V.; Maggi, L.; Vignoli, A.; Mazzoni, A.; Salvati, L.; Capone, M.; Vanni, A.; Tenori, L.; Fontanari, P.; et al. Metabo-lomic/lipidomic profiling of COVID-19 and individual response to tocilizumab. PLoS Pathog. 2021, 17, e1009243. [Google Scholar] [CrossRef]
- Caterino, M.; Costanzo, M.; Fedele, R.; Cevenini, A.; Gelzo, M.; Di Minno, A.; Andolfo, I.; Capasso, M.; Russo, R.; Annunziata, A.; et al. The Serum Metabolome of Moderate and Severe COVID-19 Patients Reflects Possible Liver Alterations Involving Carbon and Nitrogen Metabolism. Int. J. Mol. Sci. 2021, 22, 9548. [Google Scholar] [CrossRef]
- Marín-Corral, J.; Rodríguez-Morató, J.; Gomez-Gomez, A.; Pascual-Guardia, S.; Muñoz-Bermúdez, R.; Salazar-Degracia, A.; Pérez-Terán, P.; Restrepo, M.I.; Khymenets, O.; Haro, N.; et al. Metabolic Signatures Associated with Severity in Hospitalized COVID-19 Patients. Int. J. Mol. Sci. 2021, 22, 4794. [Google Scholar] [CrossRef] [PubMed]
- Elrayess, M.A.; Cyprian, F.S.; Abdallah, A.M.; Emara, M.M.; Diboun, I.; Anwardeen, N.; Schuchardt, S.; Yassine, H.M. Metabolic Signatures of Type 2 Diabetes Mellitus and Hypertension in COVID-19 Patients With Different Disease Severity. Front. Med. 2022, 8, 788687. [Google Scholar] [CrossRef] [PubMed]
- Mackie, P. The classification of viruses infecting the respiratory tract. Paediatr. Respir. Rev. 2003, 4, 84–90. [Google Scholar] [CrossRef] [PubMed]
- WHO. Influenza (Seasonal); WHO: Geneva, Switzerland, 2018; Available online: https://www.who.int/en/news-room/fact-sheets/detail/influenza-(seasonal) (accessed on 22 March 2019).
- Niessen, W. Tandem mass spectrometry of small-molecule antiviral drugs: 3. antiviral agents against herpes, influenza and other viral infections. Int. J. Mass Spectrom. 2020, 455, 116377. [Google Scholar] [CrossRef]
- Highly Pathogenic Avian Influenza A(H5N1) in Birds and Other Animals. 2015. Available online: https://www.cdc.gov/flu/avianflu/h5n1-animals.htm (accessed on 1 April 2023).
- García-Sastre, A. Lessons from Lipids in the Fight against Influenza. Cell 2013, 154, 22–23. [Google Scholar] [CrossRef] [Green Version]
- Karimi, Z.; Oskouie, A.A.; Rezaei, F.; Ajaminejad, F.; Marashi, S.M.; Azad, T. The effect of influenza virus on the metabolism of peripheral blood mononuclear cells with a metabolomics approach. J. Med. Virol. 2022, 94, 4383–4392. [Google Scholar] [CrossRef]
- Boldogh, I.; Albrecht, T.; Porter, D.D. Persistent Viral Infections. In Medical Microbiology; Baron, S., Ed.; University of Texas Medical Branch at Galveston: Galveston, TX, USA, 1996. [Google Scholar]
- Kenfack-Momo, R.; Kenmoe, S.; Takuissu, G.R.; Ebogo-Belobo, J.T.; Kengne-Ndé, C.; Mbaga, D.S.; Tchatchouang, S.; Oyono, M.G.; Kenfack-Zanguim, J.; Fogang, R.L.; et al. Epidemiology of hepatitis B virus and/or hepatitis C virus infections among people living with human immunodeficiency virus in Africa: A systematic review and meta-analysis. PLoS ONE 2022, 17, e0269250. [Google Scholar] [CrossRef]
- Xiang, Z.; Li, J.; Lu, D.; Wei, X.; Xu, X. Advances in multi-omics research on viral hepatitis. Front. Microbiol. 2022, 13, 987324. [Google Scholar] [CrossRef]
- Yang, J.; Wang, D.; Li, Y.; Wang, H.; Hu, Q.; Wang, Y. Metabolomics in viral hepatitis: Advances and review. Front. Cell. Infect. Microbiol. 2023, 13, 1189417. [Google Scholar] [CrossRef]
- Hollenbaugh, J.A.; Munger, J.; Kim, B. Metabolite profiles of human immunodeficiency virus infected CD4+ T cells and macrophages using LC–MS/MS analysis. Virology 2011, 415, 153–159. [Google Scholar] [CrossRef] [Green Version]
- WHO, HIV Data and Statistics. Available online: https://www.who.int/health-topics/hiv-aids#tab=tab_1 (accessed on 27 July 2022).
- Rasmussen, L.D.; May, M.T.; Kronborg, G.; Larsen, C.S.; Pedersen, C.; Gerstoft, J.; Obel, N. Time trends for risk of severe age-related diseases in individuals with and without HIV infection in Denmark: A nationwide population-based cohort study. Lancet HIV 2015, 2, e288–e298. [Google Scholar] [CrossRef]
- Ding, Y.; Lin, H.; Chen, X.; Zhu, B.; Xu, X.; Xu, X.; Shen, W.; Gao, M.; He, N. Comprehensive metabolomics profiling reveals common metabolic alterations underlying the four major non-communicable diseases in treated HIV infection. Ebiomedicine 2021, 71, 103548. [Google Scholar] [CrossRef]
- Liebenberg, C.; Luies, L.; Williams, A.A. Metabolomics as a Tool to Investigate HIV/TB Co-Infection. Front. Mol. Biosci. 2021, 8, 692823. [Google Scholar] [CrossRef] [PubMed]
- Hewer, R.; Vorster, J.; Steffens, F.; Meyer, D. Applying biofluid 1H NMR-based metabonomic techniques to distinguish between HIV-1 positive/AIDS patients on antiretroviral treatment and HIV-1 negative individuals. J. Pharm. Biomed. Anal. 2006, 41, 1442–1446. [Google Scholar] [CrossRef]
- Philippeos, C.; Steffens, F.E.; Meyer, D. Comparative 1H NMR-based metabonomic analysis of HIV-1 sera. J. Biomol. NMR 2009, 44, 127–137. [Google Scholar] [CrossRef]
- Hepatitis, B. 2022. Available online: https://www.who.int/news-room/fact-sheets/detail/hepatitis-b (accessed on 24 June 2022).
- Besombes, J.; Souala, F.; Bouguen, G.; Guyader, D.; Grolhier, C.; Thibault, V.; Pronier, C. Acute hepatitis B virus infection despite vaccination in a patient treated by infliximab: A case report. BMC Gastroenterol. 2022, 22, 1–5. [Google Scholar] [CrossRef]
- Lan, W.; Wang, Y.; Zhou, Z.; Sun, X.; Zhang, Y.; Zhang, F. Metabolic Regulation of Hepatitis B Virus Infection in HBV-Transgenic Mice. Metabolites 2022, 12, 287. [Google Scholar] [CrossRef]
- Pan, H.Y.; Wu, Q.Q.; Yin, Q.Q.; Dai, Y.N.; Huang, Y.C.; Zheng, W.; Hui, T.C.; Chen, M.J.; Wang, M.S.; Zhang, J.J.; et al. LC/MS-Based Global Metabolomic Identification of Serum Biomarkers Differentiating Hepatocellular Carcinoma from Chronic Hepatitis B and Liver Cirrhosis. ACS Omega 2021, 6, 1160–1170. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Zeng, Z.; Tan, H.; Feng, Q.; Zhou, Q.; Hu, J.; Li, Y.; Wang, J.; Yang, W.; Feng, J.; et al. Significant metabolic alterations in patients with hepatitis B virus replication observed via serum untargeted metabolomics shed new light on hepatitis B virus infection. J. Drug Target. 2021, 30, 442–449. [Google Scholar] [CrossRef]
- Nguyen, H.T.T.; Wimmer, R.; Le, V.Q.; Krarup, H.B. Metabolic fingerprint of progression of chronic hepatitis B: Changes in the metabolome and novel diagnostic possibilities. Metabolomics 2021, 17, 1–16. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization; Hepatitis, C. 2019. Available online: https://www.who.int/news-room/fact-sheets/detail/hepatitis-c (accessed on 1 April 2023).
- Yang, J.; Liu, H.X.; Su, Y.Y.; Liang, Z.S.; Rao, H.Y. Distribution and changes in hepatitis C virus genotype in China from 2010 to 2020. World J. Clin. Cases 2022, 10, 4480–4493. [Google Scholar] [CrossRef]
- Shanmuganathan, M.; Sarfaraz, M.O.; Kroezen, Z.; Philbrick, H.; Poon, R.; Don-Wauchope, A.; Puglia, M.; Wishart, D.; Britz-McKibbin, P. A Cross-Platform Metabolomics Comparison Identifies Serum Metabolite Signatures of Liver Fibrosis Pro-gression in Chronic Hepatitis C Patients. Front. Mol. Biosci. 2021, 8, 676349. [Google Scholar] [CrossRef]
- Fitian, A.I.; Nelson, D.R.; Liu, C.; Xu, Y.; Ararat, M.; Cabrera, R. Integrated metabolomic profiling of hepatocellular carcinoma in hepatitis C cirrhosis through GC/MS and UPLC/MS-MS. Liver Int. 2014, 34, 1428–1444. [Google Scholar] [CrossRef] [Green Version]
- Gupta, M.; Shorman, M. Cytomegalovirus; StatPearls: Treasure Island, FL, USA, 2022. [Google Scholar]
- Locci, E.; Noto, A.; Lanari, M.; Lazzarotto, T.; Fanos, V.; Atzori, L. Metabolomics: A new tool for the investigation of metabolic changes induced by cytomegalovirus. J. Matern. Neonatal Med. 2013, 26, 17–19. [Google Scholar] [CrossRef] [PubMed]
- Huang, P.; Wang, X.; Lei, M.; Ma, Y.; Chen, H.; Sun, J.; Hu, Y.; Shi, J. Metabolomics Profiles Reveal New Insights of Herpes Simplex Virus Type 1 Infection. Int. J. Mol. Sci. 2023, 24, 1521. [Google Scholar] [CrossRef] [PubMed]
- Frick, M.A.; Barba, I.; Fenoy-Alejandre, M.; López-López, P.; Baquero-Artigao, F.; Rodríguez-Molino, P.; Noguera-Julian, A.; Nicolás-López, M.; De La Fuente-Juárez, A.; Codina-Grau, M.G.; et al. 1H-NMR Urinary Metabolic Profile, A Promising Tool for the Management of Infants with Human Cytomegalovirus-Infection. Metabolites 2019, 9, 288. [Google Scholar] [CrossRef] [Green Version]
- Li, W.-W.; Shan, J.-J.; Lin, L.-L.; Xie, T.; He, L.-L.; Yang, Y.; Wang, S.-C. Disturbance in Plasma Metabolic Profile in Different Types of Human Cytomegalovirus-Induced Liver Injury in Infants. Sci. Rep. 2017, 7, 15696. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McGavern, D.B.; Kang, S.S. Illuminating viral infections in the nervous system. Nat. Rev. Immunol. 2011, 11, 318–329. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koyuncu, O.O.; Hogue, I.B.; Enquist, L.W. Virus infections in the nervous system. Cell Host Microbe 2013, 13, 379–393. [Google Scholar] [CrossRef] [Green Version]
- Kakooza-Mwesige, A.; Tshala-Katumbay, D.; Juliano, S.L. Viral infections of the central nervous system in Africa. Brain Res. Bull. 2019, 145, 2–17. [Google Scholar] [CrossRef]
- Bohmwald, K.; Gálvez, N.M.S.; Ríos, M.; Kalergis, A.M. Neurologic Alterations Due to Respiratory Virus Infections. Front. Cell. Neurosci. 2018, 12, 386. [Google Scholar] [CrossRef]
- Robinson, C.P.; Busl, K.M. Neurologic Manifestations of Severe Respiratory Viral Contagions. Crit. Care Explor. 2020, 2, e0107. [Google Scholar] [CrossRef]
- Bale, J.F., Jr. Measles, mumps, rubella, and human parvovirus B19 infections and neurologic disease. Handb. Clin. Neurol. 2014, 121, 1345–1353. [Google Scholar]
- Abdullahi, A.M.; Sarmast, S.T.; Jahan, N. Viral Infections of the Central Nervous System in Children: A Systematic Review. Cureus 2020, 12, e11174. [Google Scholar] [CrossRef] [PubMed]
- French, C.D.; Willoughby, R.E.; Pan, A.; Wong, S.J.; Foley, J.F.; Wheat, L.J.; Fernandez, J.; Encarnacion, R.; Ondrush, J.M.; Fatteh, N.; et al. NMR metabolomics of cerebrospinal fluid differentiates inflammatory diseases of the central nervous system. PLoS Neglected Trop. Dis. 2018, 12, e0007045. [Google Scholar] [CrossRef]
- Subramanian, A.; Gupta, A.; Saxena, S.; Gupta, A.; Kumar, R.; Nigam, A.; Kumar, R.; Mandal, S.K.; Roy, R. Proton MR CSF analysis and a new software as predictors for the differentiation of meningitis in children. NMR Biomed. 2005, 18, 213–225. [Google Scholar] [CrossRef] [PubMed]
- Torii, Y.; Kawano, Y.; Sato, H.; Sasaki, K.; Fujimori, T.; Kawada, J.I.; Takikawa, O.; Lim, C.K.; Guillemin, G.J.; Ohashi, Y.; et al. Quantitative metabolome profiling reveals the involvement of the kynurenine pathway in influenza-associated encephalopathy. Metabolomics 2016, 12, 84. [Google Scholar] [CrossRef]
| Variable | NMR | MS |
|---|---|---|
| Sample preparation | No sample preparation or sample extraction | Extraction, desalting, filtration |
| Number of detectable metabolites | Tens of metabolites from a single spectrum collected at or above 600 MHz | Can detect hundreds of metabolites from a single chromatogram (based on whether GC-MS or LC-MS is used) |
| Sensitivity | Lower than MS (nanomolar); lack of sensitivity | Higher than NMR (picomolar) |
| Quantification | No standard is required; linear response | Standard required (isotope-labeled standard); matrix and ionization-dependent response |
| Repeatability/Reproducibility | Both techniques are highly precise and reproducible | |
| Instrument Cost | More expensive option and takes up more space than MS | Cheaper and occupies less space than NMR |
| Specific advantages | Non-destructive detection, good replication, and structure information | Sensitivity, a high number of detectable metabolites |
| Specific disadvantages | Low sensitivity and peak overlap | Ion depression effect, no structure information, and destructive detection |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Sulaiti, H.; Almaliti, J.; Naman, C.B.; Al Thani, A.A.; Yassine, H.M. Metabolomics Approaches for the Diagnosis, Treatment, and Better Disease Management of Viral Infections. Metabolites 2023, 13, 948. https://doi.org/10.3390/metabo13080948
Al-Sulaiti H, Almaliti J, Naman CB, Al Thani AA, Yassine HM. Metabolomics Approaches for the Diagnosis, Treatment, and Better Disease Management of Viral Infections. Metabolites. 2023; 13(8):948. https://doi.org/10.3390/metabo13080948
Chicago/Turabian StyleAl-Sulaiti, Haya, Jehad Almaliti, C. Benjamin Naman, Asmaa A. Al Thani, and Hadi M. Yassine. 2023. "Metabolomics Approaches for the Diagnosis, Treatment, and Better Disease Management of Viral Infections" Metabolites 13, no. 8: 948. https://doi.org/10.3390/metabo13080948






