The Urinary Metabolomic Fingerprint in Extremely Preterm Infants on Total Parenteral Nutrition vs. Enteral Feeds
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Description
2.2. Metabolomic Profiling
2.3. Global Metabolomic Analysis
2.4. Metabolome Wide Association Analysis (MWAS)
2.5. Metabolite Set Enrichment Analysis
2.6. Time Course Comparison Analysis
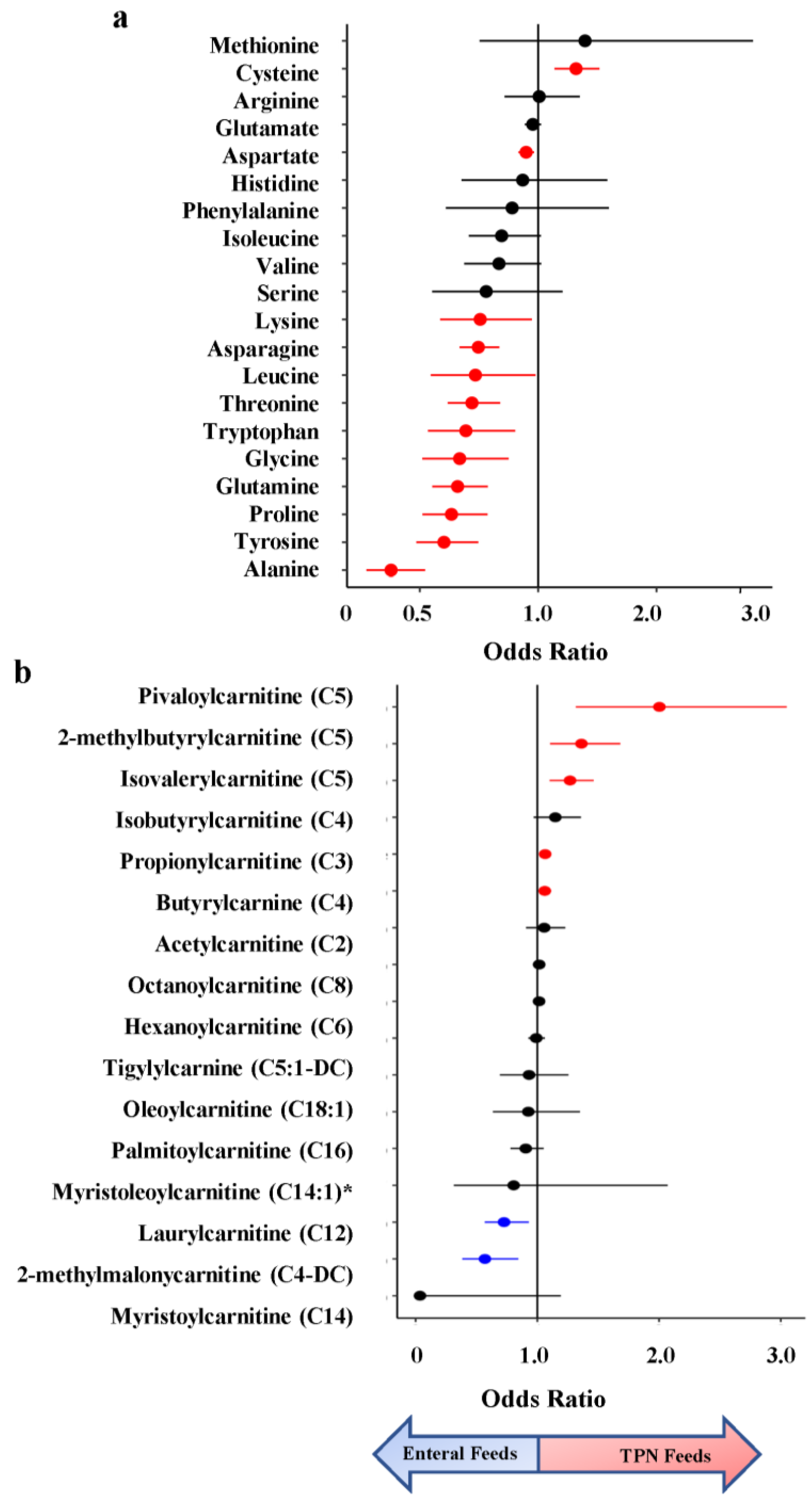
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Demerath, E.W.; Johnson, W.; Davern, B.A.; Anderson, C.G.; Shenberger, J.S.; Misra, S.; Ramel, S.E. New Body Composition Reference Charts for Preterm Infants. Am. J. Clin. Nutr. 2017, 105, 70–77. [Google Scholar] [CrossRef]
- ElHassan, N.O.; Kaiser, J.R. Parenteral Nutrition in the Neonatal Intensive Care Unit. Neoreviews 2011, 12, e130–e140. [Google Scholar] [CrossRef]
- Carnielli, V.P.; Correani, A.; Giretti, I.; D’Ascenzo, R.; Bellagamba, M.P.; Burattini, I.; Biagetti, C. Practice of Parenteral Nutrition in Preterm Infants. World Rev. Nutr. Diet. 2021, 122, 198–211. [Google Scholar] [CrossRef] [PubMed]
- Hamdan, M.; Puckett, Y. Total Parenteral Nutrition; StatPearls Publishing: Tampa, FL, USA, 2022. [Google Scholar]
- Darmaun, D.; Lapillonne, A.; Simeoni, U.; Picaud, J.-C.; Rozé, J.-C.; Saliba, E.; Bocquet, A.; Chouraqui, J.-P.; Dupont, C.; Feillet, F.; et al. Parenteral Nutrition for Preterm Infants: Issues and Strategy. Arch. Pediatr. 2018, 25, 286–294. [Google Scholar] [CrossRef]
- Jeejeebhoy, K.N. Total Parenteral Nutrition: Potion or Poison? Am. J. Clin. Nutr. 2001, 74, 160–163. [Google Scholar] [CrossRef] [PubMed]
- Lavoie, J.-C.; Chessex, P. Parenteral Nutrition and Oxidant Stress in the Newborn: A Narrative Review. Free Radic. Biol. Med. 2019, 142, 155–167. [Google Scholar] [CrossRef] [PubMed]
- Chowdary, K.V.R.; Reddy, P.N. Parenteral Nutrition: Revisited. Indian J. Anaesth. 2010, 54, 95–103. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.K.; Singhal, A.; Vaidya, U.; Banerjee, S.; Anwar, F.; Rao, S. Optimizing Nutrition in Preterm Low Birth Weight Infants-Consensus Summary. Front. Nutr. 2017, 4, 20. [Google Scholar] [CrossRef] [PubMed]
- Patel, P.; Bhatia, J. Total Parenteral Nutrition for the Very Low Birth Weight Infant. Semin. Fetal Neonatal Med. 2017, 22, 2–7. [Google Scholar] [CrossRef]
- Board on Life Sciences; Division on Earth and Life Studies; National Academies of Sciences, Engineering, and Medicine. Use of Metabolomics to Advance Research on Environmental Exposures and the Human Exposome; National Academies Press (US): Washington, DC, USA, 2016. [Google Scholar] [CrossRef]
- Schrimpe-Rutledge, A.C.; Codreanu, S.G.; Sherrod, S.D.; McLean, J.A. Untargeted Metabolomics Strategies-Challenges and Emerging Directions. J. Am. Soc. Mass Spectrom. 2016, 27, 1897–1905. [Google Scholar] [CrossRef]
- Kindschuh, W.F.; Baldini, F.; Liu, M.C.; Liao, J.; Meydan, Y.; Lee, H.H.; Heinken, A.; Thiele, I.; Thaiss, C.A.; Levy, M.; et al. Preterm Birth Is Associated with Xenobiotics and Predicted by the Vaginal Metabolome. Nat. Microbiol. 2023, 8, 246–259. [Google Scholar] [CrossRef] [PubMed]
- Steurer, M.A.; Oltman, S.; Baer, R.J.; Feuer, S.; Liang, L.; Paynter, R.A.; Rand, L.; Ryckman, K.K.; Keller, R.L.; Jelliffe-Pawlowski, L.L. Altered Metabolites in Newborns with Persistent Pulmonary Hypertension. Pediatr. Res. 2018, 84, 272–278. [Google Scholar] [CrossRef] [PubMed]
- Falaina, V.; Fotakis, C.; Boutsikou, T.; Tsiaka, T.; Moros, G.; Ouzounis, S.; Andreou, V.; Iliodromiti, Z.; Xanthos, T.; Vandenplas, Y.; et al. Urine Metabolomic Profile of Breast- versus Formula-Fed Neonates Using a Synbiotic-Enriched Formula. Int. J. Mol. Sci. 2022, 23, 10476. [Google Scholar] [CrossRef]
- López-Hernández, Y.; Oropeza-Valdez, J.J.; Blanco-Sandate, J.O.; Herrera-Van Oostdam, A.S.; Zheng, J.; Chi Guo, A.; Lima-Rogel, V.; Rajabzadeh, R.; Salgado-Bustamante, M.; Adrian-Lopez, J.; et al. The Urinary Metabolome of Healthy Newborns. Metabolites 2020, 10, 165. [Google Scholar] [CrossRef] [PubMed]
- Ballard, R.A.; Keller, R.L.; Black, D.M.; Ballard, P.L.; Merrill, J.D.; Eichenwald, E.C.; Truog, W.E.; Mammel, M.C.; Steinhorn, R.H.; Rogers, E.E.; et al. Randomized Trial of Late Surfactant Treatment in Ventilated Preterm Infants Receiving Inhaled Nitric Oxide. J. Pediatr. 2016, 168, 23–29.e4. [Google Scholar] [CrossRef]
- Poindexter, B.B.; Feng, R.; Schmidt, B.; Aschner, J.L.; Ballard, R.A.; Hamvas, A.; Reynolds, A.M.; Shaw, P.A.; Jobe, A.H.; Prematurity and Respiratory Outcomes Program. Comparisons and Limitations of Current Definitions of Bronchopulmonary Dysplasia for the Prematurity and Respiratory Outcomes Program. Ann. Am. Thorac. Soc. 2015, 12, 1822–1830. [Google Scholar] [CrossRef]
- Eicher, T.; Kinnebrew, G.; Patt, A.; Spencer, K.; Ying, K.; Ma, Q.; Machiraju, R.; Mathé, A.E.A. Metabolomics and Multi-Omics Integration: A Survey of Computational Methods and Resources. Metabolites 2020, 10, 202. [Google Scholar] [CrossRef] [PubMed]
- Torgerson, D.; Guardado, M.; Steurer, M.; Chapin, C.; Hernandez, R.D.; Ballard, P.L. The Hydrocortisone-Responsive Urinary Metabolome of Premature Infants. Pediatr. Res. 2023. [Google Scholar] [CrossRef]
- Zhang, Z.; Adelman, A.S.; Rai, D.; Boettcher, J.; Lőnnerdal, B. Amino Acid Profiles in Term and Preterm Human Milk through Lactation: A Systematic Review. Nutrients 2013, 5, 4800–4821. [Google Scholar] [CrossRef]
- Sylvester, K.G.; Kastenberg, Z.J.; Moss, R.L.; Enns, G.M.; Cowan, T.M.; Shaw, G.M.; Stevenson, D.K.; Sinclair, T.J.; Scharfe, C.; Ryckman, K.K.; et al. Acylcarnitine Profiles Reflect Metabolic Vulnerability for Necrotizing Enterocolitis in Newborns Born Premature. J. Pediatr. 2017, 181, 80–85.e1. [Google Scholar] [CrossRef]
- Esturau-Escofet, N.; Rodríguez de San Miguel, E.; Vela-Amieva, M.; García-Aguilera, M.E.; Hernández-Espino, C.C.; Macias-Kauffer, L.; López-Candiani, C.; Naveja, J.J.; Ibarra-González, I. A Longitudinal 1H NMR-Based Metabolic Profile Analysis of Urine from Hospitalized Premature Newborns Receiving Enteral and Parenteral Nutrition. Metabolites 2022, 12, 255. [Google Scholar] [CrossRef] [PubMed]
- Nilsson, A.K.; Tebani, A.; Malmodin, D.; Pedersen, A.; Hellgren, G.; Löfqvist, C.; Hansen-Pupp, I.; Uhlén, M.; Hellström, A. Longitudinal Serum Metabolomics in Extremely Premature Infants: Relationships with Gestational Age, Nutrition, and Morbidities. Front. Neurosci. 2022, 16, 830884. [Google Scholar] [CrossRef] [PubMed]
- Hughes, C.E.; Coody, T.K.; Jeong, M.-Y.; Berg, J.A.; Winge, D.R.; Hughes, A.L. Cysteine Toxicity Drives Age-Related Mitochondrial Decline by Altering Iron Homeostasis. Cell 2020, 180, 296–310.e18. [Google Scholar] [CrossRef]
- Borum, P.R. Carnitine in Parenteral Nutrition. Gastroenterology 2009, 137 (Suppl. 5), S129–S134. [Google Scholar] [CrossRef] [PubMed]
- Wilcken, B.; Wiley, V. Newborn Screening. Pathology 2008, 40, 104–115. [Google Scholar] [CrossRef] [PubMed]
- Therrell, B.L.; Padilla, C.D.; Loeber, J.G.; Kneisser, I.; Saadallah, A.; Borrajo, G.J.C.; Adams, J. Current Status of Newborn Screening Worldwide: 2015. Semin. Perinatol. 2015, 39, 171–187. [Google Scholar] [CrossRef]
- Remec, Z.I.; Trebusak Podkrajsek, K.; Repic Lampret, B.; Kovac, J.; Groselj, U.; Tesovnik, T.; Battelino, T.; Debeljak, M. Next-Generation Sequencing in Newborn Screening: A Review of Current State. Front. Genet. 2021, 12, 662254. [Google Scholar] [CrossRef]
- Oltman, S.P.; Rogers, E.E.; Baer, R.J.; Jasper, E.A.; Anderson, J.G.; Steurer, M.A.; Pantell, M.S.; Petersen, M.A.; Partridge, J.C.; Karasek, D.; et al. Newborn Metabolic Vulnerability Profile Identifies Preterm Infants at Risk for Mortality and Morbidity. Pediatr. Res. 2021, 89, 1405–1413. [Google Scholar] [CrossRef]
- Santoro, K.L.; Yakah, W.; Singh, P.; Ramiro-Cortijo, D.; Medina-Morales, E.; Freedman, S.D.; Martin, C.R. Acetaminophen and Xenobiotic Metabolites in Human Milk and the Development of Bronchopulmonary Dysplasia and Retinopathy of Prematurity in a Cohort of Extremely Preterm Infants. J. Pediatr. 2022, 244, 224–229.e3. [Google Scholar] [CrossRef]





| TOLSURF | PROP | |||||
|---|---|---|---|---|---|---|
| TPN | Enteral | p-Value | TPN | Enteral | p-Value | |
| Number of Infants | 108 | 63 | - | 80 | 63 | - |
| Age at Sample in days, mean (SD) | 27.3 (2.2) | 28.1 (2.0) | 0.013 | 28.3 (0.9) | 28.0 (0.9) | 0.14 |
| Number of Infants by Maternal Race (NHW|AA|HL) * | 50|40|18 | 35|21|7 | 0.43 | 35|30|15 | 30|25|8 | 0.62 |
| Gestational Age in weeks, mean (SD) | 25.2 (1.2) | 25.6 (1.2) | 0.034 | 25.7 (1.0) | 26.0 (1.3) | 0.11 |
| Male sex (%) | 58.3 | 58.7 | 1.00 | 51.3 | 54.0 | 0.87 |
| Birthweight (g), mean (SD) | 698 (164) | 778 (181) | 0.008 | 770 (161) | 804 (131) | 0.18 |
| On corticosteroids at day 28 (%) | 22.2 | 14.3 | 0.23 | 28.7 | 15.9 | 0.0029 |
| Biochemical Name | Super-Pathway | Meta p | Meta OR | AVG FC |
|---|---|---|---|---|
| Dexpanthenol | Xenobiotics | 6.6 × 10−15 | 11.19 | 6.30 |
| Gluconate | Xenobiotics | 6.7 × 10−14 | 12.06 | 4.83 |
| Ferulic acid 4-sulfate | Xenobiotics | 5.9 × 10−13 | 0.49 | 0.23 |
| 2,6-dihydroxybenzoic acid | Xenobiotics | 3.1 × 10−12 | 0.72 | 0.18 |
| Ascorbic acid 3-sulfate | Cofactors and Vitamins | 3.5 × 10−12 | 0.43 | 0.36 |
| 3-methyladipate | Lipid | 6.4 × 10−12 | 0.35 | 0.50 |
| N-acetylasparagine | Amino Acid | 2.6 × 10−11 | 0.41 | 0.43 |
| Methylsuccinate | Amino Acid | 3.3 × 10−11 | 0.40 | 0.35 |
| Acetylhydroquinone sulfate | Xenobiotics | 4.2 × 10−11 | 0.28 | 0.29 |
| N-succinyl-phenylalanine | Amino Acid | 4.3 × 10−11 | 0.47 | 0.28 |
| Lyxonate | Carbohydrate | 4.5 × 10−11 | 0.32 | 0.43 |
| Arabonate/xylonate | Carbohydrate | 5.7 × 10−11 | 0.22 | 0.50 |
| Isocitric lactone | Energy | 6.6 × 10−11 | 0.61 | 0.36 |
| Threonate | Cofactors and Vitamins | 7.2 × 10−11 | 0.18 | 0.61 |
| N-acetyltyrosine | Amino Acid | 1.3 × 10−10 | 5.11 | 7.08 |
| Tartronate (hydroxymalonate) | Xenobiotics | 1.7 × 10−10 | 0.36 | 0.40 |
| 3-hydroxy-2-methylpyridine sulfate | Xenobiotics | 2.8 × 10−10 | 0.61 | 0.25 |
| N-delta-acetylornithine | Amino Acid | 3.3 × 10−10 | 0.56 | 0.26 |
| N-acetylphenylalanine | Amino Acid | 3.7 × 10−10 | 3.41 | 4.02 |
| Pantothenate | Cofactors and Vitamins | 3.7 × 10−10 | 0.75 | 0.34 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guardado, M.; Steurer, M.; Chapin, C.; Hernandez, R.D.; Ballard, P.L.; Torgerson, D. The Urinary Metabolomic Fingerprint in Extremely Preterm Infants on Total Parenteral Nutrition vs. Enteral Feeds. Metabolites 2023, 13, 971. https://doi.org/10.3390/metabo13090971
Guardado M, Steurer M, Chapin C, Hernandez RD, Ballard PL, Torgerson D. The Urinary Metabolomic Fingerprint in Extremely Preterm Infants on Total Parenteral Nutrition vs. Enteral Feeds. Metabolites. 2023; 13(9):971. https://doi.org/10.3390/metabo13090971
Chicago/Turabian StyleGuardado, Miguel, Martina Steurer, Cheryl Chapin, Ryan D. Hernandez, Philip L. Ballard, and Dara Torgerson. 2023. "The Urinary Metabolomic Fingerprint in Extremely Preterm Infants on Total Parenteral Nutrition vs. Enteral Feeds" Metabolites 13, no. 9: 971. https://doi.org/10.3390/metabo13090971
APA StyleGuardado, M., Steurer, M., Chapin, C., Hernandez, R. D., Ballard, P. L., & Torgerson, D. (2023). The Urinary Metabolomic Fingerprint in Extremely Preterm Infants on Total Parenteral Nutrition vs. Enteral Feeds. Metabolites, 13(9), 971. https://doi.org/10.3390/metabo13090971







