A Basic Study of the Effects of Mulberry Leaf Administration to Healthy C57BL/6 Mice on Gut Microbiota and Metabolites
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animal Treatment
2.2. Fecal DNA Extraction and 16S-rRNA Sequencing
2.3. 16S rDNA-Based Taxonomic Analysis
2.4. Sample Preparation for 1H NMR Analysis
2.5. NMR Spectra Acquisition and Data Processing
2.6. Multivariate Analysis of the NMR Data
3. Results
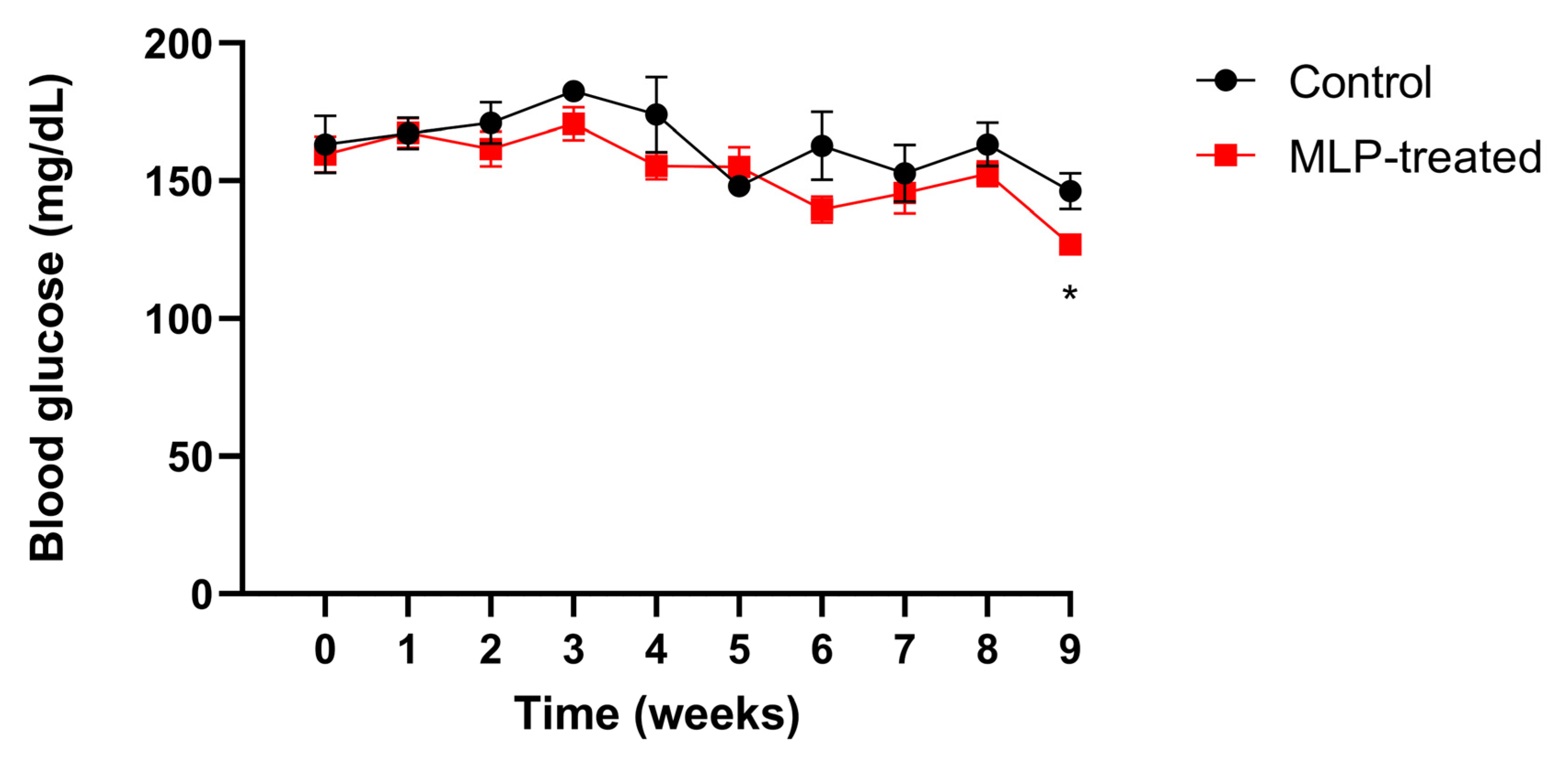
3.1. Body Weight and Blood Glucose
3.2. Effects of MLP Treatment on the Intestinal Microbiota
3.3. 1H-NMR Spectra of Mice Feces and Identification of Metabolites
3.4. Multivariate Analysis of Metabolites
3.5. Comparison of Metabolite Quantitative Value Variability
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- D’urso, G.; Mes, J.J.; Montoro, P.; Hall, R.D.; de Vos, R.C.H. Identification of Bioactive Phytochemicals in Mulberries. Metabolites 2020, 10, 7. [Google Scholar] [CrossRef]
- Thaipitakwong, T.; Numhom, S.; Aramwit, P. Mulberry Leaves and Their Potential Effects against Cardiometabolic Risks: A Review of Chemical Compositions, Biological Properties and Clinical Efficacy. Pharm. Biol. 2018, 56, 109–118. [Google Scholar] [CrossRef]
- Flaczyk, E.; Kobus-Cisowska, J.; Przeor, M.; Korczak, J.; Remiszewski, M.; Korbas, E.; Buchowski, M. Chemical Characterization and Antioxidative Properties of Polish Variety of Morus alba L. Leaf Aqueous Extracts from the Laboratory and Pilot-Scale Processes. Agric. Sci. 2013, 4, 141–147. [Google Scholar] [CrossRef]
- Ge, Q.; Chen, L.; Tang, M.; Zhang, S.; Liu, L.; Gao, L.; Ma, S.; Kong, M.; Yao, Q.; Feng, F.; et al. Analysis of Mulberry Leaf Components in the Treatment of Diabetes Using Network Pharmacology. Eur. J. Pharmacol. 2018, 833, 50–62. [Google Scholar] [CrossRef]
- Iqbal, S.; Younas, U.; Sirajuddin; Chan, K.W.; Sarfraz, R.A.; Uddin, K. Proximate Composition and Antioxidant Potential of Leaves from Three Varieties of Mulberry (Morus sp.): A Comparative Study. Int. J. Mol. Sci. 2012, 13, 6651–6664. [Google Scholar] [CrossRef] [PubMed]
- Chao, P.Y.; Lin, K.H.; Chiu, C.C.; Yang, Y.Y.; Huang, M.Y.; Yang, C.M. Inhibitive Effects of Mulberry Leaf-Related Extracts on Cell Adhesion and Inflammatory Response in Human Aortic Endothelial Cells. Evid. Based Complement. Altern. Med. 2013, 2013, 267217. [Google Scholar] [CrossRef] [PubMed]
- Vichasilp, C.; Nakagawa, K.; Sookwong, P.; Higuchi, O.; Luemunkong, S.; Miyazawa, T. Development of High 1-Deoxynojirimycin (DNJ) Content Mulberry Tea and Use of Response Surface Methodology to Optimize Tea-Making Conditions for Highest DNJ Extraction. LWT 2012, 45, 226–232. [Google Scholar] [CrossRef]
- Kimura, T.; Nakagawa, K.; Kubota, H.; Kojima, Y.; Goto, Y.; Yamagishi, K.; Oita, S.; Oikawa, S.; Miyazawa, T. Food-Grade Mulberry Powder Enriched with 1-Deoxynojirimycin Suppresses the Elevation of Postprandial Blood Glucose in Humans. J. Agric. Food Chem. 2007, 55, 5869–5874. [Google Scholar] [CrossRef]
- Liu, Q.; Li, X.; Li, C.; Zheng, Y.; Peng, G.; McPhee, D.J. 1-Deoxynojirimycin Alleviates Insulin Resistance via Activation of Insulin Signaling PI3K/AKT Pathway in Skeletal Muscle of Db/Db Mice. Molecules 2015, 20, 21700–21714. [Google Scholar] [CrossRef]
- Wu, H.; Zeng, W.; Chen, L.; Yu, B.; Guo, Y.; Chen, G.; Liang, Z. Integrated Multi-Spectroscopic and Molecular Docking Techniques to Probe the Interaction Mechanism between Maltase and 1-Deoxynojirimycin, an α-Glucosidase Inhibitor. Int. J. Biol. Macromol. 2018, 114, 1194–1202. [Google Scholar] [CrossRef]
- Cho, N.H.; Shaw, J.E.; Karuranga, S.; Huang, Y.; da Rocha Fernandes, J.D.; Ohlrogge, A.W.; Malanda, B. IDF Diabetes Atlas: Global Estimates of Diabetes Prevalence for 2017 and Projections for 2045. Diabetes Res. Clin. Pract. 2018, 138, 271–281. [Google Scholar] [CrossRef]
- Li, R.; Zhu, Q.; Wang, X.; Wang, H. Mulberry Leaf Polyphenols Alleviated High-Fat Diet-Induced Obesity in Mice. Front. Nutr. 2022, 9, 979058. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Xue, Z.; Li, S.; Zhou, J.; Liu, J.; Zhang, M.; Panichayupakaranant, P.; Chen, H. Mulberry Leaf Polysaccharides Ameliorate Obesity through Activation of Brown Adipose Tissue and Modulation of the Gut Microbiota in High-Fat Diet Fed Mice. Food Funct. 2022, 13, 561–573. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Yamasaki, M.; Katsube, T.; Shiwaku, K. Effects of Quercetin Derivatives from Mulberry Leaves: Improved Gene Expression Related Hepatic Lipid and Glucose Metabolism in Short-Term High-Fat Fed Mice. Nutr. Res. Pract. 2015, 9, 137–143. [Google Scholar] [CrossRef] [PubMed]
- Asai, A.; Nakagawa, K.; Higuchi, O.; Kimura, T.; Kojima, Y.; Kariya, J.; Miyazawa, T.; Oikawa, S. Effect of Mulberry Leaf Extract with Enriched 1-Deoxynojirimycin Content on Postprandial Glycemic Control in Subjects with Impaired Glucose Metabolism. J Diabetes Investig. 2011, 2, 318–323. [Google Scholar] [CrossRef]
- Lown, M.; Fuller, R.; Lightowler, H.; Fraser, A.; Gallagher, A.; Stuart, B.; Byrne, C.; Lewith, G. Mulberry-Extract Improves Glucose Tolerance and Decreases Insulin Concentrations in Normoglycaemic Adults: Results of a Randomised Double-Blind Placebo-Controlled Study. PLoS ONE 2017, 12, e0172239. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.G.; Ji, D.F.; Zhong, S.; Lin, T.B.; Lv, Z.Q.; Hu, G.Y.; Wang, X. 1-Deoxynojirimycin Inhibits Glucose Absorption and Accelerates Glucose Metabolism in Streptozotocin-Induced Diabetic Mice. Sci. Rep. 2013, 3, 1377. [Google Scholar] [CrossRef]
- Zhao, X.; Fu, Z.; Yao, M.; Cao, Y.; Zhu, T.; Mao, R.; Huang, M.; Pang, Y.; Meng, X.; Li, L.; et al. Mulberry (Morus alba L.) Leaf Polysaccharide Ameliorates Insulin Resistance- and Adipose Deposition-Associated Gut Microbiota and Lipid Metabolites in High-Fat Diet-Induced Obese Mice. Food Sci. Nutr. 2022, 10, 617–630. [Google Scholar] [CrossRef]
- Zheng, X.; Li, D.; Li, Y.; Chen, Y.; Zhao, Y.; Ji, S.; Guo, M.; Du, Y.; Tang, D. Mulberry Leaf Water Extract Alleviates Type 2 Diabetes in Mice via Modulating Gut Microbiota-Host Co-Metabolism of Branched-Chain Amino Acid. Phytother. Res. 2023, 37, 3195–3210. [Google Scholar] [CrossRef]
- Du, Y.; Li, D.X.; Lu, D.Y.; Zhang, R.; Zheng, X.; Xu, B.; Zhao, Y.; Ji, S.; Guo, M.; Wang, L.; et al. Morus alba L. Water Extract Changes Gut Microbiota and Fecal Metabolome in Mice Induced by High-Fat and High-Sucrose Diet plus Low-Dose Streptozotocin. Phytother. Res. 2022, 36, 1241–1257. [Google Scholar] [CrossRef]
- Zheng, J.; Zhu, L.; Hu, B.; Zou, X.; Hu, H.; Zhang, Z.; Jiang, N.; Ma, J.; Yang, H.; Liu, H. 1-Deoxynojirimycin Improves High Fat Diet-Induced Nonalcoholic Steatohepatitis by Restoring Gut Dysbiosis. J. Nutr. Biochem. 2019, 71, 16–26. [Google Scholar] [CrossRef] [PubMed]
- Qiao, Y.; Nakayama, J.; Ikeuchi, T.; Ito, M.; Kimura, T.; Kojima, K.; Takita, T.; Yasukawa, K. Kinetic Analysis of Inhibition of α-glucosidase by Leaf Powder from Morus australis and Its Component Iminosugars. Biosci. Biotechnol. Biochem. 2020, 84, 2149–2156. [Google Scholar] [CrossRef]
- Katsube, T.; Yamasaki, M.; Shiwaku, K.; Ishijima, T.; Matsumoto, I.; Abe, K.; Yamasaki, Y. Effect of Flavonol Glycoside in Mulberry (Morus alba L.) Leaf on Glucose Metabolism and Oxidative Stress in Liver in Diet-induced Obese Mice. J. Sci. Food Agric. 2010, 90, 2386–2392. [Google Scholar] [CrossRef] [PubMed]
- Herlemann, D.P.R.; Labrenz, M.; Jürgens, K.; Bertilsson, S.; Waniek, J.J.; Andersson, A.F. Transitions in Bacterial Communities along the 2000 Km Salinity Gradient of the Baltic Sea. ISME J. 2011, 5, 1571–1579. [Google Scholar] [CrossRef]
- Bolyen, E.; Rideout, J.R.; Dillon, M.R.; Bokulich, N.A.; Abnet, C.C.; Al-Ghalith, G.A.; Alexander, H.; Alm, E.J.; Arumugam, M.; Asnicar, F.; et al. Reproducible, Interactive, Scalable and Extensible Microbiome Data Science Using QIIME 2. Nat. Biotechnol. 2019, 37, 850–852. [Google Scholar] [CrossRef] [PubMed]
- Callahan, B.J.; McMurdie, P.J.; Rosen, M.J.; Han, A.W.; Johnson, A.J.A.; Holmes, S.P. DADA2: High-Resolution Sample Inference from Illumina Amplicon Data. Nat. Methods 2016, 13, 581–583. [Google Scholar] [CrossRef]
- Katoh, K.; Standley, D.M. MAFFT Multiple Sequence Alignment Software Version 7: Improvements in Performance and Usability. Mol. Biol. Evol. 2013, 30, 772–780. [Google Scholar] [CrossRef]
- Price, M.N.; Dehal, P.S.; Arkin, A.P. FastTree 2-Approximately Maximum-Likelihood Trees for Large Alignments. PLoS ONE 2010, 5, e9490. [Google Scholar] [CrossRef]
- Quast, C.; Pruesse, E.; Yilmaz, P.; Gerken, J.; Schweer, T.; Yarza, P.; Peplies, J.; Glöckner, F.O. The SILVA Ribosomal RNA Gene Database Project: Improved Data Processing and Web-Based Tools. Nucleic Acids Res. 2013, 41, D590–D596. [Google Scholar] [CrossRef]
- O’Hearn, M.; Lara-Castor, L.; Cudhea, F.; Miller, V.; Reedy, J.; Shi, P.; Zhang, J.; Wong, J.B.; Economos, C.D.; Micha, R.; et al. Incident Type 2 Diabetes Attributable to Suboptimal Diet in 184 Countries. Nat. Med. 2023, 29, 982–995. [Google Scholar] [CrossRef]
- Sheng, Y.; Zheng, S.; Ma, T.; Zhang, C.; Ou, X.; He, X.; Xu, W.; Huang, K. Mulberry Leaf Alleviates Streptozotocin-Induced Diabetic Rats by Attenuating NEFA Signaling and Modulating Intestinal Microflora. Sci. Rep. 2017, 7, 12041. [Google Scholar] [CrossRef]
- De Smet, P.A. Herbal Remedies. N. Engl. J. Med. 2002, 347, 2046–2056. [Google Scholar] [CrossRef]
- Jouad, H.; Haloui, M.; Rhiouani, H.; Hilaly, J.E.; Eddouks, M. Ethnobotanical Survey of Medicinal Plants Used for the Treatment of Diabetes, Cardiac and Renal Diseases in the North Centre Region of Morocco (Fez-Boulemane). J. Ethnopharmacol. 2001, 77, 175–182. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Xi, M.; Gao, F.; Li, M.; Dong, T.W.; Geng, Z.; Liu, C.; Huang, F.; Wang, J.; Li, X.; et al. Evaluation of Mulberry Leaves’ Hypoglycemic Properties and Hypoglycemic Mechanisms. Front. Pharmacol. 2023, 14, 1045309. [Google Scholar] [CrossRef]
- Hong, H.C.; Li, S.L.; Zhang, X.Q.; Ye, W.C.; Zhang, Q.W. Flavonoids with α-glucosidase Inhibitory Activities and Their Contents in the Leaves of Morus atropurpurea. Chin. Med. 2013, 8, 19. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, X.; Liang, C.; Hu, J.; Yu, Z. Safety Evaluation of Mulberry Leaf Extract: Acute, Subacute Toxicity and Genotoxicity Studies. Regul. Toxicol. Pharmacol. 2018, 95, 220–226. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Luo, Z.; Zhou, J.; Sun, B. Gut Microbiota and Antidiabetic Drugs: Perspectives of Personalized Treatment in Type 2 Diabetes Mellitus. Front. Cell. Infect. Microbiol. 2022, 12, 853771. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.H.; Hamaker, B.R. Maltase Has Most Versatile α-Hydrolytic Activity Among the Mucosal α-Glucosidases of the Small Intestine. J. Pediatr. Gastroenterol. Nutr. 2018, 66, S7–S10. [Google Scholar] [CrossRef]
- Hong, Y.S.; Jung, D.H.; Chung, W.H.; Nam, Y.D.; Kim, Y.J.; Seo, D.H.; Park, C.S. Human Gut Commensal Bacterium Ruminococcus Species FMB-CY1 Completely Degrades the Granules of Resistant Starch. Food Sci. Biotechnol. 2022, 31, 231–241. [Google Scholar] [CrossRef]
- Raigond, P.; Ezekiel, R.; Raigond, B. Resistant Starch in Food: A Review. J. Sci. Food Agric. 2015, 95, 1968–1978. [Google Scholar] [CrossRef]
- Tan, K.; Tesar, C.; Wilton, R.; Jedrzejczak, R.P.; Joachimiak, A. Interaction of Antidiabetic α-Glucosidase Inhibitors and Gut Bacteria α-Glucosidase. Protein Sci. 2018, 27, 1498–1508. [Google Scholar] [CrossRef]
- Qiao, Y.; Ikeda, Y.; Ito, M.; Kimura, T.; Ikeuchi, T.; Takita, T.; Yasukawa, K. Inhibition of α-Amylase and α-Glucosidase by Morus Australis Fruit Extract and Its Components Iminosugar, Anthocyanin, and Glucose. J. Food Sci. 2022, 87, 1672–1683. [Google Scholar] [CrossRef] [PubMed]
- Qiao, Y.; Ito, M.; Kimura, T.; Ikeuchi, T.; Takita, T.; Yasukawa, K. Inhibitory Effect of Morus Australis Leaf Extract and Its Component Iminosugars on Intestinal Carbohydrate-Digesting Enzymes. J. Biosci. Bioeng. 2021, 132, 226–233. [Google Scholar] [CrossRef]
- Liu, Z.; Yang, Y.; Dong, W.; Liu, Q.; Wang, R.; Pang, J.; Xia, X.; Zhu, X.; Liu, S.; Shen, Z.; et al. Investigation on the Enzymatic Profile of Mulberry Alkaloids by Enzymatic Study and Molecular Docking. Molecules 2019, 24, 1776. [Google Scholar] [CrossRef] [PubMed]
- Morrison, D.J.; Preston, T. Formation of Short Chain Fatty Acids by the Gut Microbiota and Their Impact on Human Metabolism. Gut Microbes 2016, 7, 189–200. [Google Scholar] [CrossRef]
- Liao, S.; Long, X.; Zou, Y.; Liu, F.; Li, Q. Mulberry Leaf Phenolics and Fiber Exert Anti-Obesity through the Gut Microbiota-Host Metabolism Pathway. J. Food Sci. 2021, 86, 1432–1447. [Google Scholar] [CrossRef] [PubMed]
- Kårlund, A.; Gómez-Gallego, C.; Turpeinen, A.M.; Palo-Oja, O.M.; El-Nezami, H.; Kolehmainen, M. Protein Supplements and Their Relation with Nutrition, Microbiota Composition and Health: Is More Protein Always Better for Sportspeople? Nutrients 2019, 11, 829. [Google Scholar] [CrossRef]
- Ruiz-Canela, M.; Guasch-Ferré, M.; Toledo, E.; Clish, C.B.; Razquin, C.; Liang, L.; Wang, D.D.; Corella, D.; Estruch, R.; Hernáez, Á.; et al. Plasma Branched Chain/Aromatic Amino Acids, Enriched Mediterranean Diet and Risk of Type 2 Diabetes: Case-Cohort Study within the PREDIMED Trial. Diabetologia 2018, 61, 1560–1571. [Google Scholar] [CrossRef]
- White, P.J.; McGarrah, R.W.; Herman, M.A.; Bain, J.R.; Shah, S.H.; Newgard, C.B. Insulin Action, Type 2 Diabetes, and Branched-Chain Amino Acids: A Two-Way Street. Mol. Metab. 2021, 52, 101261. [Google Scholar] [CrossRef]
- Gojda, J.; Cahova, M. Gut Microbiota as the Link between Elevated Bcaa Serum Levels and Insulin Resistance. Biomolecules 2021, 11, 1414. [Google Scholar] [CrossRef]
- Liu, Z.-Z.; Liu, Q.-H.; Liu, Z.; Tang, J.-W.; Chua, E.-G.; Li, F.; Xiong, X.-S.; Wang, M.-M.; Wen, P.-B.; Shi, X.-Y.; et al. Ethanol Extract of Mulberry Leaves Partially Restores the Composition of Intestinal Microbiota and Strengthens Liver Glycogen Fragility in Type 2 Diabetic Rats. BMC Complement. Med. Ther. 2021, 21, 172. [Google Scholar] [CrossRef] [PubMed]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gan, L.; Inamura, Y.; Shimizu, Y.; Yokoi, Y.; Ohnishi, Y.; Song, Z.; Kumaki, Y.; Kikukawa, T.; Demura, M.; Ito, M.; et al. A Basic Study of the Effects of Mulberry Leaf Administration to Healthy C57BL/6 Mice on Gut Microbiota and Metabolites. Metabolites 2023, 13, 1003. https://doi.org/10.3390/metabo13091003
Gan L, Inamura Y, Shimizu Y, Yokoi Y, Ohnishi Y, Song Z, Kumaki Y, Kikukawa T, Demura M, Ito M, et al. A Basic Study of the Effects of Mulberry Leaf Administration to Healthy C57BL/6 Mice on Gut Microbiota and Metabolites. Metabolites. 2023; 13(9):1003. https://doi.org/10.3390/metabo13091003
Chicago/Turabian StyleGan, Li, Yuga Inamura, Yu Shimizu, Yuki Yokoi, Yuki Ohnishi, Zihao Song, Yasuhiro Kumaki, Takashi Kikukawa, Makoto Demura, Masaaki Ito, and et al. 2023. "A Basic Study of the Effects of Mulberry Leaf Administration to Healthy C57BL/6 Mice on Gut Microbiota and Metabolites" Metabolites 13, no. 9: 1003. https://doi.org/10.3390/metabo13091003
APA StyleGan, L., Inamura, Y., Shimizu, Y., Yokoi, Y., Ohnishi, Y., Song, Z., Kumaki, Y., Kikukawa, T., Demura, M., Ito, M., Ayabe, T., Nakamura, K., & Aizawa, T. (2023). A Basic Study of the Effects of Mulberry Leaf Administration to Healthy C57BL/6 Mice on Gut Microbiota and Metabolites. Metabolites, 13(9), 1003. https://doi.org/10.3390/metabo13091003





