Abstract
Terminal nucleotidyltransferases (TENTs) could generate a ‘mixed tail’ or ‘U-rich tail’ consisting of different nucleotides at the 3′ end of RNA by non-templated nucleotide addition to protect or degrade cellular messenger RNA. Recently, there has been increasing evidence that the decoration of virus RNA terminus with a mixed tail or U-rich tail is a critical way to affect viral RNA stability in virus-infected cells. This paper first briefly introduces the cellular function of the TENT family and non-canonical tails, then comprehensively reviews their roles in virus invasion and antiviral immunity, as well as the significance of the TENT family in antiviral therapy. This review will contribute to understanding the role and mechanism of non-canonical RNA tailing in survival competition between the virus and host.
1. Introduction
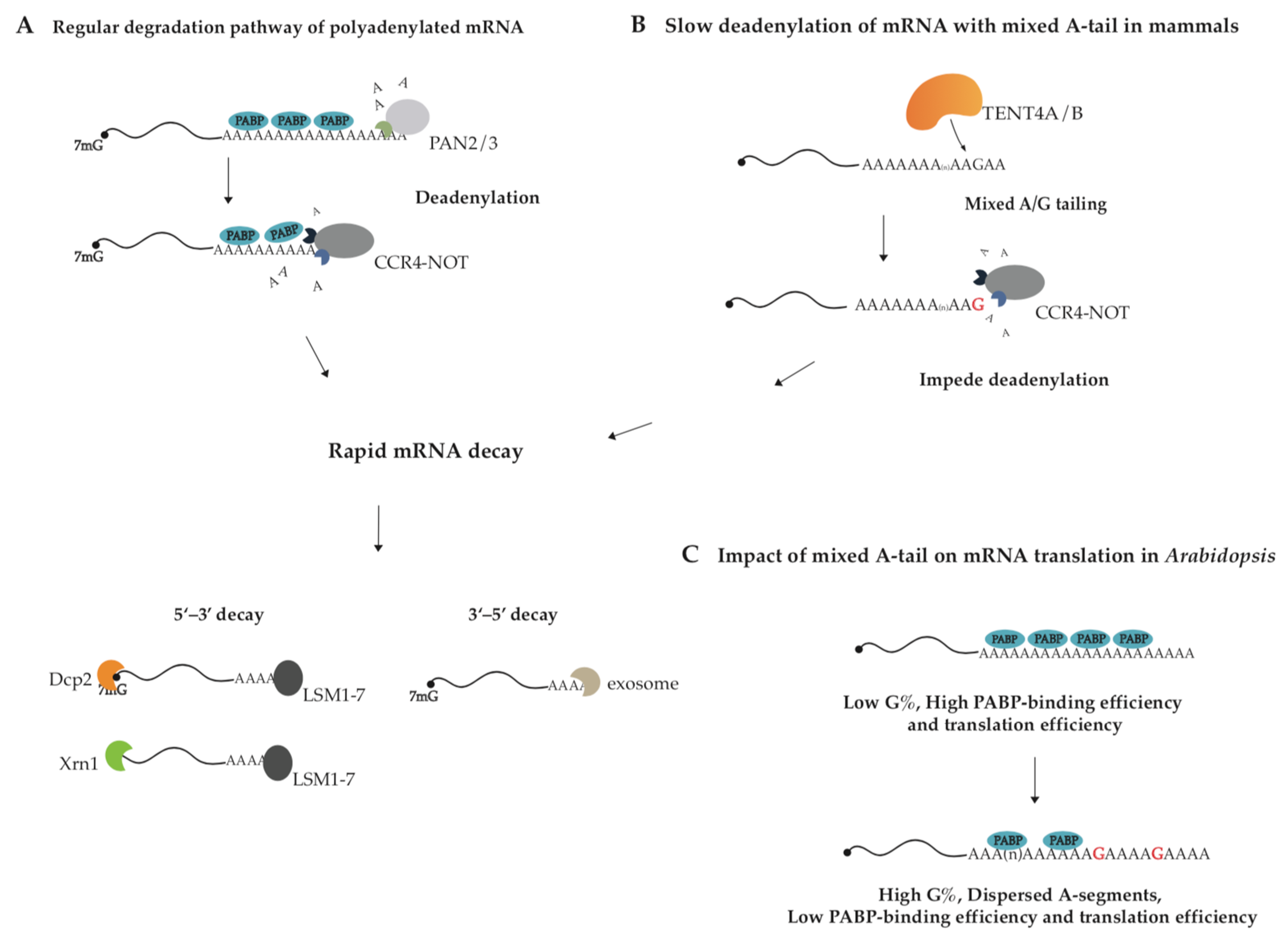
Almost all kinds of RNA in eukaryotes undergo 3′ end processing. RNA 3′ end changes dynamically in composition and length, which determine the fate of modified RNA [1,2]. The 3′ end cleavage and polyadenylation in the nucleus is essential for general mRNA maturation in eukaryotes and canonical poly(A) polymerase (PAP) adds poly(A) tail to mRNA in a transcription-termination-coupled manner [3]. The synthesized poly(A) tails are covered by Poly(A)-binding proteins (PABs/PABPs). In addition to canonical PAP, the TENT family also acts on decoration of the RNA 3′ end through non-canonical tailing, such as uridylation, mixed tailing, as well as cytoplasmic polyadenylation et al. [4,5,6], thereby exerting multiple functions. According to substrate preference for ATP or UTP, eleven TENTs in the human genome are classified into two subfamilies, non-canonical poly(A) polymerase (ncPAP) and terminal uridylyltransferase (TUTase) (Table 1) [7]. The cytoplasmic polyadenylation event by TENT2 (GLD-2) enhances the stability and translation of particular mRNAs, who possess the cytoplasmic polyadenylation element (CPE), by extending their poly(A) tails in the processes of gametogenesis, embryogenesis and long-term memory [8,9,10,11,12,13,14]. Mono-uridylation or oligo-uridylation by TUTases including TENT1 (TUT1), TENT3A (TUT4), and TENT3B (TUT7) participates in the biogenesis and turnover of A-tailed mRNAs, histone mRNAs, microRNAs, and U6 snRNA et al. [15,16,17]. Interestingly, the distinct roles of TUT4/7-mediated mono-uridylation and oligo-uridylation are well illustrated in the case of the tumor suppressor let-7 microRNA family: oligo-uridylation of pre-let-7 promotes its decay and mono-uridylation of pre-let-7 affects its maturation [18,19,20,21,22,23]. Guanosine residues in poly(A) tail of mRNA, deposited through mixed tailing by TENT4A/4B, impede the deadenylase complex CCR4-NOT and enhance the mRNA stability [24,25]. It is known that eukaryotic mRNAs are degraded through multiple pathways and the major one is mediated by 3′–5′ exonucleases (deadenylases), such as CCR4–NOT and PAN2–PAN3 (Figure 1A) [26,27,28,29]. Guanosine insertions in poly(A) tails possibly disrupt their single-stranded A-form-like helix structure and hinder deadenylase function, thus reducing the degradation rates of the transcripts [30]. The function of G-content in mixed tailing has been revealed in mammals, Arabidopsis, and certain virus-infected cells [27,31,32,33].

Table 1.
The RNA substrates and localization of human TENTs.
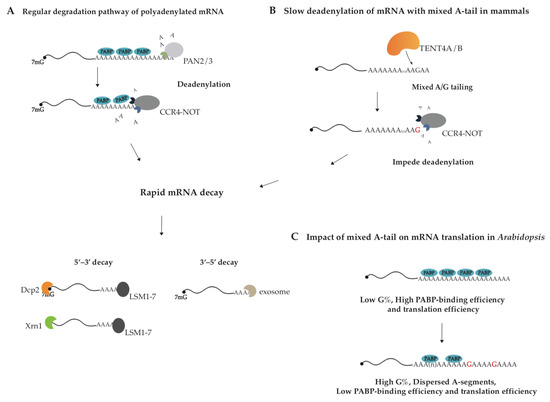
Figure 1.
The proposed model for the action mechanism of mixed tailing. (A,B) TENT4A/4B stabilizes mRNA by generating mixed A/G tailing in human cells. PAN2/3 shortens poly(A) tail to 110 nt, and CCR4-NOT removes the remaining A-residues. TENT4A/4B decorates poly(A) tail with guanosine residues. Compared with pure poly(A) tail, mixed A/G tail is more resistant to CCR4-NOT-complex-mediated deadenylation since CCR4-NOT sheds once it encounters G-residue. After deadenylation and decapping, all mRNAs are degraded from both 5′ and 3′ ends. (C) Mixed A/G tails regulate translation efficiency in Arabidopsis. Guanosines are supposed to divide the poly(A) tail into interspersed A-segments, thereby reducing binding efficiency between PABP and A-tail and translation efficiency of Arabidopsis mRNA.
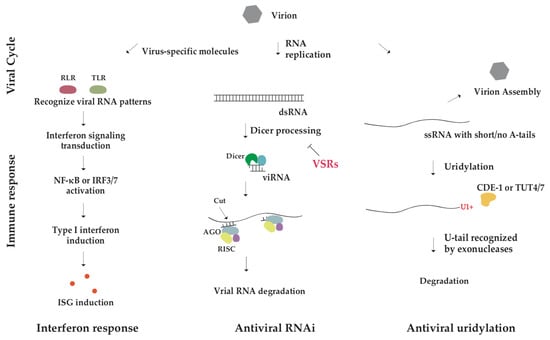
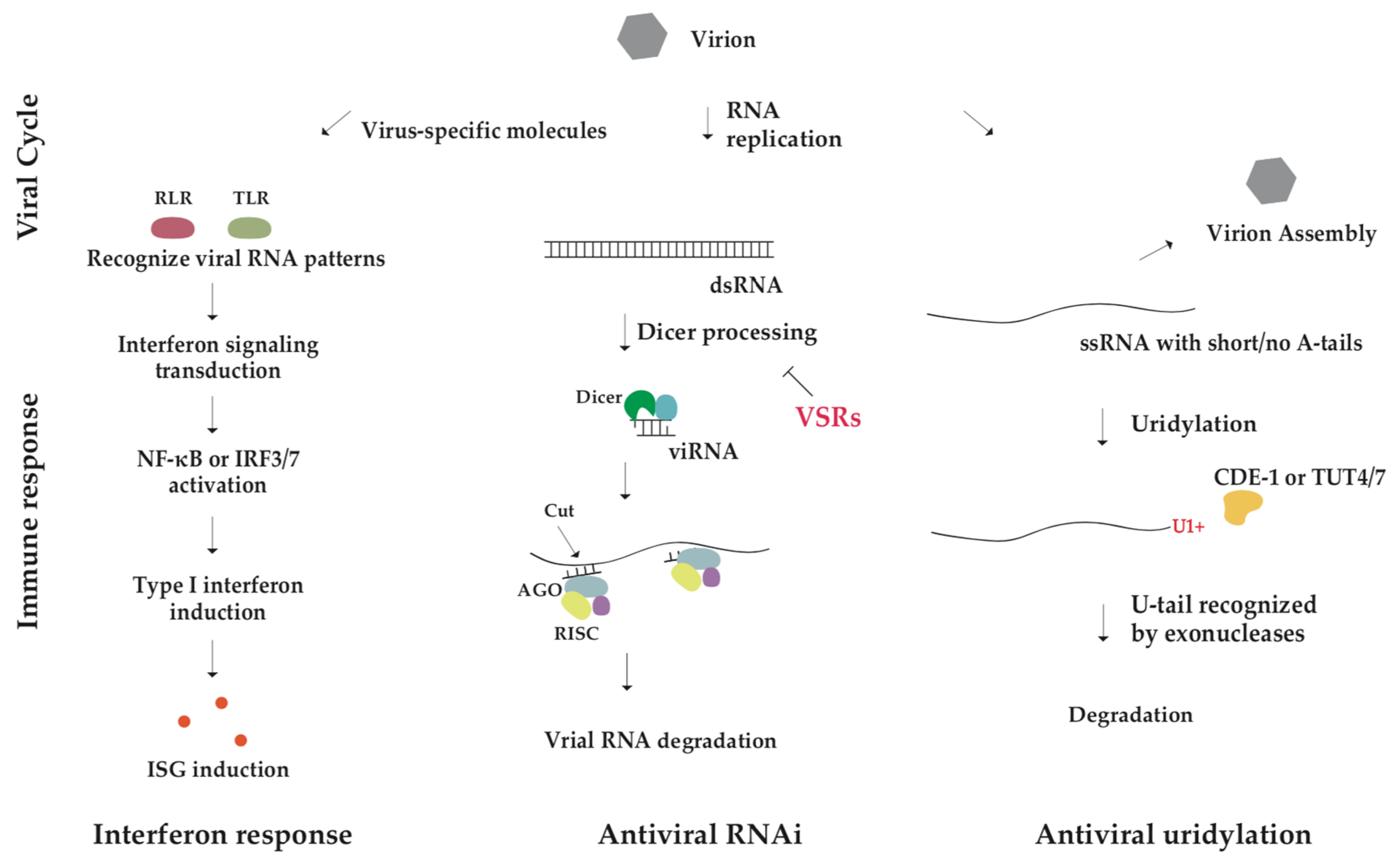
Eukaryotic cells have evolved the RNA-based antiviral immunity to escape from viral infection. One well-known mechanism is that long double-stranded RNAs (dsRNAs) derived from the virus in infected cells induce RNA interference (RNAi) to specifically remove viral RNAs [34,35]. Dicer, a member of the RNase III family, cleaves these dsRNAs into virus-derived small interfering RNAs (viRNAs), which are loaded into Argonaute (AGO) proteins to form the RNA-induced silencing complex (RISC) and thereby silencing viral RNAs [36,37,38,39]. Recent studies have identified a novel mechanism of virus–host interaction, which is conserved across animals and mediated through 3′ tailing of viral RNAs [40,41,42]. Interestingly, two types of 3′ tails, U-rich tail and mixed tail, lead to distinct consequences. The U-rich tail deposited by TUTases at the viral RNA terminus triggers viral RNA decay [40,43,44]. On the contrary, some viruses employ mixed tails to protect their RNAs from decay [45]. Increasing evidence has suggested that the length and composition of the viral RNA tail are a hotspot for evolutionary battle between viruses and their hosts.
Viral RNA 3′ tailing has emerged as an important target pathway for antiviral therapy and also the controlled mRNA 3′ tailing has great potential for improving RNA vaccine efficiency. Therefore, the comprehensive understanding is needed regarding the significance and action mechanism of RNA 3′ tailing in various organisms and virus infection. Here, we review biogenesis and function of mixed tail and U-rich tail particularly in the process of virus infection, and describe the impact of RNA 3′ tailing on virus–host survival competition and relevant antiviral therapy.
2. Mixed Tail in Viral Infection
To date, guanosine residues in poly(A) tails have been found in human cells, Arabidopsis, viral infection, and embryos from several organisms including mouse, frog, Drosophila, and zebrafish [24,32,45]. Even though the research on RNA mixed tailing is still at an early stage, the enzymes responsible for the process have been illustrated in mammals, and the function and action mechanism have been revealed in mammals, Arabidopsis, and viral infection. The experimental data have suggested that host TENT4 is employed by hepatitis A virus (HAV), hepatitis B virus (HBV), and human cytomegalovirus (HCMV) to control viral RNA stability, and inhibitors targeting TENT4 have been rapidly developing as antiviral medicine.
2.1. The Cellular Function of Mixed Tail
TENT4A and TENT4B are predominantly responsible for generating mixed tail in mammals, decorating poly(A) tail with non-adenosine nucleotides, of which guanosine is the most common [24,42]. Because TENT4A/4B is mostly found in the nucleus, but also in the cytoplasm (Table 1) [46,47], so mixed tailing more likely occurs in the nucleus. It has shown that TENT4A/4B holds relaxed nucleotide selectivity during poly(A) tail synthesis and is more selective for GTP than UTP and CTP, and thus incorporates non-adenosine residues into poly(A) tail. Guanosine residues were mainly found at the positions close to 3′ ends of long A-tails (≥25 nt). Since a single guanosine residue is sufficient to impede the CCR4-NOT complex, the complex trims the tail until exposing the guanosine at the 3′ end (Figure 1B) [24,42]. As the result, mixed tailing, also known as G-content tailing, enhances mRNA stability by slowing down mRNA degradation. It needs further examination whether mixed tailing of mRNA has conserved biogenesis and function across vertebrates.
Researchers have found that G-content in Arabidopsis poly(A) tails regulates translation efficiency but not mRNA stability of plant genes. Poly(A)-seq analysis of Arabidopsis has revealed that over 10% of poly(A) tails carry G-content, taking up 0.8–28% of each tail. Surprisingly, the data support that G-content in A-tail is negatively correlated with the binding efficiency of PABP on A-tail and further the gene with higher G-content has lower translation efficiency [31]. PABP is believed to identify the pure poly(A) primarily, and the binding of human PABP or yeast Pab1p to mRNA poly(A) tail requires 11–12 contiguous A-nucleotides [48,49,50]. Guanosine in poly(A) tail separates the tail into interspersed A-segments, supposed to reduce binding efficiency between PABP and A-tail and also translation efficiency of Arabidopsis genes (Figure 1C) [31]. The mixed tailing in mammals is generated by TENT4A/4B, whereas the producing mechanism in Arabidopsis is unclear.
The different ways of mixed tailing in regulation of mammalian and plant genes indicate the possibility of its diverse action mechanism in varying organisms. Although its overall function has been illustrated in mammal and plant, many important questions are still remained. For example, it is unknown how the preferred nucleotide incorporation undergoes during mixed tailing and how particular genes have A-tails with high G-percentage. Interestingly, the clue to gene-specific increase in G-content in A-tail has come from studies of viral infection.
2.2. The Pathological Function of Mixed Tail in Virus Infection
To survive from virus infection, hosts have evolved diverse antiviral immunity that does not solely rely on RNAi or an interferon pathway. Notably, RNA 3′ uridylation is an effective pathway for preventing viral invasion [51]. On the other hand, RNA 3′ mixed tailing is hijacked by viruses to stabilize their RNA and facilitate viral invasion [52]. Recent research indicated that RNA stability in HAV, HBV, and HCMV is closely related to TENT4A/4B. In addition, similar strategies are also used in several other viruses, including Norovirus, Saffold virus, and Kobuviruse, which indicates the active participation of TENT4A/4B in viral life cycle is a more general event [45,53,54,55].
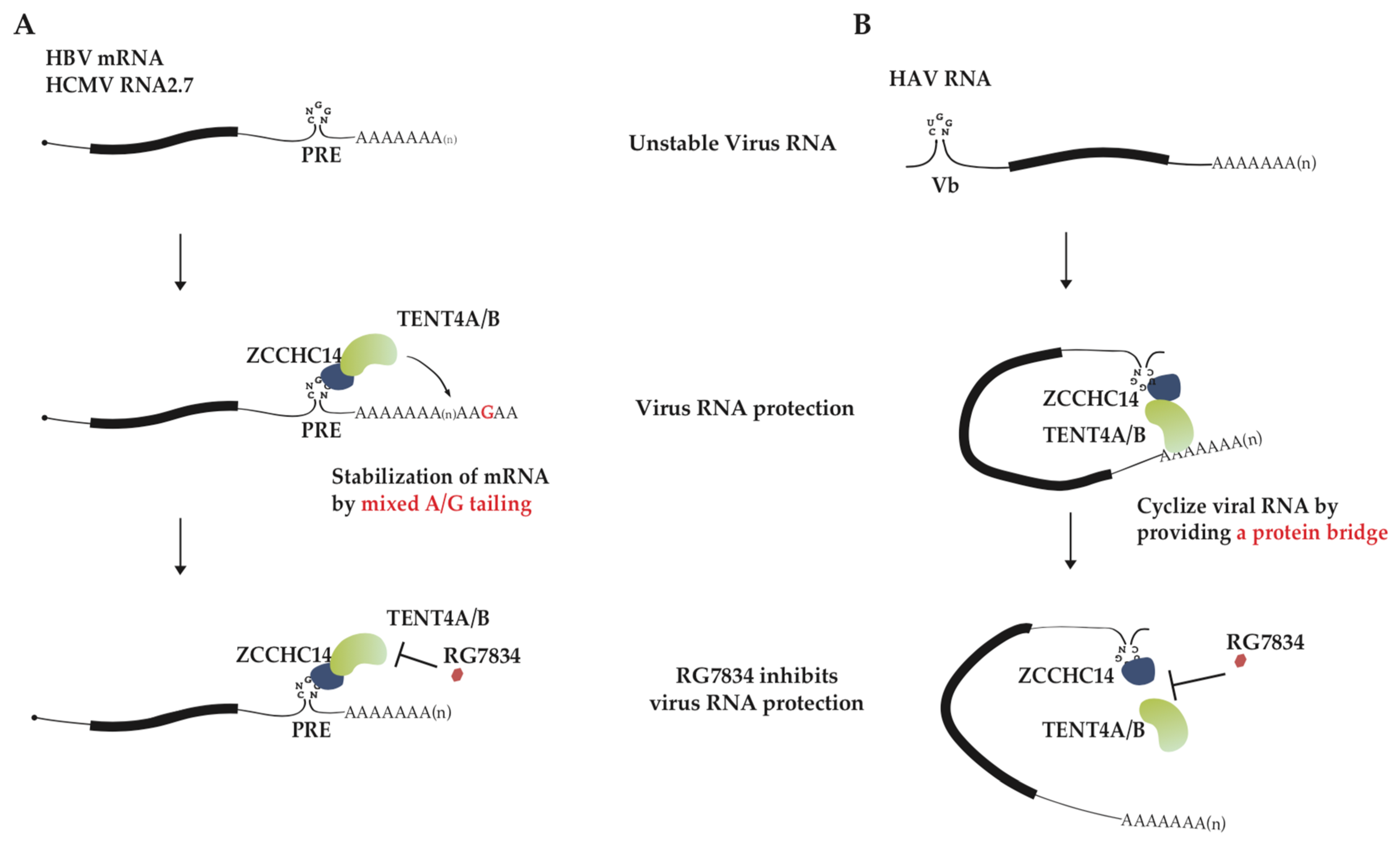
HBV and HCMV are double-stranded (ds) DNA viruses with the relaxed circular dsDNA and linear dsDNA as genomes, respectively. It had been assumed that mRNA maturation in the dsDNA viruses like HBV and HCMV might follow the processes similar to host, including 5′ capping, splicing and 3′ polyadenylation. Out of expectation, HCMV and HBV cleverly hijack host TENT4A/4B to produce mixed tail at the 3′ end of viral mRNA to facilitate their infection [45]. Remarkably, the process is well controlled and specific to viral transcripts with 3′UTR post-transcriptional regulatory element (PRE), and host RNA-binding protein (RBP) ZCCHC14 and enzyme TENT4A/4B coordinate to complete the process. The Smaug-like SAM domain in the zinc finger protein ZCCHC14 recognizes the CNGGN pentaloop in PRE. At the same time, ZCCHC14 attracts TENT4 and tethers it on viral mRNA to generate mixed tails. The non-templated addition of guanosines onto 3′ ends of HBV and HCMV mRNA enhances RNA stability by inhibiting degradation (Figure 2A). The pentaloop is present in almost all HBV mRNA species and HCMV RNA2.7, and their half-lives are significantly diminished when the enzyme TENT4A/4B is depleted [45]. The single or double knockout experiments of TENT4A/4B in HepG2 cells showed that two genes have redundant function, with TENT4B tailing HBV transcripts well when TENT4A is knocked out and TENT4A less capable of tailing when TENT4B is knocked out. In other words, TENT4B is primarily charged with stabilizing HBV mRNA at least in HepG2 cells [53]. Although this phenomenon might result from different expression levels of TENT4A and 4B in HepG2 cells, there is also another possibility. As ZCCHC14 was mainly observed in the cytoplasm, the different localization of TENT4A and 4B protein in cells led to the above phenomenon.
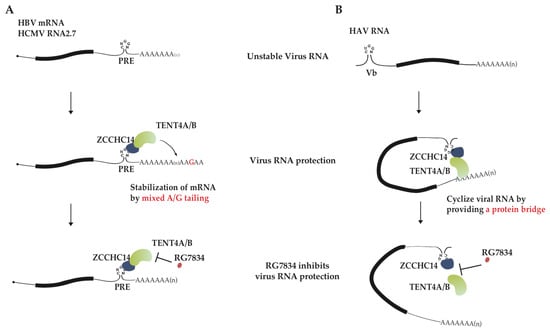
Figure 2.
The proposed models for virus RNA stabilization by ZCCHC14-TENT4A/4B complex. (A) A model for inhibition of HCMV RNA2.7 and HBV mRNA stability by RG7834. TENT4A/4B is recruited by ZCCHC14 to the stem-loop of PRE in viral mRNA 3′ UTR, and thereby generating 3′ mixed tails at viral mRNA and disturbing CCR4-NOT-mediated RNA decay. RG7834 inhibits terminal nucleotidyltransferase activity of TENT4, destabilizing HBV and HCMV transcripts. (B) A model for RG7834 inhibition of HAV RNA synthesis. ZCCHC14 binds to stem-loop Vb in the HAV 5′ UTR and TENT4 might recognize the 3′ end of polyadenylated HAV genome RNA. ZCCHC14 and TENT4 interaction serves as a bridge that facilitates functional cyclization of the genome toward synthesizing its complementary RNA. RG7834 disrupts interaction between TENT4A/4B with ZCCHC14, interrupting genome cyclization and impeding genome RNA replication.
HAV is a single-stranded (ss) RNA virus with a small positive-sense RNA as a genome. Viral genome RNA 3′ end is polyadenylated, but instead of 5′ capping, the 5′ end is covalently linked to a small viral protein VPg, the putative protein primer for minus-strand RNA synthesis. Although HAV replication cycle still remains unclear, its genome replication is expected to use RNA as template to directly synthesize complementary RNA [56]. HAV infection was also reported to require ZCCHC14 and TENT4A/4B [33,57]. However, unlike the case of HBV/HCMV, TENT4A/4B and ZCCHC14 mainly affect HAV RNA synthesis. Viral RNA poly(A) tail length, stability, and translation are unaffected but nascent viral RNA synthesis is significantly diminished by treatment of TENT4 inhibitor RG7834 [32]. ZCCHC14 could recognize a stem-loop with a CUGGN-type pentaloop in the 5′ UTR of viral genome RNA and recruit TENT4. A proposed model according to these data is that ZCCHC14-TENT4 forms a protein bridge between the 5′ UTR and 3′ end of viral genome RNA so circularizes HAV RNA and enhances viral RNA replication (Figure 2B) [32]. It remains to be determined whether HAV RNA replication is dependent on the terminal nucleotidyltransferase activity of TENT4.
In order to identify viral cis-acting elements playing roles in its RNA stability, translation and localization, high-throughput screening was conducted based on luciferase reporter system [55]. The 130 bp synthesized DNA segments of viral 3′ UTR origin were inserted into 3′ UTR of luciferase reporter for the screening and hundreds of elements were found from the experiment. Among them, Norovirus K3, Saffold virus K4, and Kobuviruse K5 elements were sensitive to TENT4 inhibitor RG7834 so all of them should be under the control of TENT4. Further comprehensive studies found that C-terminus and N-terminus of the ZCCHC2 protein interact, respectively, with K5 RNA element in 3′ UTR and TENT4 protein, thereby producing mixed tails, increasing mRNA stability and translation. Moreover, the function of K3 element is dependent on ZCCHC14 protein while K4 is insensitive to either ZCCHC14 or ZCCHC2, thus, the trans-acting factor for K4 needs further investigation [55].
The finding of TENT4A/4B as target proteins of antiviral small chemical RG7834 has greatly advanced the knowledge about the mechanism of viral gene expression [54]. Intriguingly, viruses from varying families with different genome types, sequences, and life cycles have evolved in a similar way, hiring host RBP-TENT4 complexes, to specifically stabilize viral RNA, which establishes a fantastic target pathway for developing generally workable antiviral medicine.
2.3. TENT4-ZCCHC14 and Anti-Hepatitis Virus Therapy
HBV infection is one of the most significant public health issues, with ~350 million chronic HBV patients worldwide [58,59,60]. Approximately 240 million patients with Chronic Hepatitis B (CHB) are Hepatitis B surface antigen (HbsAg) positive, exposed to the risk of cirrhosis and hepatocellular carcinoma (HCC) [61,62]. Unlike HBV, HAV infection usually causes acute hepatitis, ranging in severity from mild to severe [63,64]. In rare cases, a weakened immune system can make hepatitis A infection deadly.
Nucleotide analogues and immune modulators are widely-used antiviral agents that are effective in preventing the spread of infectious viruses, but these medicines have several disadvantages such as strong side effects and the development of drug resistance [65,66,67]. Currently, HBsAg has a major role in host immune escape and HBsAg together with HBV DNA levels are the hallmarks of chronic HBV infection, and HBsAg is the foundation for diagnosing infections, screening blood, and determining the cure for antiviral therapy [68,69,70]. So, HBsAg inhibitors have been extensively screened to overcome chronic HBV infection and they can be structurally divided into DHQ (dihydroquinazinone) and THP (tetrahydropyridine) classes [71,72].
RG7834, a small chemical in DHQ class, was developed by Roche as a HBsAg inhibitor, and could target HBV and reduce viral gene expression [73,74]. RG7834 eliminates viral antigens and DNA, having a distinct antiviral profile compared with nucleotide analogues. By reducing the viral components required to complete the virus life cycle as well as those involved in escaping the host immune system, RG7834 was believed to have the potential to inhibit HBV and improve HBV cure rates [75,76,77,78]. Indeed, oral treatment of HBV-infected humanized mice with RG7834 resulted in a 1.09 log reduction in HBsAg levels [73,74]. Meanwhile, oral RG7834 ingestion reduced HAV replication and profoundly interrupted the pathogenesis of animal models infected with HAV [32]. RG7834 was also evaluated for its safety in the first clinical trial with 49 participants, and no adverse reactions were reported (ClinicalTrials.gov NCT02604355). Unfortunately, subsequent clinical drug development failed due to its adverse neurotoxicity. Nevertheless, because recent studies have revealed TENT4A/4B as direct targets of RG7834, TENT4-ZCCHC module is currently emerging as a new therapeutic target for clinical drug development and presents a novel perspective on hepatitis virus therapy and chemoprevention.
Roche has also released a series of THP class HBsAg inhibitors, among which, the representative compound 3 inhibits HBsAg and HBV DNA synthesis in HepG2.2.15 cells [73]. Li Zhang et al. synthesized THP HBsAg Inhibitor 17i, which exhibited the excellent in vitro anti-HBV potency with low toxicity, and dramatically reduced serum HBsAg and HBV DNA levels in HBV transgenic mice [79]. In conclusion, the discovery of new antiviral chemicals and therapeutic target pathways could coordinately accelerate drug development to achieve a functional cure for patients with hepatitis.
4. Conclusions and Discussion
In eukaryotes, RNA tailing is often associated with RNA trimming or decay. In the last 30 years, there have been remarkable breakthroughs in understanding RNA uridylation, a conserved post-transcriptional gene regulation mechanism with a wide range of RNA substrates in living organisms, as an essential tool for intracellular RNA monitoring. New attention has been paid to mixed A/G tailing to date. Recent studies have been focused more on the substrate selectivity and biochemical function of TENT4. TENT4A and TENT4B are two human homologues of the yeast Trf4p protein. In yeast, the Trf4p–Air2p–Mtr4p polyadenylation (TRAMP) complex promotes nuclear surveillance of aberrant mRNAs, rRNAs, snRNAs, snoRNAs and tRNAs [99,100,101,102]. A TRAMP-like complex consisting of TENT4B, ZCCHC7 (Air1/2 homologue) and RNA helicase MTR4 is present in mammalian cells [46,103,104]. The phenomenon of TENT4A/4B producing mixed A/G tailing has been illustrated more recently and is very dissimilar to the function of TENT4A/4B to eliminate abnormal RNAs previously. In mammals, TENT4 produces mixed tailing, which disturbs CCR4-NOT complex and protects mRNA from degradation. Mixed A/G tailing has also been found in Arabidopsis. Further research needs to answer whether mixed A/G tailing is conserved in varying organisms, whether its substrates are ubiquitous or specific, as well as the underlying mechanism of its substrate selection.
The study on RNA non-A tailing illustrates the novel mechanism of virus–host interaction. Modification in viral RNA bypasses or stimulates the host machinery for RNA degradation and thus influencing infection success. The widespread presence of 3′ uridylation in eukaryotic RNA viruses suggests the uridylation-directed RNA decay pathway as a universal defense system against viruses. Perhaps in response to this threat, some viruses have evolved to modify the 3′ ends of their RNAs, which protects them against host degradation, like the mRNAs of HBV and HCMV with mixed tails and single-stranded RNAs of Flaviviridae with highly structured 3′ ends [105,106,107]. Tail modifications may also be used to regulate the activity of other transposons. RNAi targets and transposon RNAs are modified by a C. elegans poly(UG) polymerase MUT-2 by adding p(UG) tails. With over 16 nt perfectly alternating U and G nucleotides, RNA fragments attached to the p(UG) tail can act as gene-silencing agents that suppress gene expression [108,109]. In addition to tailing, another RNA modification N6-methyladenosine (m6A) has been recently discovered to have a role in the life cycles of many viruses as well as in cellular response to viral infection [110,111,112]. Parasite–host interactions are also affected by polyadenylation [98]. The fact is fascinating that viruses with varying life cycles and tissue specificities have evolved similar strategies to interfere with host defense. According to this convergent evolutionary nature, multiple viruses may possess similar tail modification mechanisms, perhaps having learned to avoid host RNA degradation pathways or partially inactivating host degradation pathways.
The involvement of TENT and its cofactor in viral life cycles may provide mechanistic insights into the development of a new category of antiviral drugs. In spite of requiring further investigation, the role of RNA tailing as an essential regulatory instrument will provide an unexpected opportunity for antiviral treatment and chemoprevention.
Author Contributions
Conceptualization, X.W. and H.J.; writing—original draft preparation, X.W. and H.J.; writing—review and editing, H.J., X.W. and A.I.; visualization, X.W.; supervision, H.J.; funding acquisition, H.J. All authors have read and agreed to the published version of the manuscript.
Funding
The work was supported by the General Program of National Natural Science Foundation of China (31970622).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Stewart, M. Polyadenylation and nuclear export of mRNAs. J. Biol. Chem. 2019, 294, 2977–2987. [Google Scholar] [CrossRef]
- Weill, L.; Belloc, E.; Bava, F.A.; Méndez, R. Translational control by changes in poly(A) tail length: Recycling mRNAs. Nat. Struct. Mol. Biol. 2012, 19, 577–585. [Google Scholar] [CrossRef] [PubMed]
- Nicholson, A.L.; Pasquinelli, A.E. Tales of Detailed Poly(A) Tails. Trends Cell Biol. 2019, 29, 191–200. [Google Scholar] [CrossRef] [PubMed]
- Slomovic, S.; Portnoy, V.; Yehudai-Resheff, S.; Bronshtein, E.; Schuster, G. Polynucleotide phosphorylase and the archaeal exosome as poly(A)-polymerases. Biochim. Biophys. Acta 2008, 1779, 247–255. [Google Scholar] [CrossRef] [PubMed]
- Edmonds, M. A history of poly A sequences: From formation to factors to function. Prog. Nucleic Acid. Res. Mol. Biol. 2002, 71, 285–389. [Google Scholar]
- Norbury, C.J. Cytoplasmic RNA: A case of the tail wagging the dog. Nat. Rev. Mol. Cell Biol. 2013, 14, 643–653. [Google Scholar] [CrossRef]
- Yu, S.; Kim, V.N. A tale of non-canonical tails: Gene regulation by post-transcriptional RNA tailing. Nat. Rev. Mol. Cell Biol. 2020, 21, 542–556. [Google Scholar] [CrossRef]
- Barnard, D.C.; Ryan, K.; Manley, J.L.; Richter, J.D. Symplekin and xGLD-2 are required for CPEB-mediated cytoplasmic polyadenylation. Cell 2004, 119, 641–651. [Google Scholar] [CrossRef]
- Suh, N.; Jedamzik, B.; Eckmann, C.R.; Wickens, M.; Kimble, J. The GLD-2 poly(A) polymerase activates gld-1 mRNA in the Caenorhabditis elegans germ line. Proc. Natl. Acad. Sci. USA 2006, 103, 15108–151123. [Google Scholar] [CrossRef]
- Benoit, P.; Papin, C.; Kwak, J.E.; Wickens, M.; Simonelig, M. PAP- and GLD-2-type poly(A) polymerases are required sequentially in cytoplasmic polyadenylation and oogenesis in Drosophila. Development 2008, 135, 1969–1979. [Google Scholar] [CrossRef]
- Kwak, J.E.; Drier, E.; Barbee, S.A.; Ramaswami, M.; Yin, J.C.; Wickens, M. GLD2 poly(A) polymerase is required for long-term memory. Proc. Natl. Acad. Sci. USA 2008, 105, 14644–14649. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.W.; Wilson, T.L.; Kimble, J. GLD-2/RNP-8 cytoplasmic poly(A) polymerase is a broad-spectrum regulator of the oogenesis program. Proc. Natl. Acad. Sci. USA 2010, 107, 17445–17450. [Google Scholar] [CrossRef] [PubMed]
- Sartain, C.V.; Cui, J.; Meisel, R.P.; Wolfner, M.F. The poly(A) polymerase GLD2 is required for spermatogenesis in Drosophila melanogaster. Development 2011, 138, 1619–1629. [Google Scholar] [CrossRef]
- Cui, J.; Sartain, C.V.; Pleiss, J.A.; Wolfner, M.F. Cytoplasmic polyadenylation is a major mRNA regulator during oogenesis and egg activation in Drosophila. Dev. Biol. 2013, 383, 121–131. [Google Scholar] [CrossRef]
- Menezes, M.R.; Balzeau, J.; Hagan, J.P. 3′ RNA Uridylation in Epitranscriptomics, Gene Regulation, and Disease. Front. Mol. Biosci. 2018, 5, 61. [Google Scholar] [CrossRef] [PubMed]
- Rissland, O.S.; Mikulasova, A.; Norbury, C.J. Efficient RNA polyuridylation by noncanonical poly(A) polymerases. Mol. Cell. Biol. 2007, 27, 3612–3624. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Yang, Y.; Piao, W.; Jin, H. Uridylation: A vital way for cellular RNA surveillance. Hereditas 2022, 44, 449–465. [Google Scholar] [PubMed]
- Ustianenko, D.; Chiu, H.S.; Treiber, T.; Weyn-Vanhentenryck, S.M.; Treiber, N.; Meister, G.; Sumazin, P.; Zhang, C. LIN28 Selectively Modulates a Subclass of Let-7 MicroRNAs. Mol. Cell 2018, 71, 271–283.e5. [Google Scholar] [CrossRef]
- Liu, X.; Zheng, Q.; Vrettos, N.; Maragkakis, M.; Alexiou, P.; Gregory, B.D.; Mourelatos, Z. A MicroRNA precursor surveillance system in quality control of MicroRNA synthesis. Mol. Cell 2014, 55, 868–879. [Google Scholar] [CrossRef]
- Heo, I.; Ha, M.; Lim, J.; Yoon, M.J.; Park, J.E.; Kwon, S.C.; Chang, H.; Kim, V.N. Mono-uridylation of pre-microRNA as a key step in the biogenesis of group II let-7 microRNAs. Cell 2012, 151, 521–532. [Google Scholar] [CrossRef]
- Liu, M.; Sun, Z.; Tang, Y.; Zhang, S.; Luo, J. The Regulation of Exosome-Mediated miR-132-3p/miR-132-3p-UUU on Radiation-Induced Esophageal Injury. Radiat. Res. 2023, 23, 231–241. [Google Scholar] [CrossRef]
- Liu, L.; Wang, X.; Zhao, W.; Li, Q.; Li, J.; Chen, H.; Shan, G. Systematic characterization of small RNAs associated with C. elegans Argonautes. Sci. China Life Sci. 2023, 66, 1303–1322. [Google Scholar] [CrossRef] [PubMed]
- Lipińska-Zubrycka, L.; Grochowski, M.; Bähler, J.; Małecki, M. Pervasive mRNA uridylation in fission yeast is catalysed by both Cid1 and Cid16 terminal uridyltransferases. PLoS ONE 2023, 18, e0285576. [Google Scholar] [CrossRef] [PubMed]
- Lim, J.; Kim, D.; Lee, Y.S.; Ha, M.; Lee, M.; Yeo, J.; Chang, H.; Song, J.; Ahn, K.; Kim, V.N. Mixed tailing by TENT4A and TENT4B shields mRNA from rapid deadenylation. Science 2018, 361, 701–704. [Google Scholar] [CrossRef]
- Hyrina, A.; Jones, C.; Chen, D.; Clarkson, S.; Cochran, N.; Feucht, P.; Hoffman, G.; Lindeman, A.; Russ, C.; Sigoillot, F.; et al. A Genome-wide CRISPR Screen Identifies ZCCHC14 as a Host Factor Required for Hepatitis B Surface Antigen Production. Cell Rep. 2019, 29, 2970–2978. [Google Scholar] [CrossRef] [PubMed]
- Narayanan, K.; Makino, S. Interplay between viruses and host mRNA degradation. Biochim. Biophys. Acta 2013, 1829, 732–741. [Google Scholar] [CrossRef] [PubMed]
- Yi, H.; Park, J.; Ha, M.; Lim, J.; Chang, H.; Kim, V.N. PABP Cooperates with the CCR4-NOT Complex to Promote mRNA Deadenylation and Block Precocious Decay. Mol. Cell 2018, 70, 1081–1088. [Google Scholar] [CrossRef]
- Webster, M.W.; Chen, Y.H.; Stowell, J.A.W.; Alhusaini, N.; Sweet, T.; Graveley, B.R.; Coller, J.; Passmore, L.A. mRNA Deadenylation Is Coupled to Translation Rates by the Differential Activities of Ccr4-Not Nucleases. Mol. Cell 2018, 70, 1089–1100. [Google Scholar] [CrossRef]
- Chen, C.Y.; Shyu, A.B. Mechanisms of deadenylation-dependent decay. Wiley Interdiscip. Rev. RNA 2011, 2, 167–183. [Google Scholar] [CrossRef]
- Passmore, L.A.; Coller, J. Roles of mRNA poly(A) tails in regulation of eukaryotic gene expression. Nat. Rev. Mol. Cell Biol. 2022, 23, 93–106. [Google Scholar] [CrossRef]
- Zhao, T.; Huan, Q.; Sun, J.; Liu, C.; Hou, X.; Yu, X.; Silverman, I.M.; Zhang, Y.; Gregory, B.D.; Liu, C.M.; et al. Impact of poly(A)-tail G-content on Arabidopsis PAB binding and their role in enhancing translational efficiency. Genome Biol. 2019, 20, 189–197. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Misumi, I.; Shiota, T.; Sun, L.; Lenarcic, E.M.; Kim, H.; Shirasaki, T.; Hertel-Wulff, A.; Tibbs, T.; Mitchell, J.E.; et al. The ZCCHC14/TENT4 complex is required for hepatitis A virus RNA synthesis. Proc. Natl. Acad. Sci. USA 2022, 119, 22045–22061. [Google Scholar] [CrossRef] [PubMed]
- Kulsuptrakul, J.; Wang, R.; Meyers, N.L.; Ott, M.; Puschnik, A.S. A genome-wide CRISPR screen identifies UFMylation and TRAMP-like complexes as host factors required for hepatitis A virus infection. Cell Rep. 2021, 34, 108859–108867. [Google Scholar] [CrossRef] [PubMed]
- Ashe, A.; Sarkies, P.; Le Pen, J.; Tanguy, M.; Miska, E.A. Antiviral RNA Interference against Orsay Virus Is neither Systemic nor Transgenerational in Caenorhabditis elegans. J. Virol. 2015, 89, 12035–12046. [Google Scholar] [CrossRef]
- Ding, S.-W. RNA-based antiviral immunity. Nat. Rev. Immunol. 2010, 10, 632–644. [Google Scholar] [CrossRef]
- Iwakawa, H.O.; Tomari, Y. Life of RISC: Formation, action, and degradation of RNA-induced silencing complex. Mol. Cell 2022, 82, 30–43. [Google Scholar] [CrossRef]
- Wilson, R.C.; Doudna, J.A. Molecular mechanisms of RNA interference. Annu. Rev. Biophys. 2013, 42, 217–239. [Google Scholar] [CrossRef]
- MacKay, C.R.; Wang, J.P.; Kurt-Jones, E.A. Dicer’s role as an antiviral: Still an enigma. Curr. Opin. Immunol. 2014, 26, 49–55. [Google Scholar] [CrossRef]
- Koralewska, N.; Ciechanowska, K.; Pokornowska, M.; Figlerowicz, M.; Kurzyńska-Kokorniak, A. Human ribonuclease Dicer—Structure and functions. Postep. Biochem. 2019, 65, 173–182. [Google Scholar] [CrossRef][Green Version]
- Le Pen, J.; Jiang, H.; Di Domenico, T.; Kneuss, E.; Kosałka, J.; Leung, C.; Morgan, M.; Much, C.; Rudolph, K.L.M.; Enright, A.J.; et al. Terminal uridylyltransferases target RNA viruses as part of the innate immune system. Nat. Struct. Mol. Biol. 2018, 25, 778–786. [Google Scholar] [CrossRef]
- Huo, Y.; Shen, J.; Wu, H.; Zhang, C.; Guo, L.; Yang, J.; Li, W. Widespread 3′-end uridylation in eukaryotic RNA viruses. Sci. Rep. 2016, 6, 25454–25461. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.; Lim, J.; Ha, M.; Kim, V.N. TAIL-seq: Genome-wide determination of poly(A) tail length and 3′ end modifications. Mol. Cell 2014, 53, 1044–1052. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.C.; Shen, Y.R.; Chang, C.C.; Guo, X.Y.; Young, Y.Y.; Lai, T.Y.; Yu, I.S.; Lee, C.Y.; Chuang, T.H.; Tsai, H.Y.; et al. Terminal uridyltransferase 7 regulates TLR4-triggered inflammation by controlling Regnase-1 mRNA uridylation and degradation. Nat. Commun. 2021, 12, 3878–3885. [Google Scholar] [CrossRef]
- Gupta, A.; Li, Y.; Chen, S.H.; Papas, B.N.; Martin, N.P.; Morgan, M. TUT4/7-mediated uridylation of a coronavirus subgenomic RNAs delays viral replication. Commun. Biol. 2023, 6, 438–444. [Google Scholar] [CrossRef]
- Kim, D.; Lee, Y.S.; Jung, S.J.; Yeo, J.; Seo, J.J.; Lee, Y.Y.; Lim, J.; Chang, H.; Song, J.; Yang, J.; et al. Viral hijacking of the TENT4-ZCCHC14 complex protects viral RNAs via mixed tailing. Nat. Struct. Mol. Biol. 2020, 27, 581–588. [Google Scholar] [CrossRef] [PubMed]
- Sudo, H.; Nozaki, A.; Uno, H.; Ishida, Y.; Nagahama, M. Interaction properties of human TRAMP-like proteins and their role in pre-rRNA 5′ETS turnover. FEBS Lett. 2016, 590, 2963–2972. [Google Scholar] [CrossRef] [PubMed]
- Fasken, M.B.; Leung, S.W.; Banerjee, A.; Kodani, M.O.; Chavez, R.; Bowman, E.A.; Purohit, M.K.; Rubinson, M.E.; Rubinson, E.H.; Corbett, A.H. Air1 zinc knuckles 4 and 5 and a conserved IWRXY motif are critical for the function and integrity of the Trf4/5-Air1/2-Mtr4 polyadenylation (TRAMP) RNA quality control complex. J. Biol. Chem. 2011, 286, 37429–37445. [Google Scholar] [CrossRef]
- Tang, T.T.L.; Passmore, L.A. Recognition of Poly(A) RNA through Its Intrinsic Helical Structure. Cold Spring Harb. Symp. Quant. Biol. 2019, 84, 21–30. [Google Scholar] [CrossRef] [PubMed]
- Huch, S.; Nissan, T. Interrelations between translation and general mRNA degradation in yeast. Wiley Interdiscip. Rev. RNA 2014, 5, 747–763. [Google Scholar] [CrossRef]
- Kajjo, S.; Sharma, S.; Chen, S.; Brothers, W.R.; Cott, M.; Hasaj, B.; Jovanovic, P.; Larsson, O.; Fabian, M.R. PABP prevents the untimely decay of select mRNA populations in human cells. EMBO J. 2022, 41, e108650–e108661. [Google Scholar] [CrossRef]
- Yeo, J.; Kim, V.N. U-tail as a guardian against invading RNAs. Nat. Struct. Mol. Biol. 2018, 25, 903–905. [Google Scholar] [CrossRef] [PubMed]
- Block, T.M.; Young, J.A.T.; Javanbakht, H.; Sofia, M.J.; Zhou, T. Host RNA quality control as a hepatitis B antiviral target. Antiviral Res. 2021, 186, 104972–104980. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Lee, A.C.H.; Guo, F.; Kondratowicz, A.S.; Micolochick Steuer, H.M.; Miller, A.; Bailey, L.D.; Wang, X.; Chen, S.; Kultgen, S.G.; et al. Host Poly(A) Polymerases PAPD5 and PAPD7 Provide Two Layers of Protection That Ensure the Integrity and Stability of Hepatitis B Virus RNA. J. Virol. 2021, 95, e0057421–e0057429. [Google Scholar] [CrossRef] [PubMed]
- Mueller, H.; Lopez, A.; Tropberger, P.; Wildum, S.; Schmaler, J.; Pedersen, L.; Han, X.; Wang, Y.; Ottosen, S.; Yang, S.; et al. PAPD5/7 Are Host Factors That Are Required for Hepatitis B Virus RNA Stabilization. Hepatology 2019, 69, 1398–1411. [Google Scholar] [CrossRef] [PubMed]
- Seo, J.J.; Jung, S.J.; Yang, J.; Choi, D.E.; Kim, V.N. Functional viromic screens uncover regulatory RNA elements. Cell 2023, 186, 3291–3306. [Google Scholar] [CrossRef]
- McKnight, K.L.; Lemon, S.M. Hepatitis A Virus Genome Organization and Replication Strategy. Cold Spring Harb. Perspect. Med. 2018, 8, 23–41. [Google Scholar] [CrossRef]
- Das, A.; Barrientos, R.; Shiota, T.; Madigan, V.; Misumi, I.; McKnight, K.L.; Sun, L.; Li, Z.; Meganck, R.M.; Li, Y.; et al. Gangliosides are essential endosomal receptors for quasi-enveloped and naked hepatitis A virus. Nat. Microbiol. 2020, 5, 1069–1078. [Google Scholar] [CrossRef]
- Abutaleb, A.; Kottilil, S. Hepatitis A: Epidemiology, Natural History, Unusual Clinical Manifestations, and Prevention. Gastroenterol. Clin. N. Am. 2020, 49, 191–199. [Google Scholar] [CrossRef]
- De Clercq, E.; Férir, G.; Kaptein, S.; Neyts, J. Antiviral treatment of chronic hepatitis B virus (HBV) infections. Viruses 2010, 2, 1279–1305. [Google Scholar] [CrossRef]
- Ganem, D.; Prince, A.M. Hepatitis B virus infection—Natural history and clinical consequences. N. Engl. J. Med. 2004, 350, 1118–1129. [Google Scholar] [CrossRef]
- Yuen, M.F.; Chen, D.S.; Dusheiko, G.M.; Janssen, H.L.A.; Lau, D.T.Y.; Locarnini, S.A.; Peters, M.G.; Lai, C.L. Hepatitis B virus infection. Nat. Rev. Dis. Primers 2018, 4, 18035–18041. [Google Scholar] [CrossRef] [PubMed]
- Trépo, C.; Chan, H.L.; Lok, A. Hepatitis B virus infection. Lancet 2014, 384, 2053–2063. [Google Scholar] [CrossRef] [PubMed]
- Maynard, J.E. Hepatitis A. Yale J. Biol. Med. 1976, 49, 227–233. [Google Scholar]
- Mathiesen, L.R. The hepatitis A virus infection. Liver 1981, 1, 81–109. [Google Scholar] [CrossRef]
- Nassal, M. HBV cccDNA: Viral persistence reservoir and key obstacle for a cure of chronic hepatitis B. Gut 2015, 64, 1972–1984. [Google Scholar] [CrossRef] [PubMed]
- Karatayli, E.; Karatayli, S.C.; Cinar, K.; Gokahmetoglu, S.; Güven, K.; Idilman, R.; Yurdaydin, C.; Bozdayi, A.M. Molecular characterization of a novel entecavir mutation pattern isolated from a multi-drug refractory patient with chronic hepatitis B infection. J. Clin. Virol. Off. Publ. Pan Am. Soc. Clin. Virol. 2012, 53, 130–134. [Google Scholar] [CrossRef]
- Seto, W.K.; Lo, Y.R.; Pawlotsky, J.M.; Yuen, M.F. Chronic hepatitis B virus infection. Lancet 2018, 392, 2313–2324. [Google Scholar] [CrossRef]
- Férir, G.; Kaptein, S.; Neyts, J.; De Clercq, E. Antiviral treatment of chronic hepatitis B virus infections: The past, the present and the future. Rev. Med. Virol. 2008, 18, 19–34. [Google Scholar] [CrossRef]
- Warner, N.; Locarnini, S.; Xu, H. The role of hepatitis B surface antibodies in HBV infection, disease and clearance. Future Virol. 2020, 8, 22–34. [Google Scholar] [CrossRef]
- Kuhns, M.C.; Holzmayer, V.; Anderson, M.C.; McNamara, A.L.; Sauleda, S.; Mbanya, D.; Duong, P.T.; Dung, N.T.T.; Cloherty, G.A. Molecular and Serological Characterization of Hepatitis B Virus (HBV)-Positive Samples with Very Low or Undetectable Levels of HBV Surface Antigen. Viruses 2021, 13, 2053. [Google Scholar] [CrossRef]
- Zhang, Q.; Huang, J.; Chow, H.Y.; Wang, J.; Zhang, Y.; Fung, Y.M.E.; Ren, Q.; Li, X. Development of DHQ-based chemical biology probe to profile cellular targets for HBV. Bioorg. Med. Chem. Lett. 2020, 30, 127615–127621. [Google Scholar] [CrossRef] [PubMed]
- Zhou, T.; Block, T.; Liu, F.; Kondratowicz, A.S.; Sun, L.; Rawat, S.; Branson, J.; Guo, F.; Steuer, H.M.; Liang, H.; et al. HBsAg mRNA degradation induced by a dihydroquinolizinone compound depends on the HBV posttranscriptional regulatory element. Antivir. Res. 2018, 149, 191–201. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Zhou, C.; Jiang, M.; Wang, Y.; Wang, J.; Cheng, Z.; Wang, M.; Liu, Y.; Liang, C.; Wang, J.; et al. Discovery of RG7834: The First-in-Class Selective and Orally Available Small Molecule Hepatitis B Virus Expression Inhibitor with Novel Mechanism of Action. J. Med. Chem. 2018, 61, 10619–10634. [Google Scholar] [CrossRef] [PubMed]
- Mueller, H.; Wildum, S.; Luangsay, S.; Walther, J.; Lopez, A.; Tropberger, P.; Ottaviani, G.; Lu, W.; Parrott, N.J.; Zhang, J.D.; et al. A novel orally available small molecule that inhibits hepatitis B virus expression. J. Hepatol. 2018, 68, 412–420. [Google Scholar] [CrossRef] [PubMed]
- Bopst, M.; Dinklo, T.; Funk, J.; Greiter-Wilke, A.; Lenz, B.; Kustermann, S.; Jiang, T.; Xie, J. Unexpected neurotoxicity in chronic toxicity studies with a HBV viral expression inhibitor. Regul. Toxicol. Pharmacol. 2023, 141, 105407. [Google Scholar] [CrossRef]
- Zhou, Y.H.; Yu, J.; Zhou, L.; Xu, B.; Dai, Y.; Wang, H.; Zhou, W.; Zhao, H. Prevalence of antibody to hepatitis B surface antigen among qualified blood donors in Nanjing, China. Hum. Vaccin. Immunother. 2023, 19, 2206774–2206778. [Google Scholar] [CrossRef]
- Hu, Y.; Sun, F.; Yuan, Q.; Du, J.; Hu, L.; Gu, Z.; Zhou, Q.; Du, X.; He, S.; Sun, Y.; et al. Discovery and preclinical evaluations of GST-HG131, a novel HBV antigen inhibitor for the treatment of chronic hepatitis B infection. Bioorg. Med. Chem. Lett. 2022, 75, 128977–128987. [Google Scholar] [CrossRef]
- Qin, X.; Yang, L.; Ma, X.; Jiang, B.; Wu, S.; Wang, A.; Xu, S.; Wu, W.; Song, H.; Du, N.; et al. Identification of dihydroquinolizinone derivatives with cyclic ether moieties as new anti-HBV agents. Eur. J. Med. Chem. 2022, 238, 114518–114521. [Google Scholar] [CrossRef]
- Zhang, L.; Ge, X.; Jin, H.; Lu, D.; Chen, S.; Zhang, Y.; Wang, X.; Xu, H.; Ao, W.; Zhang, Y. Discovery, optimization and biological evaluation of novel HBsAg production inhibitors. Eur. J. Med. Chem. 2023, 256, 115387. [Google Scholar] [CrossRef]
- Thompson, M.R.; Kaminski, J.J.; Kurt-Jones, E.A.; Fitzgerald, K.A. Pattern recognition receptors and the innate immune response to viral infection. Viruses 2011, 3, 920–940. [Google Scholar] [CrossRef]
- Mesev, E.V.; LeDesma, R.A.; Ploss, A. Decoding type I and III interferon signalling during viral infection. Nat. Microbiol. 2019, 4, 914–924. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Perez, A.C.; Stempel, M.; Chan, B.; Brinkmann, M.M. One Step Ahead: Herpesviruses Light the Way to Understanding Interferon-Stimulated Genes (ISGs). Front. Microbiol. 2020, 11, 124. [Google Scholar] [CrossRef] [PubMed]
- Schneider, W.M.; Chevillotte, M.D.; Rice, C.M. Interferon-stimulated genes: A complex web of host defenses. Annu. Rev. Immunol. 2014, 32, 513–545. [Google Scholar] [CrossRef] [PubMed]
- Wilkins, C.; Dishongh, R.; Moore, S.C.; Whitt, M.A.; Chow, M.; Machaca, K. RNA interference is an antiviral defence mechanism in Caenorhabditis elegans. Nature 2005, 436, 1044–1047. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Li, W.X.; Ding, S.W. Induction and Suppression of RNA Silencing by an Animal Virus. Science 2002, 296, 1319–1321. [Google Scholar] [CrossRef]
- Ding, S.W.; Voinnet, O. Antiviral immunity directed by small RNAs. Cell 2007, 130, 413–426. [Google Scholar] [CrossRef]
- Maillard, P.V.; Ciaudo, C.; Marchais, A.; Li, Y.; Jay, F.; Ding, S.W.; Voinnet, O. Antiviral RNA Interference in Mammalian Cells. Science 2013, 342, 235–238. [Google Scholar] [CrossRef]
- Berkhout, B. RNAi-mediated antiviral immunity in mammals. Curr. Opin. Virol. 2018, 32, 9–14. [Google Scholar] [CrossRef]
- Li, Y.; Lu, J.; Han, Y.; Fan, X.; Ding, S.W. RNA interference functions as an antiviral immunity mechanism in mammals. Science 2013, 342, 231–234. [Google Scholar] [CrossRef]
- Rissland, O.S.; Norbury, C.J. Decapping is preceded by 3′ uridylation in a novel pathway of bulk mRNA turnover. Nat. Struct. Mol. Biol. 2009, 16, 616–623. [Google Scholar] [CrossRef]
- Wickens, M.; Kwak, J.E. Molecular biology. A tail tale for U. Science 2008, 319, 1344–1345. [Google Scholar] [CrossRef][Green Version]
- Joly, A.C.; Garcia, S.; Hily, J.M.; Koechler, S.; Demangeat, G.; Garcia, D.; Vigne, E.; Lemaire, O.; Zuber, H.; Gagliardi, D. An extensive survey of phytoviral RNA 3′ uridylation identifies extreme variations and virus-specific patterns. Plant Physiol. 2023, 193, 271–290. [Google Scholar] [CrossRef]
- Warkocki, Z.; Krawczyk, P.S.; Adamska, D.; Bijata, K.; Garcia-Perez, J.L.; Dziembowski, A. Uridylation by TUT4/7 Restricts Retrotransposition of Human LINE-1s. Cell 2018, 174, 1537–1548. [Google Scholar] [CrossRef] [PubMed]
- Strzyz, P. TUT-TUTting retrotransposons. Nat. Rev. Mol. Cell Biol. 2018, 19, 618–625. [Google Scholar] [CrossRef] [PubMed]
- Jupin, I.; Bouzoubaa, S.; Richards, K.; Jonard, G.; Guilley, H. Multiplication of beet necrotic yellow vein virus RNA 3 lacking a 3′ poly(A) tail is accompanied by reappearance of the poly(A) tail and a novel short U-rich tract preceding it. Virology 1990, 178, 281–284. [Google Scholar] [CrossRef] [PubMed]
- Pyle, J.D.; Mandadi, K.K.; Scholthof, K.B.G. Panicum Mosaic Virus and Its Satellites Acquire RNA Modifications Associated with Host-Mediated Antiviral Degradation. mBio 2019, 10, 01900–01913. [Google Scholar] [CrossRef] [PubMed]
- Viegas, S.C.; Silva, I.J.; Apura, P.; Matos, R.G.; Arraiano, C.M. Surprises in the 3′-end: ‘U’ can decide too! FEBS J. 2015, 282, 3489–3499. [Google Scholar] [CrossRef]
- Rehwinkel, J. Is anti-viral defence the evolutionary origin of mRNA turnover? Bioessays 2016, 38, 817–824. [Google Scholar] [CrossRef]
- Berman-Booty, L.D.; Sargeant, A.M.; Rosol, T.J.; Rengel, R.C.; Clinton, S.K.; Chen, C.S.; Kulp, S.K. A review of the existing grading schemes and a proposal for a modified grading scheme for prostatic lesions in TRAMP mice. Toxicol. Pathol. 2012, 40, 5–17. [Google Scholar] [CrossRef]
- Molleston, J.M.; Sabin, L.R.; Moy, R.H.; Menghani, S.V.; Rausch, K.; Gordesky-Gold, B.; Hopkins, K.C.; Zhou, R.; Jensen, T.H.; Wilusz, J.E.; et al. A conserved virus-induced cytoplasmic TRAMP-like complex recruits the exosome to target viral RNA for degradation. Genes Dev. 2016, 30, 1658–1670. [Google Scholar] [CrossRef]
- Schmidt, K.; Butler, J.S. Nuclear RNA surveillance: Role of TRAMP in controlling exosome specificity. Wiley Interdiscip. Rev. RNA 2013, 4, 217–231. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, R.; Takeuchi, R.; Takata, K.; Shimanouchi, K.; Abe, Y.; Kanai, Y.; Ruike, T.; Ihara, A.; Sakaguchi, K. TRF4 is involved in polyadenylation of snRNAs in Drosophila melanogaster. Mol. Cell. Biol. 2008, 28, 6620–6631. [Google Scholar] [CrossRef] [PubMed]
- Rammelt, C.; Bilen, B.; Zavolan, M.; Keller, W. PAPD5, a noncanonical poly(A) polymerase with an unusual RNA-binding motif. RNA 2011, 17, 1737–1746. [Google Scholar] [CrossRef] [PubMed]
- Lubas, M.; Christensen, M.S.; Kristiansen, M.S.; Domanski, M.; Falkenby, L.G.; Lykke-Andersen, S.; Andersen, J.S.; Dziembowski, A.; Jensen, T.H. Interaction profiling identifies the human nuclear exosome targeting complex. Mol. Cell 2011, 43, 624–637. [Google Scholar] [CrossRef]
- Manokaran, G.; Finol, E.; Wang, C.; Gunaratne, J.; Bahl, J.; Ong, E.Z.; Tan, H.C.; Sessions, O.M.; Ward, A.M.; Gubler, D.J.; et al. Dengue subgenomic RNA binds TRIM25 to inhibit interferon expression for epidemiological fitness. Science 2015, 350, 217–221. [Google Scholar] [CrossRef]
- Brinton, M.A.; Basu, M. Functions of the 3′ and 5′ genome RNA regions of members of the genus Flavivirus. Virus Res. 2015, 206, 108–119. [Google Scholar] [CrossRef]
- Liu, Z.Y.; Qin, C.F. Structure and function of cis-acting RNA elements of flavivirus. Rev. Med. Virol. 2020, 30, e2092–e2099. [Google Scholar] [CrossRef]
- Preston, M.A.; Porter, D.F.; Chen, F.; Buter, N.; Lapointe, C.P.; Keles, S.; Kimble, J.; Wickens, M. Unbiased screen of RNA tailing activities reveals a poly(UG) polymerase. Nat. Methods 2019, 16, 437–445. [Google Scholar] [CrossRef]
- Shukla, A.; Yan, J.; Pagano, D.J.; Dodson, A.E.; Fei, Y.; Gorham, J.; Seidman, J.G.; Wickens, M.; Kennedy, S. poly(UG)-tailed RNAs in genome protection and epigenetic inheritance. Nature 2020, 582, 283–2888, reprinted in Nature 2021, 592, E27–E34. [Google Scholar] [CrossRef]
- Lee, M.; Kim, B.; Kim, V.N. Emerging roles of RNA modification: M(6)A and U-tail. Cell 2014, 158, 980–987. [Google Scholar] [CrossRef]
- Lu, M.; Xue, M.; Wang, H.T.; Kairis, E.L.; Ahmad, S.; Wei, J.; Zhang, Z.; Liu, Q.; Zhang, Y.; Gao, Y.; et al. Nonsegmented Negative-Sense RNA Viruses Utilize N(6)-Methyladenosine (m(6)A) as a Common Strategy To Evade Host Innate Immunity. J. Virol. 2021, 95, 01939–01947. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.; Cheng, W.; Zhao, F.; Tang, M.; Diao, Y.; Xu, R. Association of N6-methyladenosine with viruses and related diseases. Virol. J. 2019, 16, 133–136. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).