Toxicological Effects of Thimerosal and Aluminum in the Liver, Kidney, and Brain of Zebrafish (Danio rerio)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Experimental Conditions
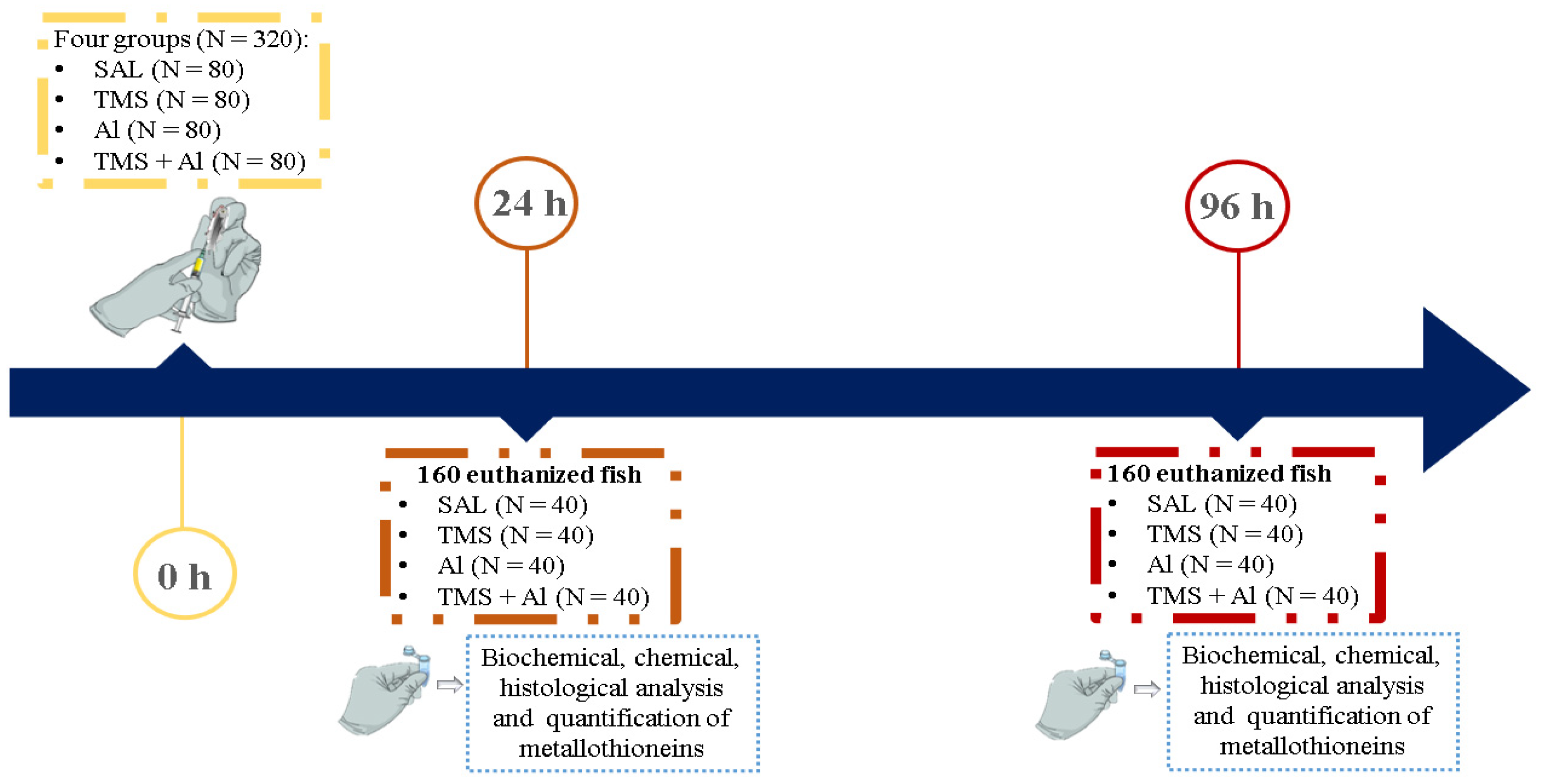
2.3. Experimental Design
2.3.1. Thimerosal and Aluminum Hydroxide
2.3.2. Mortality Test
2.3.3. Exposure to TMS and/or Al
2.4. Analysis of Biochemical Biomarkers
2.4.1. Sample Preparation
2.4.2. Quantification of Total Protein
2.4.3. Superoxide Dismutase (SOD) Activity
2.4.4. Glutathione S-Transferase (GST) Activity
2.4.5. Glutathione Peroxidase (GPx) Activity
2.4.6. Acetylcholinesterase (AChE) Activity
2.4.7. Reduced Glutathione (GSH) Concentration
2.4.8. Quantification of Metallothionein (MT)
2.5. Histopathological Analysis
2.6. Metal Quantification
2.7. Statistical Analysis
3. Results
3.1. Experimental Conditions
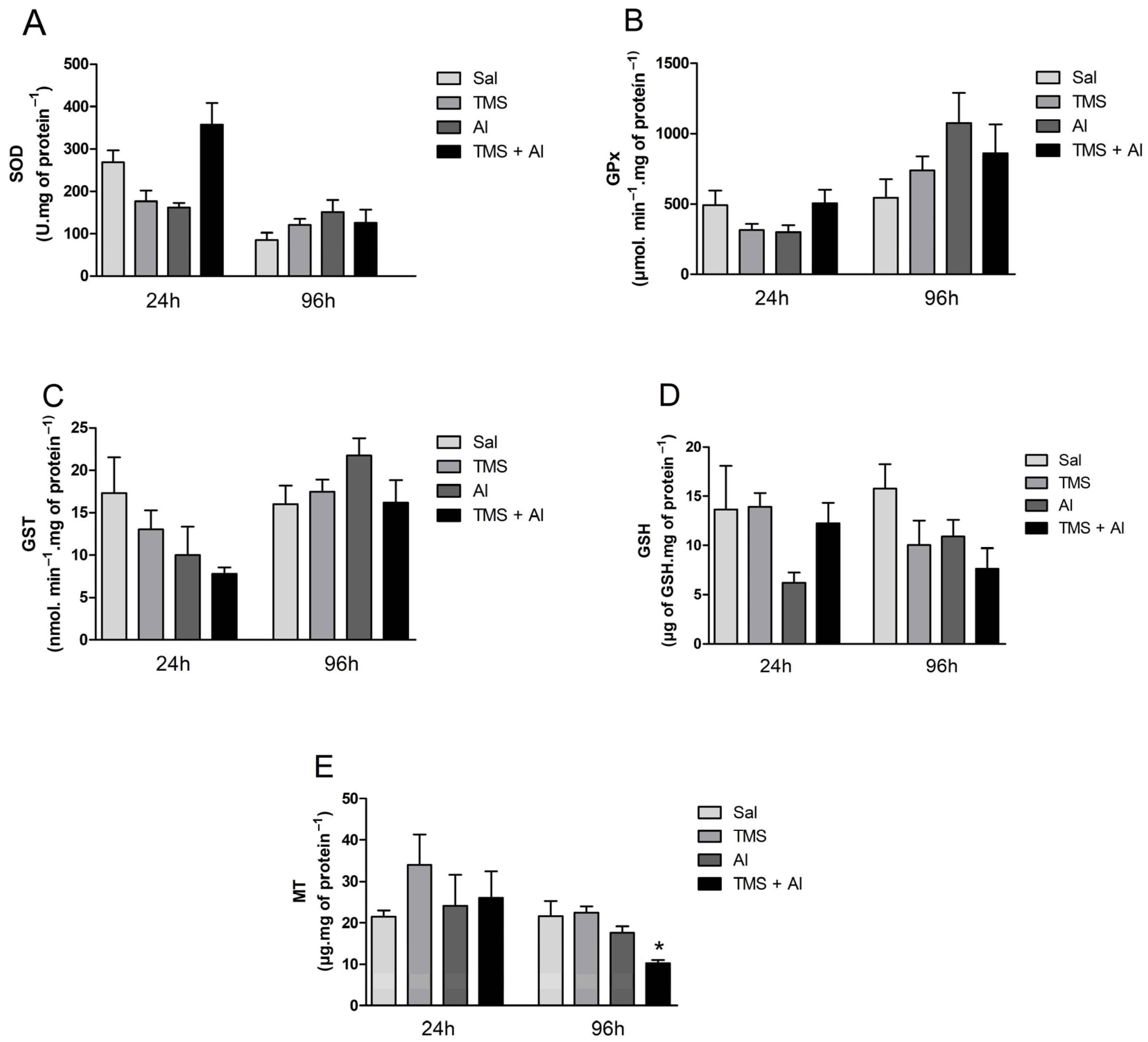
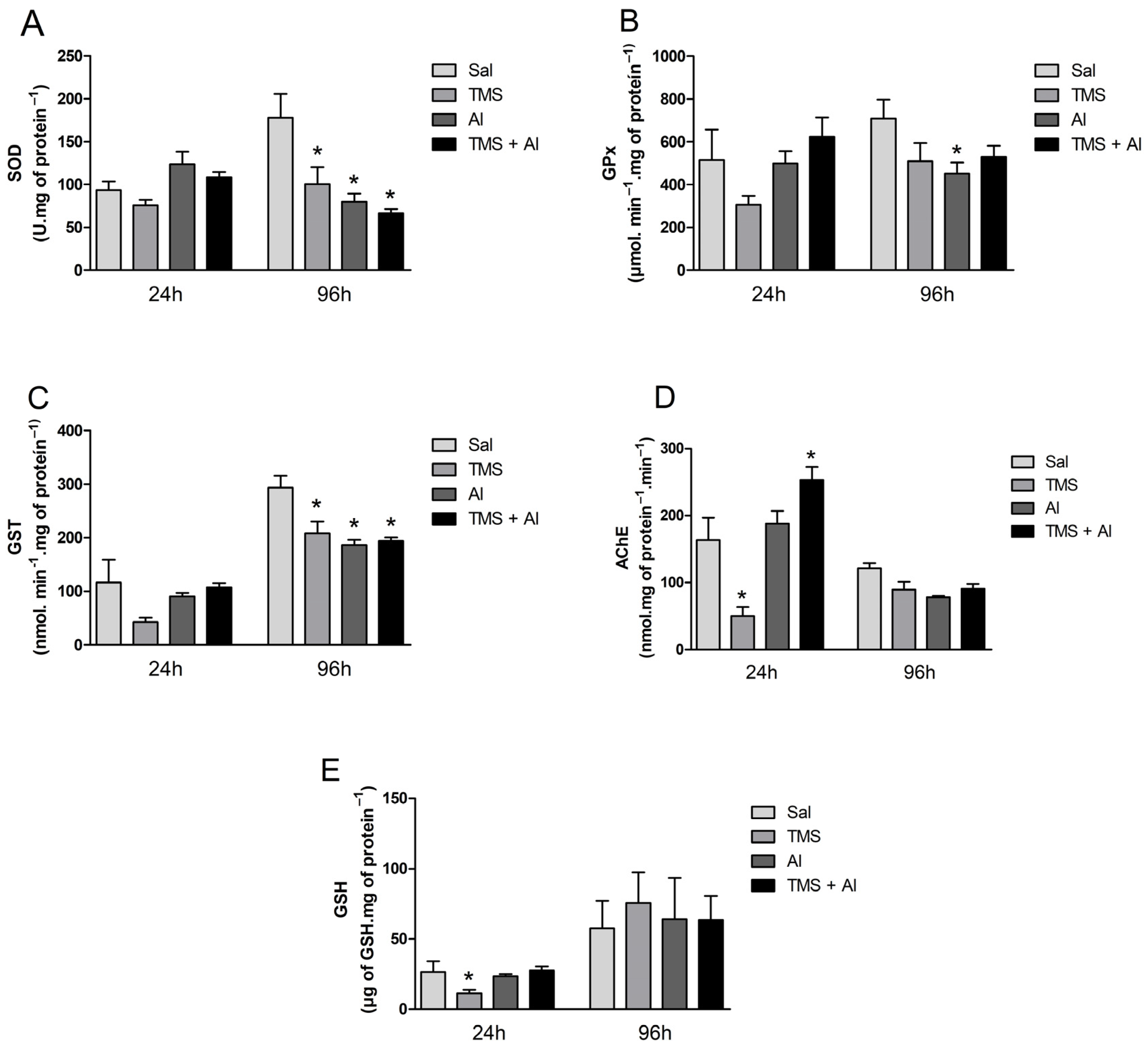
3.2. Biochemical Analysis
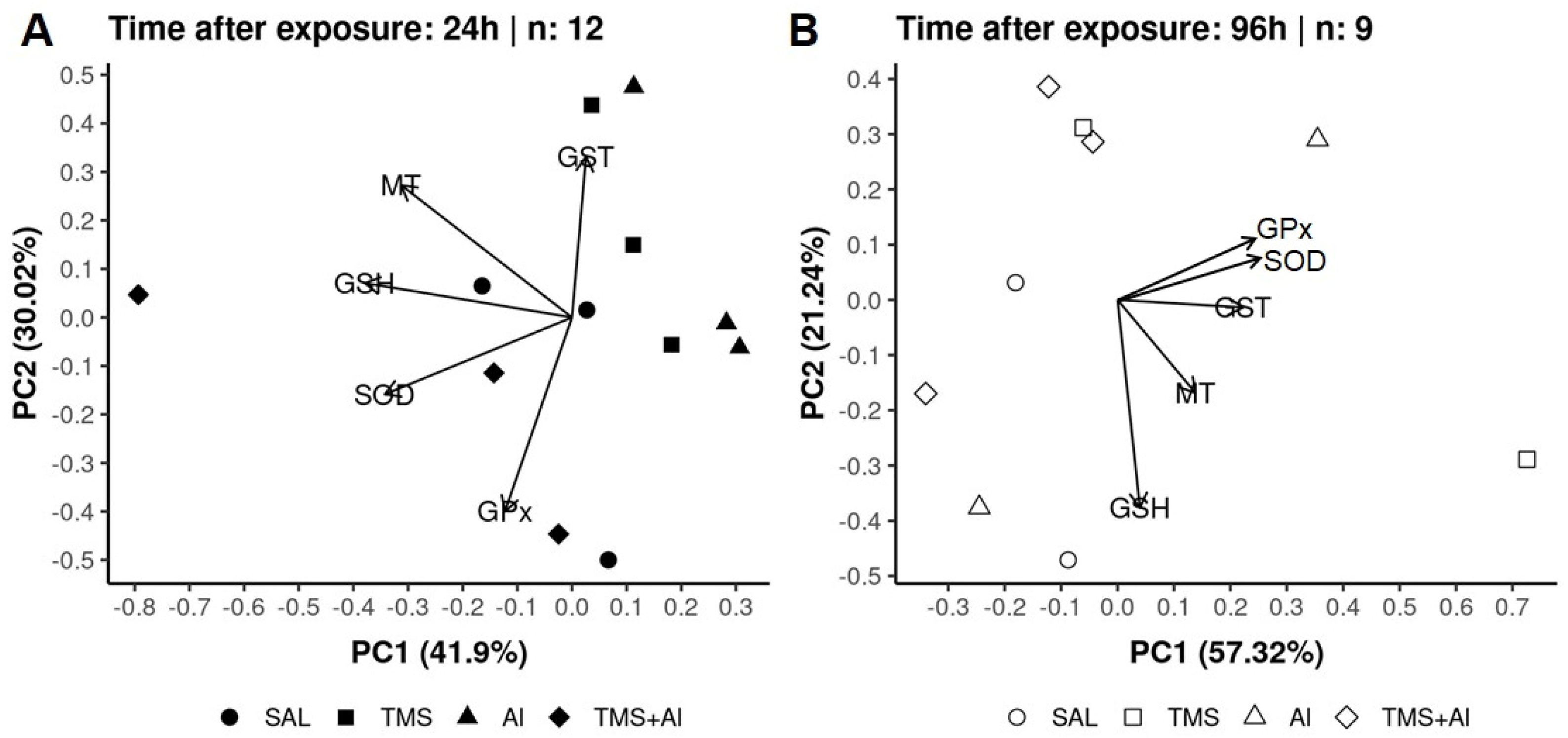
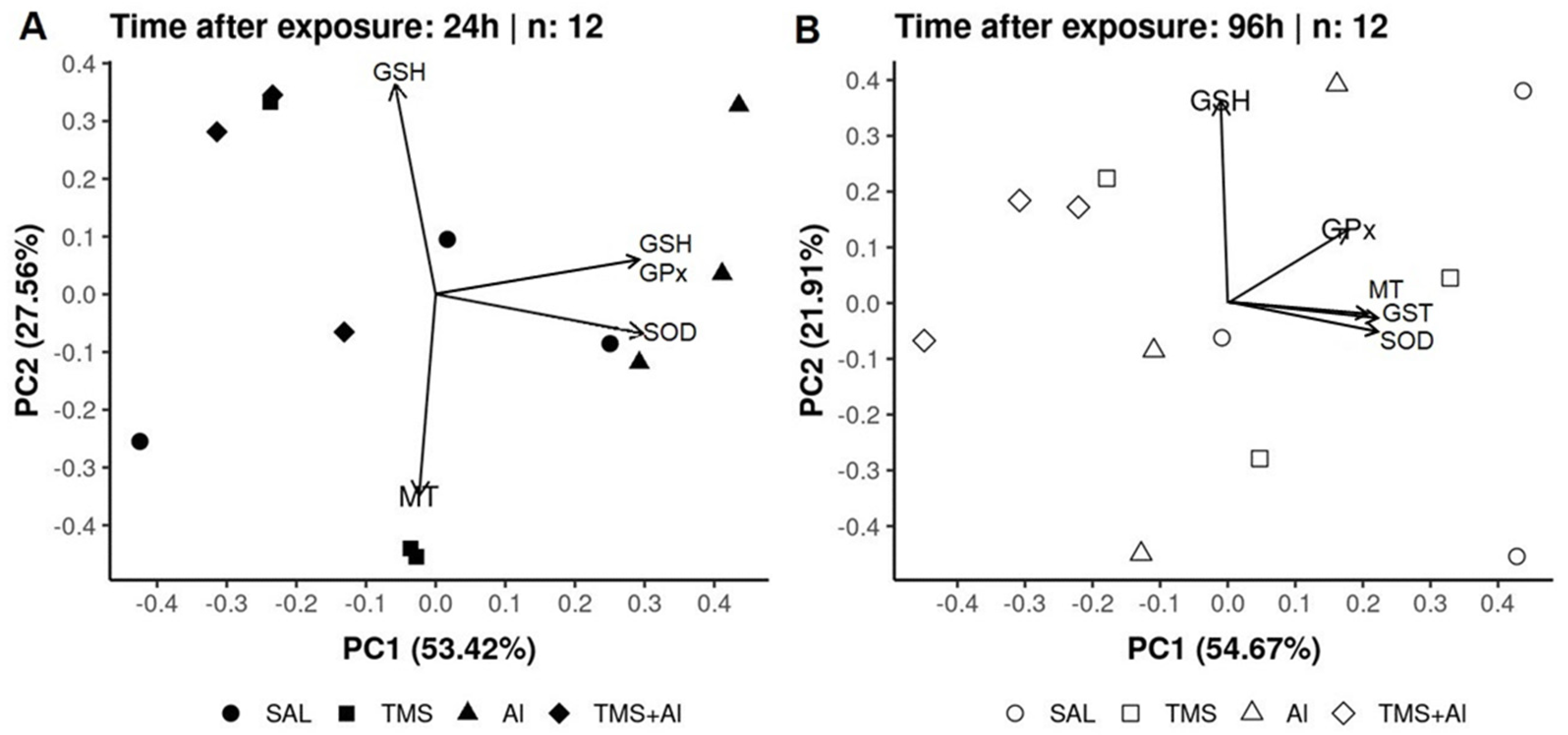
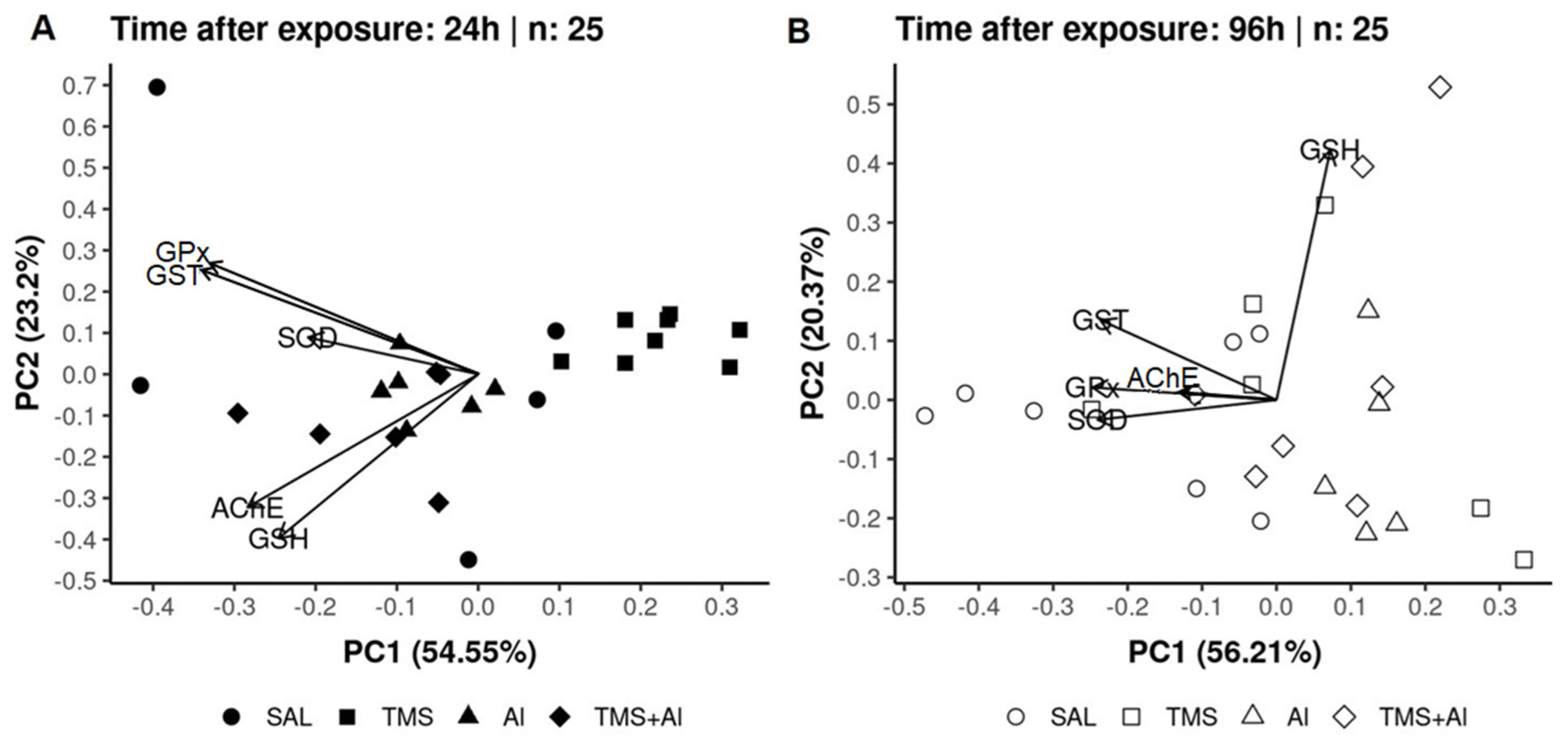
3.3. Principal Component Analysis (PCA)
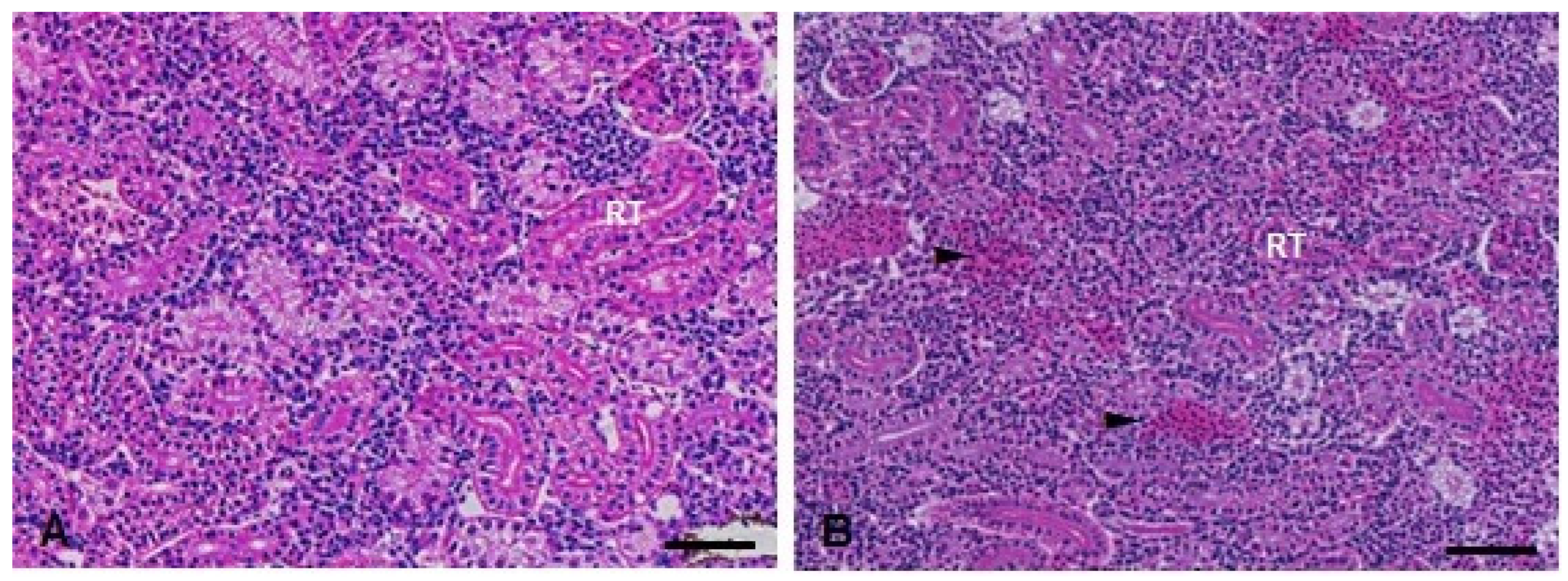
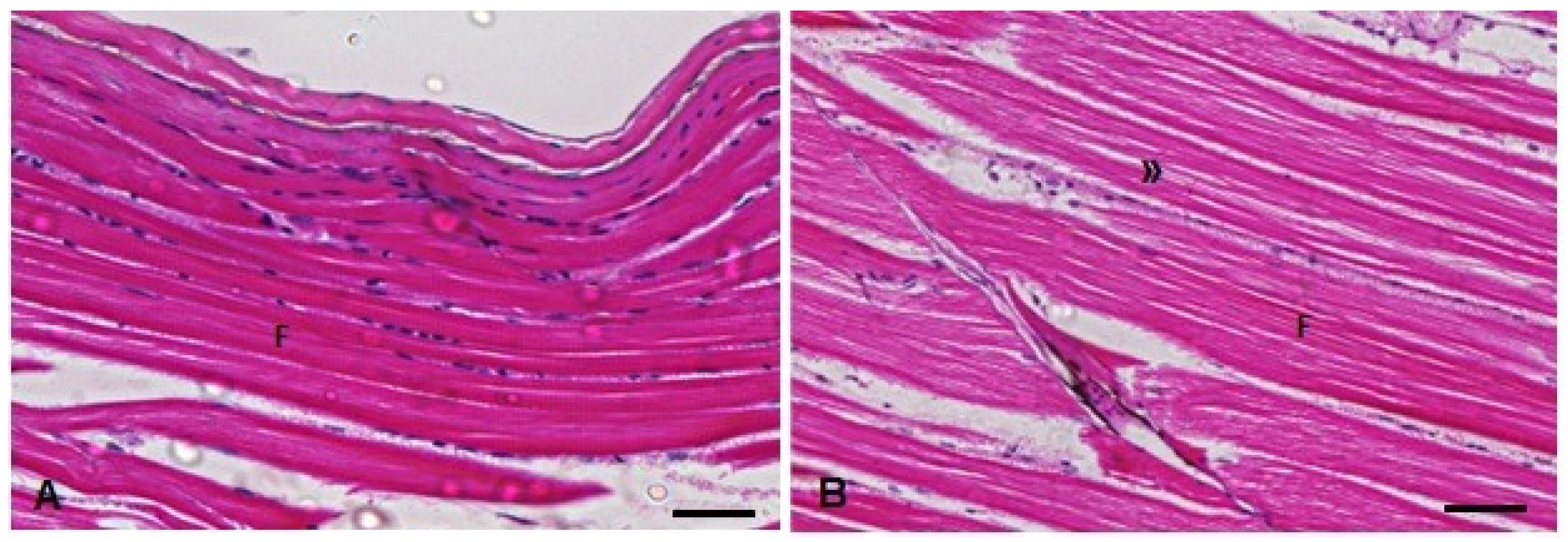
3.4. Histopathological Analysis
3.5. Hg and Al Quantification
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Lessa, S.C.; Dórea, G.F. Bioética e vacinação infantil em massa. Rev. Bioét. 2013, 21, 226–236. [Google Scholar] [CrossRef]
- Stone, C.A., Jr.; Rukasin, C.R.F.; Beachkofsky, T.M. Immune-mediated adverse reactions to vaccines. Br. J. Clin. Pharmacol. 2019, 85, 2694–2706. [Google Scholar] [CrossRef]
- Dórea, J.G. Safety of Thimerosal in vaccines: For whom and how many doses? Thérapie 2011, 66, 95–96. [Google Scholar] [CrossRef] [PubMed]
- Dórea, J.G. Low-dose Thimerosal in pediatric vaccines: Adverse effects in perspective. Environ. Res. 2017, 152, 280–293. [Google Scholar] [CrossRef] [PubMed]
- Dórea, J.G. Low-dose Thimerosal (ethyl-mercury) is still used in infants vaccines: Should we be concerned with this form of exposure? J. Trace Elemen. Med. Biol. 2018, 49, 134–139. [Google Scholar] [CrossRef]
- Dórea, J.G. Neurotoxic effects of combined exposures to aluminum and mercury in early life (infancy). Environ. Res. 2020, 188, 109734. [Google Scholar] [CrossRef]
- Garçon, N.; Leroux-Roels, G.; Cheng, W. Vaccine adjuvants. Perspec. Vaccinol. 2011, 1, 89–113. [Google Scholar] [CrossRef]
- Christensen, D. Vaccine adjuvants: Why and how. Hum. Vaccines Immunother. 2016, 12, 2709–2711. [Google Scholar] [CrossRef]
- Ghimire, T.R. The mechanisms of action of vaccines containing aluminum adjuvants: An in vitro vs in vivo paradigm. Springerplus 2015, 4, 1–18. [Google Scholar] [CrossRef]
- McFarland, G.; Joie, E.L.; Thomas, P. Acute exposure and chronic retention of aluminum in three vaccine schedules and effects of genetic and environmental variation. J. Trace Elemen. Med. Biol. 2020, 58, 126444. [Google Scholar] [CrossRef]
- Nabi, S. Methylmercury and thimerosal. In Toxic Effects of Mercury; Springer: New Delhi, India, 2014; pp. 219–232. [Google Scholar]
- Rocha, V.B.; Scherrer, M.A.R. Thimerosal: Current sources of contact in Brazil. An. Bras. Dermatol. 2014, 89, 376–378. [Google Scholar] [CrossRef]
- Oliveira, C.S.; Nogara, P.A.; Ardisson-Araújo, D.M.; Aschner, M.; Rocha, J.B.T.; Dórea, J.G. Neurodevelopmental Effects of Mercury. Adv. Neurotoxicology 2018, 2, 27–86. [Google Scholar]
- Alves, R.R.; Gaspar, A.; Ferreira, M.B. Reacções alérgicas a vacinas. Rev. Port. Imunoalergologia 2007, 15, 465–483. [Google Scholar]
- Aps, L.R.M.M.; Piantola, M.A.F.; Pereira, S.A.; Castro, J.T.; Santos, F.A.; Ferreira, L.C.S. Eventos adversos de vacinas e as consequências da não vacinação: Uma análise crítica. Rev. Saúde Pública 2018, 52, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Agência Nacional de Vigilância Sanitária (ANVISA), Bulário Eletrônico. 2013. Available online: https://consultas.anvisa.gov.br/#/bulario/ (accessed on 19 June 2022).
- Guimarães, L.E.; Baker, B.; Perricone, C. Vaccines, adjuvants and autoimmunity. Pharmacol. Res. 2015, 100, 190–209. [Google Scholar]
- Chagas, S.R.; Dall’Agnol, M.; Pessoa, A.V.C.; Ramis-Vidal, M.G.; Pascoal, L.M. Vacinas e suas reações adversas: Revisão. Pubvet 2019, 13, 153. [Google Scholar] [CrossRef]
- Melnick, J.G.; Yurkerwich, K.; Buccella, D. Molecular structures of thimerosal (Merthiolate) and other arylthiolate mercury alkyl compounds. Inorg. Chem. 2008, 47, 6421–6426. [Google Scholar] [CrossRef]
- Geier, D.A.; Sykes, L.K.; Geier, M.R. A review of Thimerosal (Merthiolate) and its ethylmercury breakdown product: Specific historical considerations regarding safety and effectiveness. J. Toxicol. Environ. Health Part B 2007, 10, 575–596. [Google Scholar] [CrossRef]
- Ramos, A.; Dos Santos, M.M.; de Macedo, G.T.; Wildner, G.; Prestes, A.S.; Masuda, C.A.; Corte, C.L.D.; Rocha, J.B.T.; Barbosa, N.V. Methyl and Ethylmercury elicit oxidative stress and unbalance the antioxidant system in Saccharomyces cerevisiae. Chem-Biol. Interac. 2020, 315, 108867. [Google Scholar] [CrossRef]
- Geier, D.A.; King, P.G.; Hooker, B.S.; Dórea, J.G.; Kern, J.K.; Sykes, L.K.; Geier, M.R. Thimerosal: Clinical, epidemiologic and biochemical studies. Clin. Chim. Acta 2015, 444, 212–220. [Google Scholar] [CrossRef]
- Dórea, J.G.; Farina, M.; Rocha, J.B. Toxicity of ethylmercury (and Thimerosal): A comparison with methylmercury. J. Appl. Toxicol. 2013, 33, 700–711. [Google Scholar]
- Oliveira, C.S.; Piccoli, B.C.; Aschner, M.; Rocha, J.B.T. Chemical speciation of selenium and mercury as a determinant of their neurotoxicity. Neurotoxicol. Met. 2017, 53–83. [Google Scholar]
- Nogara, P.A.; Oliveira, C.S.; Schmitz, G.L.; Piquini, P.C.; Farina, M.; Aschner, M.; Rocha, J.B.T. Methylmercury’s chemistry: From the environment to the mammalian brain. Biochim. Biophys. Acta 2019, 1863, 129284. [Google Scholar]
- Dórea, J.G.; Marques, R.; Brandão, K. Neonate exposure to Thimerosal mercury from Hepatitis B vaccines. J. Perinatol. 2009, 26, 523–527. [Google Scholar] [CrossRef]
- Howe, K.; Clark, M.D.; Torroja, C.F.; Torrance, J.; Berthelot, C.; Muffato, M.; Collins, J.E.; Humphray, S.; Mclaren, K.; Matthews, L.; et al. The zebrafish reference genome sequence and its relationship to the human genome. Nature 2013, 496, 498–503. [Google Scholar]
- Lee, J.; Freeman, J.L. Zebrafish as a model for investigating developmental lead (Pb) neurotoxicity as a risk factor in adult neurodegenerative disease: A mini-review. Neurotoxicology 2014, 43, 57–64. [Google Scholar]
- Herman, P.; Fehér, M.; Molnár, Á.; Harangi, S.; Sajtos, Z.; Stündl, L.; Fábián, I.; Baranyai, E. Iron and manganese retention of juvenile zebrafish (Danio rerio) exposed to contaminated dietary zooplankton (Daphnia pulex)—A model experiment. Biol. Trace Elem. Res. 2021, 199, 732–743. [Google Scholar] [PubMed]
- Mahieu, S.; del Carmen Contini, M.; Gonzalez, M.; Millen, M.; Elias, M.M. Aluminum toxicity. Hematological effects. Toxicol. Lett. 2000, 111, 235–242. [Google Scholar] [CrossRef] [PubMed]
- Shaw, C.A.; Petrik, M.S. Aluminum hydroxide injections lead to motor deficits and motor neuron degeneration. J. Inorg. Biochem. 2009, 103, 1555–1562. [Google Scholar]
- Bohrer, D.; Schmidt, M.; Marques, R.C. Distribution of aluminum in hair of Brazilian infants and correlation to aluminum-adjuvanted vaccine exposure. Clin. Chim. Acta 2014, 428, 9–13. [Google Scholar] [CrossRef] [PubMed]
- Vinay, T.N.; Park, C.S.; Kim, H.Y.; Jung, S.-J. Toxicity and dose determination of quillaja saponin, aluminum hydroxide, and squalene in olive flounder (Paralichthys olivaceus). Vet. Immunol. Immunopathol. 2014, 158, 73–85. [Google Scholar] [CrossRef] [PubMed]
- Hornig, M.; Chian, D.; Lipkin, W.I. Neurotoxic effects of postnatal thimerosal are mouse strain-dependent. Mol. Psychiatry 2004, 9, 833–845. [Google Scholar] [CrossRef] [PubMed]
- Geier, D.A.; King, P.G.; Geier, M.R. Mitochondrial dysfunction, impaired oxidative-reduction activity, degeneration, and death in human neuronal and fetal cells induced by low-level exposure to thimerosal and other metal compounds. Toxicol. Environ. Chem. 2009, 91, 735–749. [Google Scholar] [CrossRef]
- Olczak, M.; Duszczyk, M.; Mierzejewski, P.; Bobrowicz, T.; Majewska, M.D. Neonatal administration of a vaccine preservative, thimerosal, produces lasting impairment of nociception and apparent activation of opioid system in rats. Brain Res. 2009, 1301, 143–151. [Google Scholar] [CrossRef] [PubMed]
- Bianchini, M.C.; Gularte, C.O.A.; Nogara, P.A.; Krum, B.N.; Gayer, M.C.; Bridi, J.C.; Roos, D.H.; Roehrs, R.; Fachinetto, R.; Pinton, S.; et al. Thimerosal inhibits Drosophila melanogaster tyrosine hydroxylase (DmTyrH) leading to changes in dopamine levels and impaired motor behavior: Implications for neurotoxicity. Metallomics 2019, 11, 362–374. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Gao, R.; Yuan, Z.; Zhao, Z. Mechanism of pyrogallol autoxidation and determination of superoxide dismutase enzyme activity. Bioelectrochem. Bioenerg. 1998, 45, 41–45. [Google Scholar] [CrossRef]
- Keen, J.H.; Habig, W.H.; Jakoby, W.B. Mechanism for the several activities of the glutathione S-transferases. J. Biol. Chem. 1976, 51, 6183–6188. [Google Scholar] [CrossRef]
- Paglia, D.E.; Valentine, W.N. Studies on the quantitative and qualitative characterization of erythrocyte glutathione peroxidase. J. Lab. Clin. Med. 1967, 70, 58–169. [Google Scholar]
- Ellman, G.L.; Courtney, K.D.; Andres, V., Jr. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem. Pharmacol. 1961, 7, 88–95. [Google Scholar] [CrossRef]
- Sedlak, J.; Lindsay, R.H. Estimation of total, protein-bound, and nonprotein sulfhydryl groups in tissue with Ellman’s reagent. Anal. Biochem. 1968, 25, 192–205. [Google Scholar] [CrossRef]
- Viarengo, A.; Ponzano, E.; Dondero, F.; Fabbri, R. A simple spectrophotometric method for metallothionein evaluation in marine organisms: An application to Mediterranean and Antarctic mollusks. Mar. Environ. Res. 1997, 44, 69–84. [Google Scholar] [CrossRef]
- Bernet, D.; Schmidt, H.; Meier, W.; Burkhardt-Homl, P.; Wahli, T. Histopathology in fish: Proposal for a protocol to assess aquatic pollution. J. Fish Dis. 1999, 22, 25–34. [Google Scholar] [CrossRef]
- Mela, M.; Guiloski, I.C.; Doria, H.B.; Rabitto, I.S.; Silva, C.A.; Maraschi, A.C.; Prodocimo, V.; Freire, C.A.; Randi, M.A.F.; Oliveira, C.A.; et al. Risks of waterborne copper exposure to a cultivated freshwater Neotropical catfish (Rhamdia quelen). Ecotoxicol. Environ. Saf. 2013, 88, 108–116. [Google Scholar] [CrossRef] [PubMed]
- EPA3050B. Environmental Protection Agency (EPA, EUA), (Method 3050B: Acid Digestion of Sediments, Sludges and Soils, Revision 2). Environ. Prot. Agency 1996, 2, 3–5. [Google Scholar]
- Wickham, H.; François, R.; Henry, L. dplyr: A Grammar of Data Manipulation. R Package Version 1.0.9. 2022. Available online: https://CRAN.R-project.org/package=dplyr (accessed on 15 August 2022).
- Wickham, H. ggplot2: Elegant Graphics for Data Analysis; Springer: New York, NY, USA, 2016; ISBN 978-3-319-24277-4. Available online: https://ggplot2.tidyverse.org (accessed on 15 August 2022).
- Tang, Y.; Horikoshi, M.; Li, W. ggfortify: Unified Interface to Visualize Statistical Result of Popular R Packages. R J. 2016, 8, 474–485. [Google Scholar] [CrossRef]
- Horikoshi, M.; Tang, Y. ggfortify: Data Visualization Tools for Statistical Analysis Results. 2018. Available online: https://CRAN.R-project.org/package=ggfortify (accessed on 30 July 2022).
- Pedersen, T.L. Patchwork: The Composer of Plots. R Package Version 1.1.1. 2020. Available online: https://CRAN.R-project.org/package=patchwork (accessed on 30 July 2022).
- Veiga, M.; Bohrer, D.; Banderó, C.R.; Oliveira, S.M.R.; Nascimento, P.C.; Mattiazzi, P.; Mello, C.F.; Lenza, Q.F.; Oliveira, M.S. Accumulation, elimination, and effects of parenteral exposure to aluminum in newborn and adult rats. J. Inorg. Biochem. 2013, 128, 215–220. [Google Scholar] [CrossRef]
- Dórea, J.G. Exposure to mercury and aluminum in early life: Developmental vulnerability as a modifying factor in neurologic and immunologic effects. Int. J. Environ. Res. Public Health 2015, 12, 1295–1313. [Google Scholar] [CrossRef]
- Food and Drug Administration (FDA). Thimerosal and Vaccines. Available online: https://www.fda.gov/vaccines-blood-biologics/safety-availability-biologics/thimerosal-and-vaccines (accessed on 7 August 2023).
- Schlenk, D.; Zhang, Y.S.; Nix, J. Expression of hepatic metallothionein messenger RNA in feral and caged fish species correlates with muscle mercury levels. Ecotoxicol. Environ. Saf. 1995, 31, 282–286. [Google Scholar] [CrossRef]
- Alam, R.; Abu Zeid, E.; Khalifa, B.A.; Arisha, A.H.; Reda, R.M. Dietary exposure to methyl mercury chloride induces alterations in hematology, biochemical parameters, and mRNA expression of antioxidant enzymes and metallothionein in Nile tilapia. Environ. Sci. Pollut. Res. 2021, 28, 31391–31402. [Google Scholar] [CrossRef]
- Thirumoorthy, N.; Kumar, K.M.; Sundar, A.S. Metallothionein: An overview. World J. Gastroenterol. 2007, 13, 993. [Google Scholar] [CrossRef] [PubMed]
- Meister, A.M.E.A. Glutathione. Annu. Rev. Biochem. 1983, 52, 711–760. [Google Scholar] [CrossRef] [PubMed]
- Bounous, G.; Sukkar, S.; Molson, J.H. The antioxidant system. Anticancer Res. 2003, 23, 1411–1416. [Google Scholar] [PubMed]
- Ajsuvakova, O.P.; Tinkov, A.A.; Aschner, M.; Rocha, J.B.T.; Michalke, B.; Skalnaya, M.G.; Skalny, A.V.; Butnariu, M.; Dadar, M.; Sarac, I.; et al. Sulfhydryl groups as targets of mercury toxicity. Coord. Chem. Rev. 2020, 417, 213343. [Google Scholar] [CrossRef]
- Ijaz, M.U.; Ashraf, A.; Ismail, H.; Imram, M.; Al-Ghanim, K.A.; Raulf, A.; Al-Misned, F.; Ahmed, Z.; Samad, A.; Riaz, M.N.; et al. Administration of vaccine preservative Thimerosal produces impairment in rat liver. Braz. Arch. Biol. Technol. 2021, 64, e21190689. [Google Scholar] [CrossRef]
- Decker, K. Mechanisms and mediators in hepatic necrosis. Gastroenterol. Jpn. 1993, 28, 20–25. [Google Scholar] [CrossRef]
- Fridovich, I. Superoxide radical and superoxide dismutases. Annu. Rev. Biochem. 1995, 64, 97–112. [Google Scholar] [CrossRef]
- Fanucchi, M.V. Development of Antioxidant and Xenobiotic Metabolizing Enzyme Systems. Lung 2014, 223–231. [Google Scholar]
- Strange, R.C.; Spiteri, M.A.; Ramachandran, S. Glutathione-S-transferase family of enzymes. Mut. Res.—Fundam. Mol. Mech. Mutagen. 2001, 482, 21–26. [Google Scholar] [CrossRef]
- Ohtawa, M.; Seko, M.; Tarayama, F. Effect of aluminum ingestion on lipid peroxidation in rats. Chem. Pharm. Bull. 1983, 31, 1415–1418. [Google Scholar] [CrossRef]
- Ijaz, M.U.; Majeed, S.A.; Asharaf, A.; Ali, T.; Al-Ghanim, K.A.; Asad, F.; Zafar, S.; Ismail, M.; Samad, A.; Ahmed, Z.; et al. Toxicological effects of Thimerosal on rat kidney: A histological and biochemical study. Braz. J. Biol. 2021, 83, e242942. [Google Scholar] [CrossRef]
- El-Shelby, A.; Elbaghdady, H.A. Effect of oil pollution on serum growth hormone (GH) levels, histology and ultrastructure of muscles of the Nile Tilapia (Oreochromis niloticus). Egypt. J. Aquat. Biol. Fish 2011, 15, 145–158. [Google Scholar] [CrossRef]
- Mota, W.M.; Barros, M.L.; Cunha, P.E.L.; Santana, M.V.A.; Stevam, C.S.; Leopoldo, P.T.G.; Fernandes, R.P.M. Avaliação da inibição da acetilcolinesterase por extratos de plantas medicinais. Rev. Bras. Plantas Med. 2012, 14, 624–628. [Google Scholar] [CrossRef]
- Richetti, S.K.; Rosemberg, D.B.; Ventura-Lima, J.; Monserrat, J.M.; Bogo, M.R.; Bonan, C.D. Acetylcholinesterase activity and antioxidant capacity of zebrafish brain is altered by heavy metal exposure. Neurotoxicology 2011, 32, 116–122. [Google Scholar] [CrossRef] [PubMed]
- Hassanein, N.M.; El-Sanhory, H.M.; Ali, A.A. Postnatal exposure to vaccines’ additives (Thimerosal and Al(OH)3) impairs male offspring’ spatial learning and memory tasks under normal and prenatal stress conditions: Are there negative impact of maternal selenium supplement? Middle East J. Appl. Sci. 2018, 8, 1451–1470. [Google Scholar]
- Gonzalez, P.; Dominique, Y.; Massabuau, J.C.; Boudou, A.; Bourdineaud, J.P. Comparative effects of dietary methylmercury on gene expression in liver, skeletal muscle, and brain of the zebrafish (Danio rerio). Environ. Sci. Technol. 2005, 39, 3972–3980. [Google Scholar] [CrossRef] [PubMed]
- Monteiro, D.A.; Rantin, F.T.; Kalinin, A.L. Inorganic mercury exposure: Toxicological effects, oxidative stress biomarkers and bioaccumulation in the tropical freshwater fish matrinxã, Brycon amazonicus (Spix and Agassiz, 1829). Ecotoxicology 2010, 19, 105–123. [Google Scholar] [CrossRef] [PubMed]



| Groups | 24 h | 96 h |
|---|---|---|
| Liver | ||
| Sal | 0.0 (0.0, 0.0) | 0.0 (0.0, 0.0) |
| TMS | 3.0 (0.0, 6.0) | 0.0 (0.0, 6.0) |
| Al | 0.0 (0.0, 6.0) | 0.0 (0.0, 6.0) |
| TMS + Al | 6.0 (0.0, 6.0) | 6.0 (6.0, 6.0) * |
| Kidney | ||
| Sal | 0.0 (0.0, 0.0) | 0.0 (0.0, 0.0) |
| TMS | 0.0 (0.0, 3.0) | 0.0 (0.0, 1.0) |
| Al | 4.0 (4.0, 4.0) | 4.0 (0.0, 4.0) * |
| TMS + Al | 7.0 (4.0, 8.0) * | 4.0 (3.0, 4.0) * |
| Muscle | ||
| Sal | 0.0 (0.0, 0.0) | 0.0 (0.0, 0.0) |
| TMS | 2.0 (0.0, 4.0) | 2.0 (0.0, 5.0) * |
| Al | 0.0 (0.0, 0.0) | 0.0 (0.0, 0.0) |
| TMS + Al | 4.0 (1.0, 7.0) | 4.0 (0.0, 4.0) * |
| Brain | ||
| Sal | 0.0 (0.0, 0.0) | 0.0 (0.0, 0.0) |
| TMS | 0.0 (0.0, 0.0) | 0.0 (0.0, 8.0) |
| Al | 0.0 (0.0, 0.0) | 0.0 (0.0, 0.0) |
| TMS + Al | 3.0 (0.0, 8.0) * | 7.0 (0.0, 9.0) * |
| Groups | Whole Fish (µg Hg/g Fish) | Whole Fish (µg Al/g Fish) |
|---|---|---|
| 24 h | ||
| Sal | 0.03 ± 0.02 | 0.29 ± 0.27 |
| TMS | 0.19 ± 0.15 | 0.12 ± 0.12 |
| Al | 0.03 ± 0.01 | 0.21 ± 0.06 |
| TMS + Al | 0.38 ± 0.15 * | 32.38 ± 17.48 * |
| 96 h | ||
| Sal | 0.04 ± 0.01 | 0.18 ± 0.17 |
| TMS | 0.26 ± 0.08 * | 0.12 ± 0.14 |
| Al | 0.05 ± 0.01 | 6053.0 ± 10,476.0 |
| TMS + Al | 0.05 ± 0.04 | 9.32 ± 8.41 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Galiciolli, M.E.A.; Silva, J.F.; Prodocimo, M.M.; Laureano, H.A.; Calado, S.L.d.M.; Oliveira, C.S.; Guiloski, I.C. Toxicological Effects of Thimerosal and Aluminum in the Liver, Kidney, and Brain of Zebrafish (Danio rerio). Metabolites 2023, 13, 975. https://doi.org/10.3390/metabo13090975
Galiciolli MEA, Silva JF, Prodocimo MM, Laureano HA, Calado SLdM, Oliveira CS, Guiloski IC. Toxicological Effects of Thimerosal and Aluminum in the Liver, Kidney, and Brain of Zebrafish (Danio rerio). Metabolites. 2023; 13(9):975. https://doi.org/10.3390/metabo13090975
Chicago/Turabian StyleGaliciolli, Maria Eduarda Andrade, Juliana Ferreira Silva, Maritana Mela Prodocimo, Henrique Aparecido Laureano, Sabrina Loise de Morais Calado, Claudia Sirlene Oliveira, and Izonete Cristina Guiloski. 2023. "Toxicological Effects of Thimerosal and Aluminum in the Liver, Kidney, and Brain of Zebrafish (Danio rerio)" Metabolites 13, no. 9: 975. https://doi.org/10.3390/metabo13090975






