Resistant Potato Starch Supplementation Reduces Serum Free Fatty Acid Levels and Influences Bile Acid Metabolism
Abstract
1. Introduction
2. Materials and Methods
2.1. Investigational Products
2.2. Study Participants
2.3. Clinical Trial Design and Execution
2.4. FFA and Ketone Body Analysis
2.5. Bile Acid Analysis
2.6. Statistical Analysis
3. Results
3.1. RPS Decreases Circulating Free Fatty Acid Levels
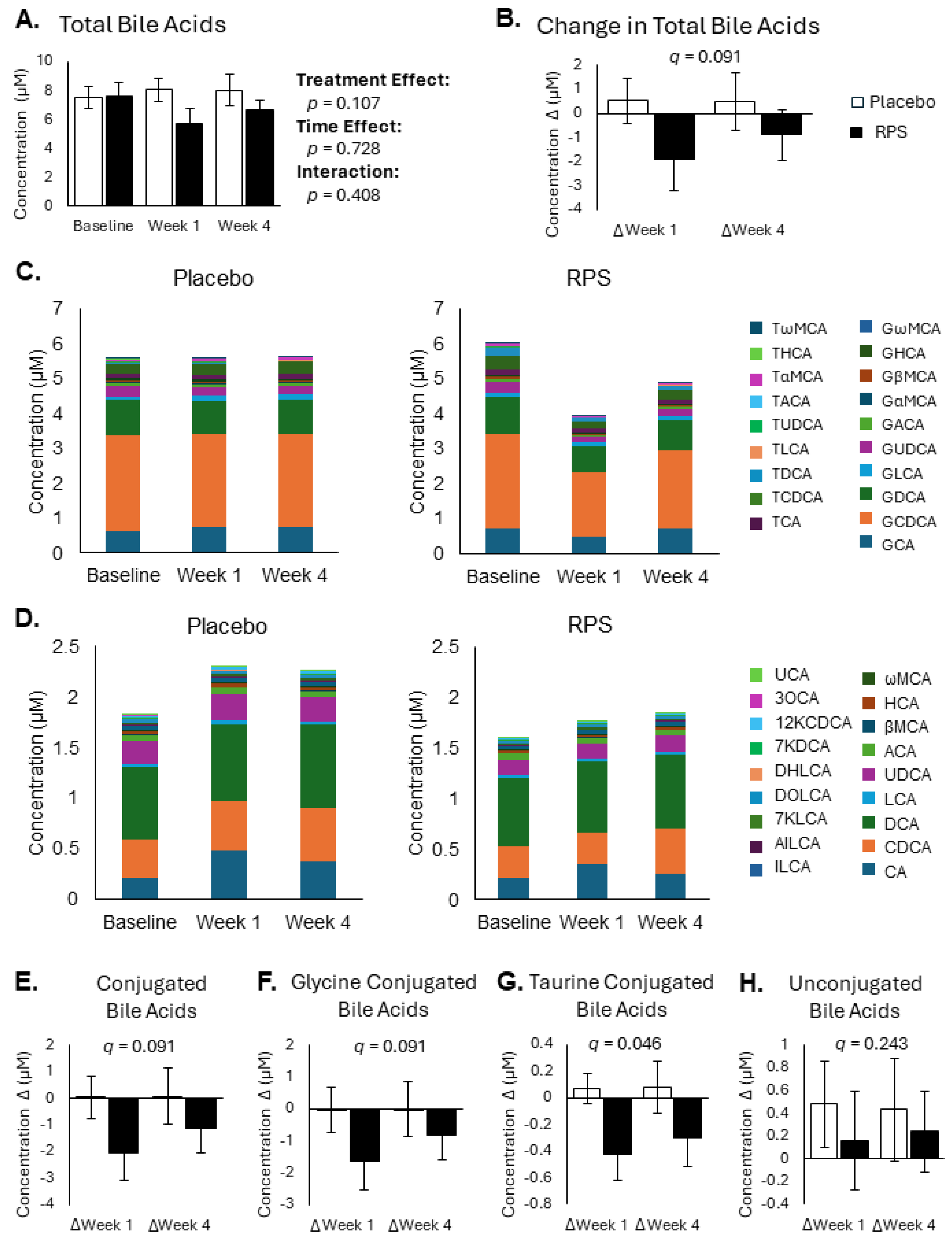
3.2. Conjugated Secondary BAs Were Affected by RPS
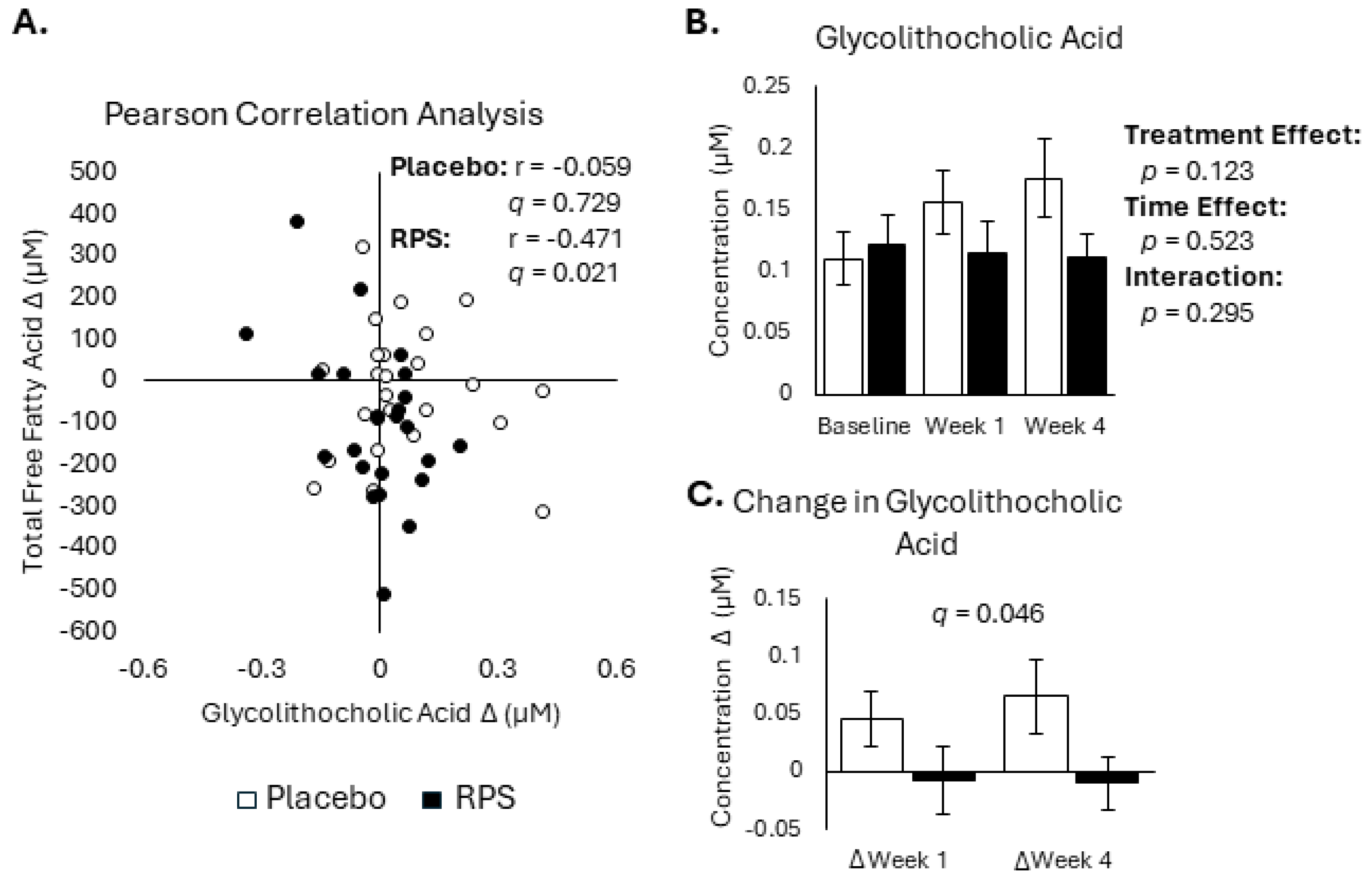
3.3. Decreased FFA Levels Correlate with Reductions in Bile Acids
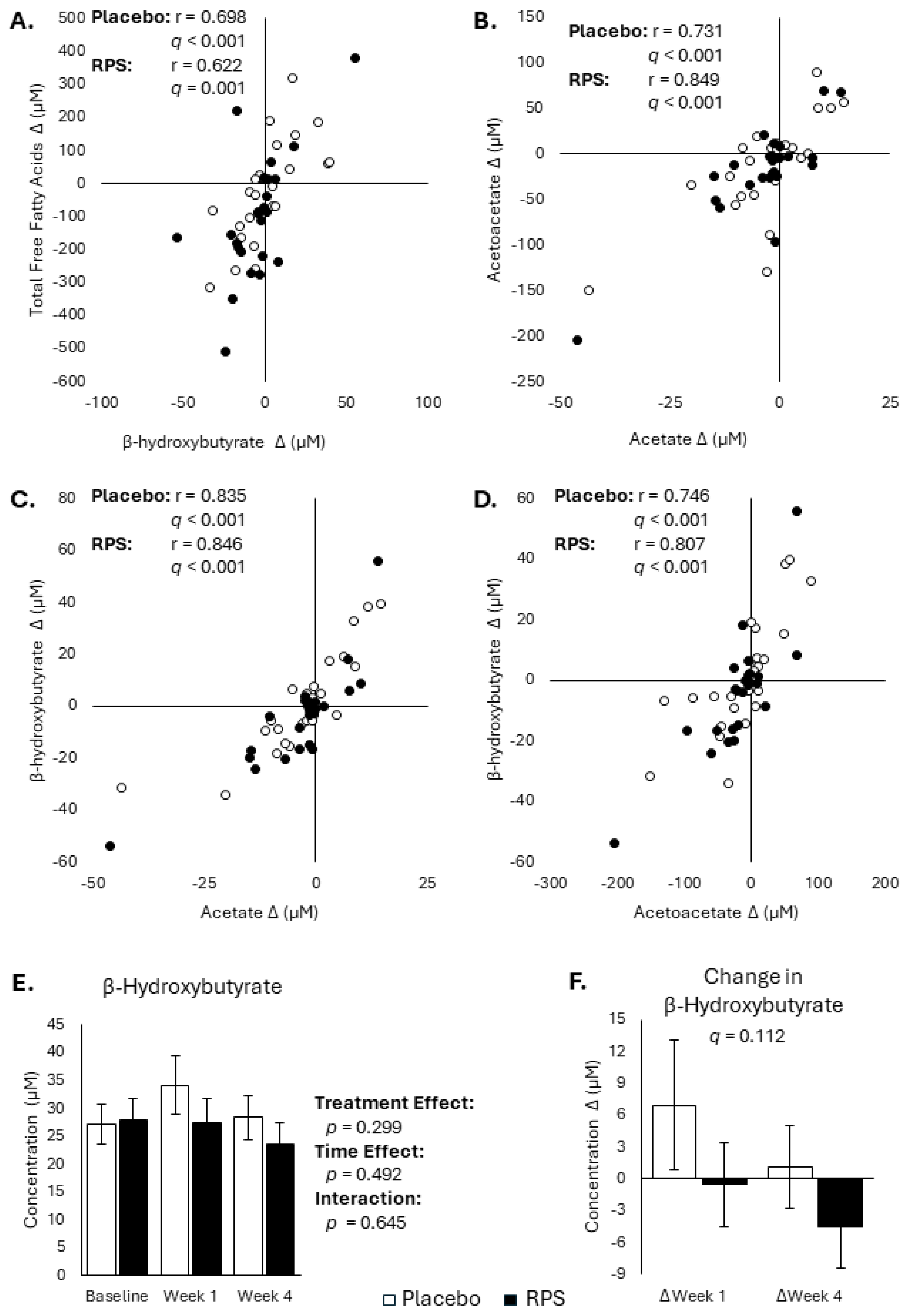
3.4. Changes in FFAs and Ketone Body Are Inter-Correlated
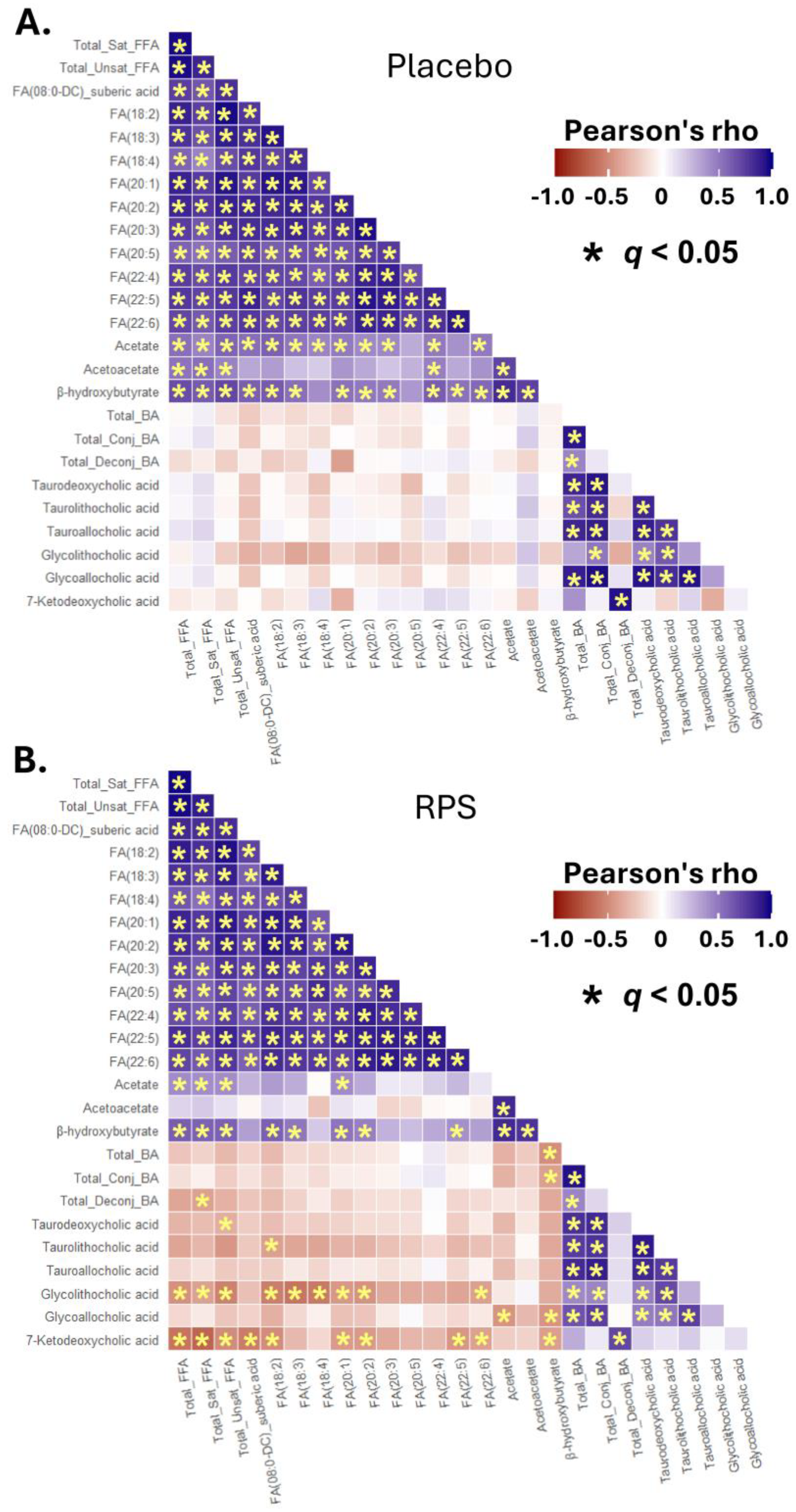
3.5. Inter-Correlations between Changes in Select BAs, FFAs, and Ketone Bodies
4. Discussion
5. Conclusions
6. Patents
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fonesca, V.A. Defining and Characterizing the Progression of Type 2 Diabetes. Diabetes Care 2009, 32, S151–S156. [Google Scholar] [CrossRef] [PubMed]
- Johnson, J.D. On the causal relationships between hyperinsulinaemia, insulin resistance, obesity and dysglycaemia in type 2 diabetes. Diabeologia 2021, 64, 2138–2146. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, E.; Li, S.; Lamptey, R.; Webb, D.R.; Davies, M.J. Type 2 diabetes. Lancet 2022, 400, 1803–1820. [Google Scholar] [CrossRef] [PubMed]
- Titchenell, P.M.; Quinn, Q.J.; Lu, M.; Chu, Q.; Lu, W.; Li, C.; Chen, H.; Monks, B.R.; Chen, J.; Rabinowitz, J.D.; et al. Direct Hepatocyte Insulin Signaling Is Required for Lipogenesis but Is Dispensable for the Suppression of Glucose Production. Cell Metab. 2016, 23, 1154–1166. [Google Scholar] [CrossRef] [PubMed]
- Henderson, G.C. Plasma Free Fatty Acid Concentration as a Modifiable Risk Factor for Metabolic Disease. Nutrients 2021, 13, 2590. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention. National Diabetes Statistics Report. Available online: https://www.cdc.gov/diabetes/php/data-research/?CDC_AAref_Val=https://www.cdc.gov/diabetes/data/statistics-report/index.html (accessed on 23 July 2024).
- Maiya, M.; Adorno, A.; Toulabi, S.B.; Tucker, W.J.; Patterson, M.A. Resistant starch improves cardiometabolic disease outcomes: A narrative review of randomized trials. Nutr. Res. 2023, 114, 20–40. [Google Scholar] [CrossRef]
- Balentine, D. High Amylose Maize Starch and Reduced Risk Type 2 Diabetes Mellitus. Available online: https://www.fda.gov/media/103626/download (accessed on 5 August 2024).
- Englyst, H.N.; Kingman, S.M.; Hudson, G.J.; Cummings, J.H. Measurement of resistant starch in vitro and in vivo. Br. J. Nutr. 1996, 75, 749–755. [Google Scholar] [CrossRef]
- Sajilata, M.G.; Singhal, R.S.; Kulkarni, P.R. Resistant Starch—A Review. Comp. Rev. Food Sci. Food Saf. 2006, 5, 1–17. [Google Scholar] [CrossRef]
- Baghurst, P.A.; Baghurst, K.I.; Record, S.J. Dietary Fibre, non-starch polysaccharides and resistant starch. A review. Food Aust. 1996, 48, S3–S35. [Google Scholar]
- Alfa, M.J.; Strang, D.; Tappia, P.S.; Graham, M.; Van Domselaar, G.; Forbes, J.D.; Laminman, V.; Olson, N.; DeGagne, P.; Bray, D.; et al. A randomized trial to determine the impact of a digestion resistant starch composition on the gut microbiome in older and mid-age adults. Clin. Nutr. 2018, 37, 797–807. [Google Scholar] [CrossRef]
- Zhang, L.; Ouyang, Y.; Li, H.; Shen, L.; Ni, Y.; Fang, Q.; Wu, G.; Qian, L.; Xiao, Y.; Zhang, J.; et al. Metabolic phenotypes and the gut microbiota in response to dietary resistant starch type 2 in normal-weight subjects: A randomized crossover trial. Sci. Rep. 2019, 9, 4736. [Google Scholar] [CrossRef] [PubMed]
- Hughes, R.L.; Horn, W.H.; Finnegan, P.; Newman, J.W.; Marco, M.L.; Keim, N.L.; Kable, M.E. Resistant Starch Type 2 from Wheat Reduces Postprandial Glycemic Response with Concurrent Alterations in Gut Microbiota Composition. Nutrients 2021, 13, 645. [Google Scholar] [CrossRef] [PubMed]
- Gondalia, S.J.; Wymond, B.; Benassi-Evans, B.; Berbezy, P.; Bird, A.R.; Belobrajdic, D.P. Substitution of Refined Conventional Wheat Flour with Wheat High in Resistant Starch Modulates the Intestinal Microbiota and Fecal Metabolites in Healthy Adults: A Randomized, Controlled Trial. J. Nutr. 2022, 152, 1426–1437. [Google Scholar] [CrossRef] [PubMed]
- DeMartino, P.; Johnston, E.A.; Petersen, K.S.; Kris-Etherton, P.M.; Cockburn, D.W. Additional Resistant Starch from One Potato Side Dish per Day Alters the Gut Microbiota but Not Fecal Short-Chain Fatty Acid Concentrations. Nutrients 2022, 14, 721. [Google Scholar] [CrossRef] [PubMed]
- Bush, J.R.; Baisley, J.; Harding, S.V.; Alfa, M.J. Consumption of Solnul™ Resistant Potato Starch Produces a Prebiotic Effect in a Randomized, Placebo-Controlled Clinical Trial. Nutrients 2023, 15, 1582. [Google Scholar] [CrossRef]
- DeMartino, P.; Cockburn, D.W. Resistant starch: Impact on the gut microbiome and health. Curr. Opin. Biotechnol. 2020, 61, 66–71. [Google Scholar] [CrossRef]
- Bodinham, C.L.; Al-Mana, N.M.; Smith, L.; Robertson, M.D. Endogenous plasma glucagon-like peptide-1 following acute dietary fibre consumption. Br. J. Nutr. 2013, 110, 1429–1433. [Google Scholar] [CrossRef]
- Tachon, S.; Zhou, J.; Keenan, M.; Martin, R.; Marco, M.L. The intestinal microbiota in aged mice is modulated by dietary resistant starch and correlated with improvements in host responses. FEMS Microbiol. Ecol. 2013, 83, 299–309. [Google Scholar] [CrossRef]
- Vidrine, K.; Ye, J.; Martin, R.J.; McCutcheon, K.L.; Raggio, A.M.; Pelkman, C.; Durham, H.A.; Zhou, J.; Senevirathne, R.N.; Williams, C.; et al. Resistant starch from high amylose maize (HAM-RS2) and dietary butyrate reduce abdominal fat by a different apparent mechanism. Obesity 2014, 22, 344–348. [Google Scholar] [CrossRef]
- Robertson, M.D.; Bickerton, A.S.; Dennis, A.L.; Vidal, H.; Frayn, K.N. Insulin sensitizing effects of dietary resistant starch and effects on skeletal muscle and adipose tissue metabolism. Am. J. Clin. Nutr. 2005, 82, 559–567. [Google Scholar] [CrossRef]
- Maki, K.C.; Pelkman, C.L.; Finocchiaro, E.T.; Kelley, K.M.; Lawless, A.L.; Schild, A.L.; Rains, T.M. Resistant starch from high-amylose maize increases insulin sensitivity in overweight and obese men. J. Nutr. 2012, 142, 717–723. [Google Scholar] [CrossRef]
- Robertson, M.D.; Wright, J.W.; Loizon, E.; Debard, C.; Vidal, H.; Shojaee-Moradie, F.; Russell-Jones, D.; Umpleby, A.M. Insulin-sensitizing effects on muscle and adipose tissue after dietary fiber intake in men and women with metabolic syndrome. J. Clin. Endocrinol. Metab. 2012, 97, 3326–3332. [Google Scholar] [CrossRef] [PubMed]
- Gower, B.A.; Bergman, R.; Stefanovski, D.; Darnell, B.; Ovalle, F.; Fisher, G.; Sweatt, S.K.; Resuehr, H.S.; Pelkman, C. Baseline insulin sensitivity affects response to high-amylose maize resistant starch in women: A randomized, controlled trial. Nutr. Metab. 2016, 13, 2. [Google Scholar] [CrossRef]
- Alfa, M.J.; Strang, D.; Tappia, P.S.; Olson, N.; DeGagne, P.; Bray, D.; Murray, B.-L.; Hiebert, B. A Randomized Placebo Controlled Clinical Trial to Determine the Impact of Digestion Resistant Starch MSPrebiotic® on Glucose, Insulin, and Insulin Resistance in Elderly and Mid-Age Adults. Front. Med. 2018, 4, 260. [Google Scholar] [CrossRef] [PubMed]
- Costa, E.S.; França, C.N.; Fonseca, F.A.H.; Kato, J.T.; Bianco, H.T.; Freitas, T.T.; Fonseca, H.A.R.; Figueiredo Neto, A.M.; Izar, M.C. Beneficial effects of green banana biomass consumption in patients with pre-diabetes and type 2 diabetes: A randomised controlled trial. Br. J. Nutr. 2019, 121, 1365–1375. [Google Scholar] [CrossRef]
- Sanders, L.M.; Dicklin, M.R.; Palacios, O.M.; Maki, C.E.; Wilcox, M.L.; Maki, K.C. Effects of potato resistant starch intake on insulin sensitivity, related metabolic markers and appetite ratings in men and women at risk for type 2 diabetes: A pilot cross-over randomised controlled trial. J. Hum. Nutr. Diet. 2021, 34, 94–105. [Google Scholar] [CrossRef] [PubMed]
- García-Vázquez, C.; Ble-Castillo, J.L.; Arias-Córdova, Y.; Ramos-García, M.; Olvera-Hernández, V.; Guzmán-Priego, C.G.; Martínez-López, M.C.; Jiménez-Domínguez, G.; Hernández-Becerra, J.A. Effects of resistant starch on glycemic response, postprandial lipemia and appetite in subjects with type 2 diabetes. Eur. J. Nutr. 2023, 62, 2269–2278. [Google Scholar] [CrossRef]
- Li, H.; Zhang, L.; Li, J.; Wu, Q.; Qian, L.; He, J.; Ni, Y.; Kovatcheva-Datchary, P.; Yuan, R.; Liu, S.; et al. Resistant starch intake facilitates weight loss in humans by reshaping the gut microbiota. Nat. Metab. 2024, 6, 578–579. [Google Scholar] [CrossRef]
- Bush, J.R.; Han, J.E.; Deehan, E.C.; Harding, S.V.; Maiya, M.; Baisley, J.; Schibli, D.; Goodlett, D.R. Resistant potato starch supplementation reduces serum histamine levels in healthy adults with links to attenuated intestinal permeability. J. Funct. Foods 2023, 108, 105740. [Google Scholar] [CrossRef]
- Bush, J.R.; Alfa, M.J. Consumption of resistant potato starch produces changes in gut microbiota that correlate with improvements in abnormal bowel symptoms: A secondary analysis of a clinical trial. BMC Nutr. 2024. submitted. [Google Scholar]
- Nelson, A.B.; Queathem, E.D.; Puchalska, P.; Crawford, P.A. Metabolic Messengers: Ketone bodies. Nat. Metab. 2023, 5, 2062–2074. [Google Scholar] [CrossRef] [PubMed]
- Mohanty, I.; Allaband, C.; Mannochio-Russo, H.; El Abiead, Y.; Hagey, L.R.; Knight, R.; Dorrestein, P.C. The changing metabolic landscape of bile acids—keys to metabolism and immune regulation. Nat. Rev. Gastroenterol. Hepatol. 2024, 21, 493–516. [Google Scholar] [CrossRef] [PubMed]
- Tong, C.; Ma, Z.; Chen, H.; Gao, H. Toward an understanding of potato starch structure, function, biosynthesis, and applications. Food Front. 2023, 4, 980–1000. [Google Scholar] [CrossRef]
- Deehan, E.C.; Yang, C.; Perez-Muñoz, M.E.; Nguyen, N.K.; Cheng, C.C.; Triador, L.; Zhang, Z.; Bakal, J.A.; Walter, J. Precision Microbiome Modulation with Discrete Dietary Fiber Structures Directs Short-Chain Fatty Acid Production. Cell Host Microbe 2020, 27, 389–404. [Google Scholar] [CrossRef]
- Han, J.; Gagnon, S.; Eckle, T.; Borchers, C.H. Metabolomic analysis of key central carbon metabolism carboxylic acids as their 3-nitrophenylhydrazones by UPLC/ESI-MS. Electrophoresis 2013, 34, 2891–2900. [Google Scholar] [CrossRef]
- Han, J.; Lin, K.; Sequeira, C.; Borchers, C.H. An isotope-labeled chemical derivatization method for the quantitation of short-chain fatty acids in human feces by liquid chromatography-tandem mass spectrometry. Anal. Chim. Acta 2015, 854, 86–94. [Google Scholar] [CrossRef]
- Liu, T.; Wang, R.-X.; Han, J.; Hao, C.-Z.; Qiu, Y.-L.; Yan, Y.-Y.; Li, L.-T.; Wang, N.-L.; Gong, J.-Y.; Lu, Y.; et al. Comprehensive bile acid profiling in hereditary intrahepatic cholestasis: Genetic and clinical correlations. Liver Int. 2018, 38, 1676–1685. [Google Scholar] [CrossRef]
- Storey, J.D.; Tibshirani, R. Statistical significance for genome wide studies. Proc. Natl. Acad. Sci. USA 2003, 100, 9440–9445. [Google Scholar] [CrossRef]
- Wickham, H. ggplot2: Elegant Graphics for Data Analysis, 2nd ed.; Springer International Publishing: Cham, Switzerland, 2016. [Google Scholar] [CrossRef]
- Sayin, S.I.; Wahlström, A.; Felin, J.; Jäntti, S.; Marschall, H.-U.; Bamberg, K.; Angelin, B.; Hyötyläinen, T.; Orešič, M.; Bäckhed, F. Gut microbiota regulates bile acid metabolism by reducing the levels of tauro-beta-muricholic acid, a naturally occurring FXR antagonist. Cell Metab. 2013, 17, 225–235. [Google Scholar] [CrossRef]
- Shapiro, H.; Kolodziejcczyk, A.A.; Halstuch, D.; Elinav, E. Bile acids in glucose metabolism in health and disease. J. Exp. Med. 2018, 215, 383–396. [Google Scholar] [CrossRef]
- Petersen, A.Ø.; Julienne, H.; Hyötyläinen, T.; Sen, P.; Fan, Y.; Pedersen, H.K.; Jäntti, S.; Hansen, T.H.; Nielsen, T.; Jørgensen, T.; et al. Conjugated C-6 hydroxylated bile acids in serum relate to human metabolic health and gut Clostridia species. Sci. Rep. 2021, 11, 13252. [Google Scholar] [CrossRef] [PubMed]
- Bodinham, C.L.; Smith, L.; Thomas, E.L.; Bell, J.D.; Swann, J.R.; Costabile, A.; Russell-Jones, D.; Umpleby, A.M.; Robertson, M.D. Efficacy of increased resistant starch consumption in human type 2 diabetes. Endocr. Connect. 2014, 3, 75–84. [Google Scholar] [CrossRef] [PubMed]
- Maziarz, M.P.; Preisendanz, S.; Juma, S.; Imrhan, V.; Prasad, C.; Vijayagopal, P. Resistant starch lowers postprandial glucose and leptin in overweight adults consuming a moderate-to-high-fat diet: A randomized-controlled trial. Nutr. J. 2017, 16, 14. [Google Scholar] [CrossRef] [PubMed]
- Miketinas, D.C.; Shankar, K.; Maiya, M.; Patterson, M.A. Usual Dietary Intake of Resistant Starch in US Adults from NHANES 2015–2016. J. Nutr. 2020, 150, 2738–3747. [Google Scholar] [CrossRef]
- Wang, K.; Hasjim, J.; Wu, A.C.; Henry, R.J.; Gilbert, R.G. Variation in amylose fine structure of starches from different botanical sources. J. Agric. Food Chem. 2014, 62, 4443–4453. [Google Scholar] [CrossRef]
- Blennow, A.; Engelsen, S.B.; Munck, L.; Mollera, B.L. Starch molecular structure and phosphorylation investigated by a combined chromatographic and chemometric approach. Carbohydr. Polym. 2000, 41, 163–174. [Google Scholar] [CrossRef]
- Perez, S.; Bertoft, E. The molecular structures of starch components and their contribution to the architecture of starch granules: A comprehensive review. Starch 2010, 62, 389–420. [Google Scholar] [CrossRef]
- Vamadevan, V.; Bertoft, E. Structure-function relationships of starch components. Starch 2015, 67, 55–68. [Google Scholar] [CrossRef]
- Dhital, S.; Shrestha, A.K.; Gidley, M.J. Relationship between granule size and in vitro digestibility of maize and potato starches. Carbohydr. Polym. 2010, 82, 480–488. [Google Scholar] [CrossRef]
- Hanes, D.; Nowinski, B.; Lamb, J.J.; Larson, I.A.; McDonald, D.; Knight, R.; Song, S.J.; Patno, N. The gastrointestinal and microbiome impact of a resistant starch blend from potato, banana, and apple fibers: A randomized clinical trial using smart caps. Front. Nutr. 2022, 9, 987216. [Google Scholar] [CrossRef]
- Yoon, H.S.; Cho, C.H.; Yun, M.S.; Jang, S.J.; You, H.J.; Kim, J.-H.; Han, D.; Cha, K.H.; Moon, S.H.; Lee, K.; et al. Akkermansia muciniphila secretes a glucagon-like peptide-1-inducing protein that improves glucose homeostasis and ameliorates metabolic disease in mice. Nat. Microbiol. 2021, 6, 563–573. [Google Scholar] [CrossRef] [PubMed]
- Rideout, T.C.; Harding, S.V.; Raslawsky, A.; Rempel, C.B. Dietary Resistant Starch Supplementation Increases High-Density Lipoprotein Particle Number in Pigs Fed a Western Diet. J. Diet. Suppl. 2016, 14, 334–345. [Google Scholar] [CrossRef] [PubMed]
- Lambert, J.E.; Parks, E.J. Postprandial metabolism of meal triglyceride in humans. Biochim. Biophys. Acta 2012, 1821, 721–726. [Google Scholar] [CrossRef] [PubMed]
- Makki, K.; Deehan, E.C.; Walter, J.; Bäckhed, F. The Impact of Dietary Fiber on Gut Microbiota in Host Health and Disease. Cell Host Microbe 2018, 23, 705–715. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bush, J.R.; Iwuamadi, I.; Han, J.; Schibli, D.J.; Goodlett, D.R.; Deehan, E.C. Resistant Potato Starch Supplementation Reduces Serum Free Fatty Acid Levels and Influences Bile Acid Metabolism. Metabolites 2024, 14, 536. https://doi.org/10.3390/metabo14100536
Bush JR, Iwuamadi I, Han J, Schibli DJ, Goodlett DR, Deehan EC. Resistant Potato Starch Supplementation Reduces Serum Free Fatty Acid Levels and Influences Bile Acid Metabolism. Metabolites. 2024; 14(10):536. https://doi.org/10.3390/metabo14100536
Chicago/Turabian StyleBush, Jason R., Izuchukwu Iwuamadi, Jun Han, David J. Schibli, David R. Goodlett, and Edward C. Deehan. 2024. "Resistant Potato Starch Supplementation Reduces Serum Free Fatty Acid Levels and Influences Bile Acid Metabolism" Metabolites 14, no. 10: 536. https://doi.org/10.3390/metabo14100536
APA StyleBush, J. R., Iwuamadi, I., Han, J., Schibli, D. J., Goodlett, D. R., & Deehan, E. C. (2024). Resistant Potato Starch Supplementation Reduces Serum Free Fatty Acid Levels and Influences Bile Acid Metabolism. Metabolites, 14(10), 536. https://doi.org/10.3390/metabo14100536









