Wheat Peptides as Catalysts for Athletic Performance Improvement in Cross-Country Skiers: A Randomized Controlled Trial
Abstract
1. Introduction
2. Materials and Methods
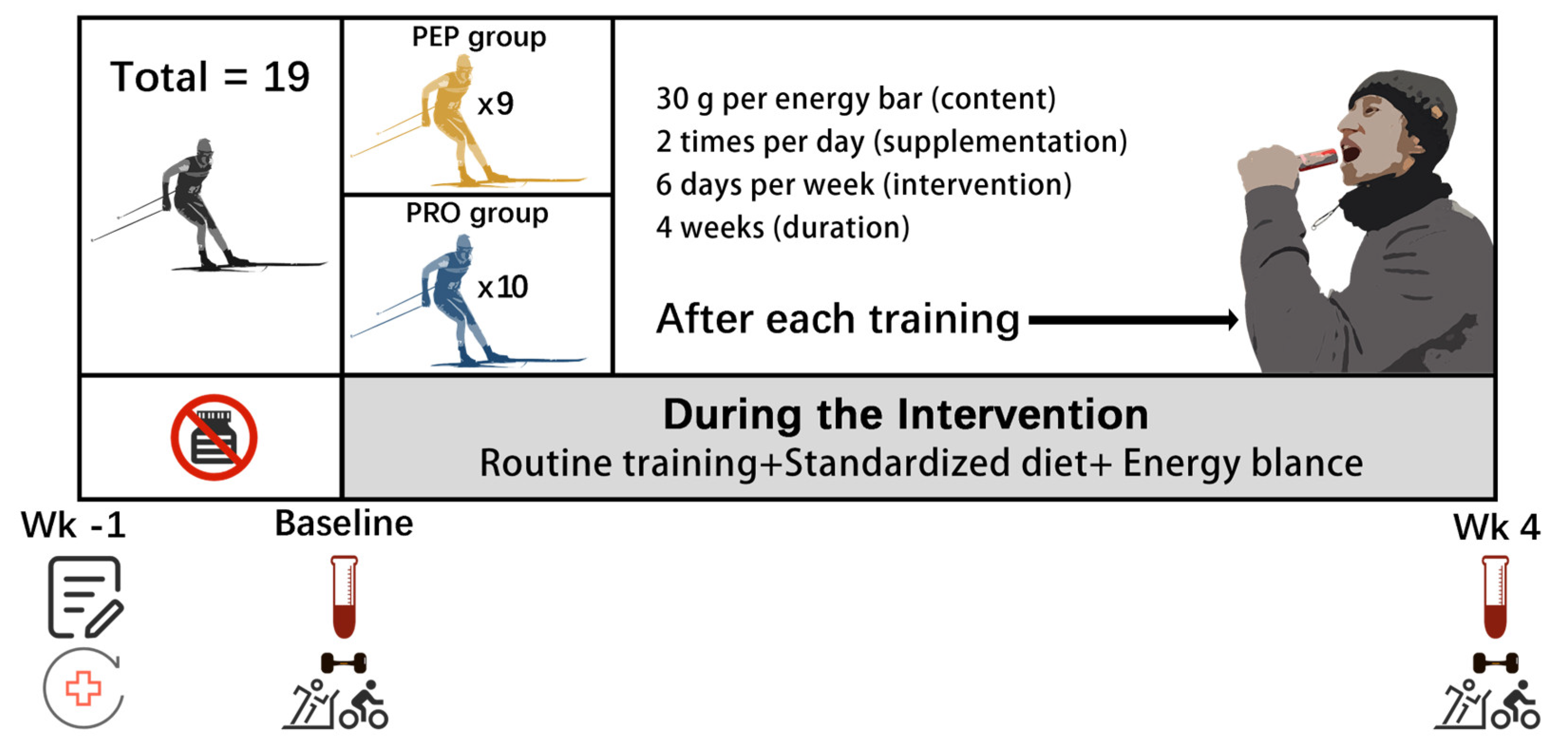
2.1. Participants
2.2. Study Design
2.3. Supplementation Protocol
2.4. Blood Collection
2.5. Athletic Performance Tests
2.5.1. Sport-Specific Ability Tests
- (1)
- The 30 m Sprint: The completion time for a maximum-effort 30 m sprint was recorded using TC-Timers (Brower, Sandy, USA);
- (2)
- The Hexagonal Agility Test: The time taken to complete three full hexagonal circuits was recorded using a Seiko SVAS009 stopwatch (Seiko Instruments Inc, Tokyo, Japan);
- (3)
- Relative Bench Press Strength: The maximum bench press weight relative to body weight was recorded;
- (4)
- The 1500 m Run: The completion time for a 1500 m run on a standard track was recorded with a Seiko SVAS009 stopwatch (Seiko Instruments Inc, Tokyo, Japan);
- (5)
- The 10 km Roller Skating Event: The completion time for a 10 km roller skating event was recorded with a Seiko SVAS009 stopwatch (Seiko Instruments Inc, Tokyo, Japan).
2.5.2. Aerobic Capacity Tests
- (1)
- Pre-exercise measurements: We measured the resting heart rate using monitors (Firstbeat, Jyväskylä, Finland), blood glucose using glucometers (Ecoing R2, Aikang Biotechnology Co., Ltd., Håangzhou, China), and blood lactate using portable lactate analyzers (EKF Lactate Scout 4, EKF Diagnostics, Cardiff, UK);
- (2)
- Warm-up: Subjects performed a 5–10 min warm-up prior to treadmill testing;
- (3)
- Preparation: We collected subjects’ personal details, attached heart rate monitors, and connected a respiratory mask and gas analyzer (METAMAX3B, Cortex Biophysical GmbH, Leipzig, Germany);
- (4)
- Testing protocol: The test started with a 2 min warm-up at 6 km/h and 0% grade, followed by incremental increases in speed and grade, during which we monitored heart rate, respiratory exchange ratio (RER), and Rating of Perceived Exertion (RPE);
- (5)
- Test termination: We ended testing when specific criteria were met, including an oxygen uptake plateau, small differences between stages, attainment of the maximum heart rate, high RER and RPE, or a subject’s inability to continue;
- (6)
- Post-exercise measurements: We recorded post-exercise blood glucose, RPE, heart rate, and blood lactate at 0, 5, and 10 min;
- (7)
- Data analysis: We analyzed VO2max, ventilatory threshold, and time to anaerobic threshold (AT), as well as heart rate, blood glucose, and lactate at AT.
2.5.3. Anaerobic Capacity and Strength Tests
- (1)
- Subjects performed a two-minute warm-up increasing their heart rate to 150–160 bpm, including 2–3 maximum 4–8 s sprints;
- (2)
- After two minutes of rest, subjects performed an all-out 30 s sprint against a constant load;
- (3)
- Revolutions were recorded every 5 s during the test as times were called, and power was calculated using the formula;
- (4)
- Post-test, the load was reduced, and subjects performed light activity for 2–3 min.
- (1)
- Peak power: Highest five-second power recorded;
- (2)
- Average power: Mean of the 6 five-second power values;
- (3)
- Fatigue index: (Peak power−minimum power)/peak power × 100;
- (4)
- The blood glucose level immediately after exercise and the blood lactate levels at 0, 5, 7, and 9 min after exercise.
2.6. Metabolomics Experiment
2.6.1. Metabolite Extraction
- (1)
- A 20 μL volume of each sample and standard was combined with 120 μL of protein precipitation solution, shaken at 1200 rpm for 30 min, and centrifuged at 18,000 g at 4 °C for 30 min;
- (2)
- A 30 μL volume of supernatant was transferred to a 96-well plate with 20 μL of derivatization reagent and 20 μL of EDC working solution;
- (3)
- The plate was sealed and incubated at 40 °C with shaking at 1200 rpm for 60 min;
- (4)
- The plate was centrifuged at 4000 g at 4 °C for 5 min, after which 30 μL was transferred to a new plate with 90 μL of dilution solvent and shaken at 600 rpm for 10 min;
- (5)
- The new plate was centrifuged at 4000 g at 4 °C for 30 min, sealed, and prepared for analysis.
2.6.2. UPLC-MS Analysis
2.7. Statistical Analysis
3. Results
3.1. Anthropometric and Physiological Parameters
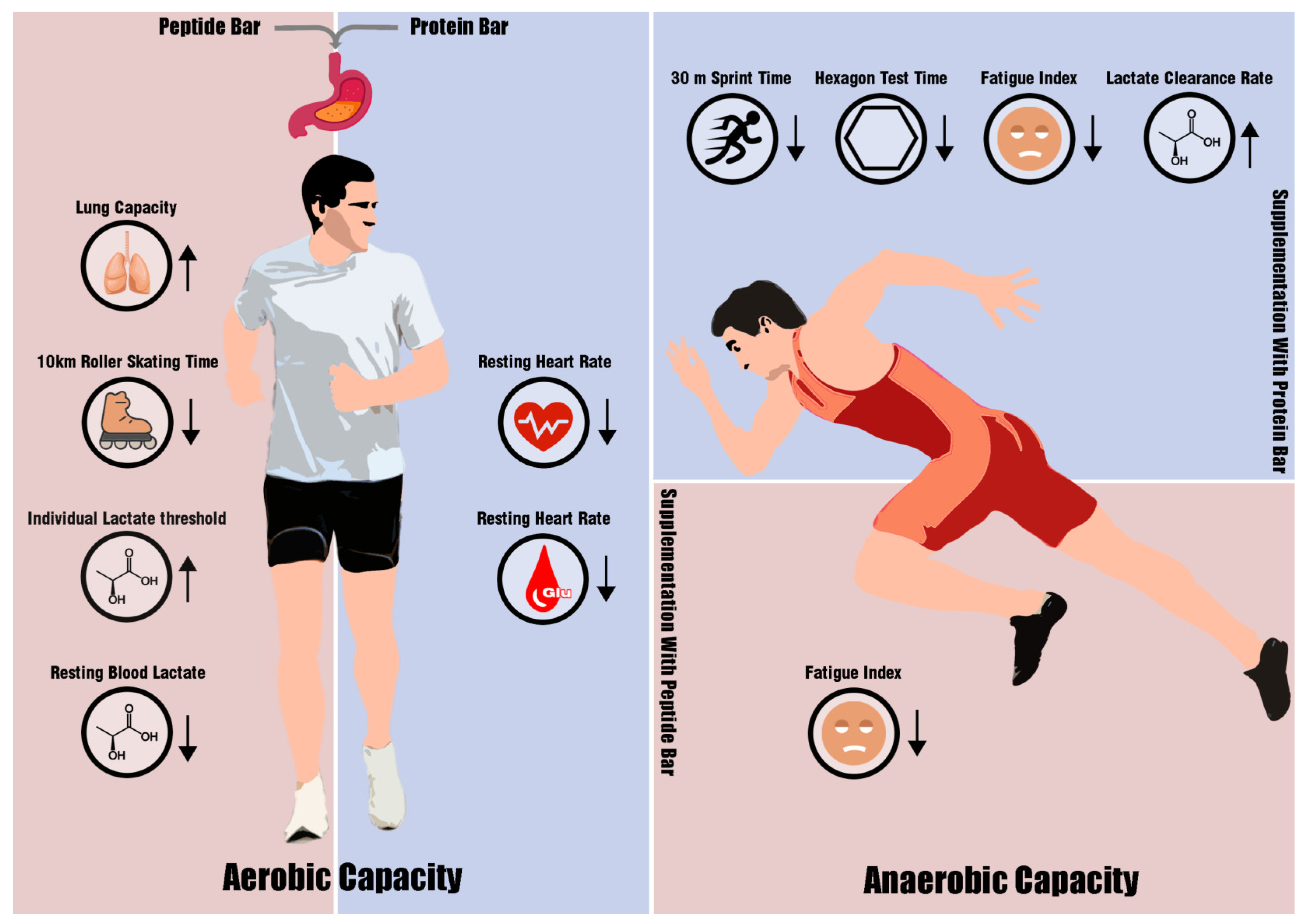
3.2. Athletic Performance
3.2.1. Aerobic Capacity
3.2.2. Anaerobic Capacity and Strength
3.3. Metabolomic Analysis
3.3.1. Multivariate Analysis
3.3.2. Univariate Analysis
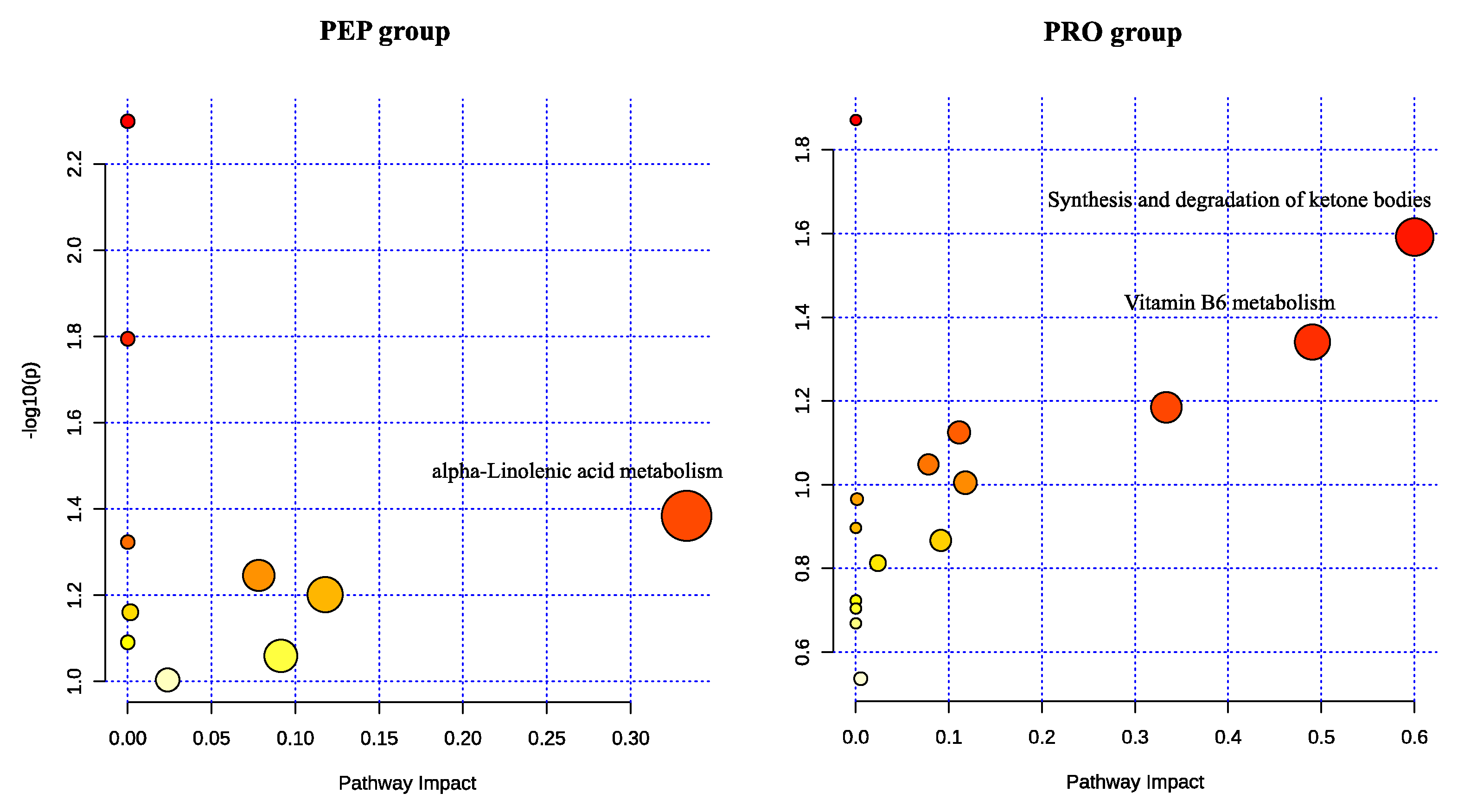
3.3.3. Functional Analysis of Metabolic Pathways
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Holmberg, H.-C. The elite cross-country skier provides unique insights into human exercise physiology. Scand. J. Med. Sci. Sports 2015, 25, 100–109. [Google Scholar] [CrossRef] [PubMed]
- Sandbakk, Ø.; Holmberg, H.-C. Physiological Capacity and Training Routines of Elite Cross-Country Skiers: Approaching the Upper Limits of Human Endurance. Int. J. Sports Physiol. Perform. 2017, 12, 1003–1011. [Google Scholar] [CrossRef] [PubMed]
- Hébert-Losier, K.; Zinner, C.; Platt, S.; Stöggl, T.; Holmberg, H.C. Factors that Influence the Performance of Elite Sprint Cross-Country Skiers. Sports Med. 2017, 47, 319–342. [Google Scholar] [CrossRef]
- Losnegard, T. Energy system contribution during competitive cross-country skiing. Eur. J. Appl. Physiol. 2019, 119, 1675–1690. [Google Scholar] [CrossRef]
- Sandbakk, Ø.; Holmberg, H.-C.; Leirdal, S.; Ettema, G. Metabolic rate and gross efficiency at high work rates in world class and national level sprint skiers. Eur. J. Appl. Physiol. 2010, 109, 473–481. [Google Scholar] [CrossRef]
- Rusko, H. The Handbooks of Sports Medicine and Science: Cross Country Skiing; John Wiley & Sons: Hoboken, NJ, USA, 2008. [Google Scholar]
- Heikura, I.A.; Kettunen, O.; Garthe, I.; Holmlund, H.; Sandbakk, S.B.; Valtonen, M.; Ihalainen, J.K. Energetic demands and nutritional strategies of elite cross-country skiers during tour de ski: A narrative review. J. Sci. Sport Exerc. 2021, 3, 224–237. [Google Scholar] [CrossRef]
- Tarnopolsky, M. Protein Requirements for Endurance Athletes. Eur. J. Sport Sci. 2004, 4, 1–15. [Google Scholar] [CrossRef]
- Moore, D.R. Nutrition to Support Recovery from Endurance Exercise: Optimal Carbohydrate and Protein Replacement. Curr. Sports Med. Rep. 2015, 14, 294–300. [Google Scholar] [CrossRef]
- Moore, D.R.; Camera, D.M.; Areta, J.L.; Hawley, J.A. Beyond muscle hypertrophy: Why dietary protein is important for endurance athletes. Appl. Physiol. Nutr. Metab. 2014, 39, 987–997. [Google Scholar] [CrossRef]
- Lin, Y.-N.; Tseng, T.-T.; Knuiman, P.; Chan, W.P.; Wu, S.-H.; Tsai, C.-L.; Hsu, C.-Y. Protein supplementation increases adaptations to endurance training: A systematic review and meta-analysis. Clin. Nutr. 2021, 40, 3123–3132. [Google Scholar] [CrossRef]
- Matthews, D.M.; Adibi, S.A. Peptide Absorption. Gastroenterology 1976, 71, 151–161. [Google Scholar] [CrossRef]
- Addison, J.M.; Burston, D.; Matthews, D. Evidence for active transport of the dipeptide glycylsarcosine by hamster jejunum in vitro. Clin. Sci. 1972, 43, 907–911. [Google Scholar] [CrossRef]
- Gardner, M. Absorption of amino acids and peptides from a complex mixture in the isolated small intestine of the rat. J. Physiol. 1975, 253, 233–256. [Google Scholar] [CrossRef]
- Tagari, H.; Webb, K., Jr.; Theurer, B.; Huber, T.; De Young, D.; Cuneo, P.; Santos, J.; Simas, J.; Sadik, M.; Alio, A. Mammary uptake, portal-drained visceral flux, and hepatic metabolism of free and peptide-bound amino acids in cows fed steam-flaked or dry-rolled sorghum grain diets. J. Dairy Sci. 2008, 91, 679–697. [Google Scholar] [CrossRef]
- Kitakaze, T.; Sakamoto, T.; Kitano, T.; Inoue, N.; Sugihara, F.; Harada, N.; Yamaji, R. The collagen derived dipeptide hydroxyprolyl-glycine promotes C2C12 myoblast differentiation and myotube hypertrophy. Biochem. Biophys. Res. Commun. 2016, 478, 1292–1297. [Google Scholar] [CrossRef]
- Zdzieblik, D.; Oesser, S.; Baumstark, M.W.; Gollhofer, A.; König, D. Collagen peptide supplementation in combination with resistance training improves body composition and increases muscle strength in elderly sarcopenic men: A randomised controlled trial. Br. J. Nutr. 2015, 114, 1237–1245. [Google Scholar] [CrossRef]
- Clifford, T.; Ventress, M.; Allerton, D.M.; Stansfield, S.; Tang, J.C.Y.; Fraser, W.D.; Vanhoecke, B.; Prawitt, J.; Stevenson, E. The effects of collagen peptides on muscle damage, inflammation and bone turnover following exercise: A randomized, controlled trial. Amino Acids 2019, 51, 691–704. [Google Scholar] [CrossRef]
- Hansen, M.; Bangsbo, J.; Jensen, J.; Bibby, B.M.; Madsen, K. Effect of Whey Protein Hydrolysate on Performance and Recovery of Top-Class Orienteering Runners. Int. J. Sport Nutr. Exerc. Metab. 2015, 25, 97–109. [Google Scholar] [CrossRef]
- Jendricke, P.; Kohl, J.; Centner, C.; Gollhofer, A.; König, D. Influence of Specific Collagen Peptides and Concurrent Training on Cardiometabolic Parameters and Performance Indices in Women: A Randomized Controlled Trial. Front. Nutr. 2020, 7, 580918. [Google Scholar] [CrossRef]
- Oosthuyse, T.; Carstens, M.; Millen, A.M.E. Whey or Casein Hydrolysate with Carbohydrate for Metabolism and Performance in Cycling. Int. J. Sports Med. 2015, 36, 636–646. [Google Scholar] [CrossRef]
- Lollo, P.C.B.; Amaya-Farfan, J.; Faria, I.C.; Salgado, J.V.V.; Chacon-Mikahil, M.P.T.; Cruz, A.G.; Oliveira, C.A.F.; Montagner, P.C.; Arruda, M. Hydrolysed whey protein reduces muscle damage markers in Brazilian elite soccer players compared with whey protein and maltodextrin. A twelve-week in-championship intervention. Int. Dairy J. 2014, 34, 19–24. [Google Scholar] [CrossRef]
- Qu, Y.; Ji, H.; Song, W.; Peng, S.; Zhan, S.; Wei, L.; Chen, M.; Zhang, D.; Liu, S. The anti-fatigue effect of the Auxis thazard oligopeptide via modulation of the AMPK/PGC-1α pathway in mice. Food Funct. 2022, 13, 1641–1650. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Z.-Q.; Geng, Z.-H.; Liu, J.-X.; Guo, S.-T. Compressed food with added functional oligopeptides improves performance during military endurance training. Asia Pac. J. Clin. Nutr. 2017, 26, 1066–1075. [Google Scholar] [PubMed]
- Morgan, P.T.; Breen, L. The role of protein hydrolysates for exercise-induced skeletal muscle recovery and adaptation: A current perspective. Nutr. Metab. 2021, 18, 44. [Google Scholar] [CrossRef]
- Cohen, J. Statistical Power Analysis for the Behavioral Sciences; Academic Press: Cambridge, MA, USA, 2013. [Google Scholar]
- Worley, B.; Powers, R. Multivariate analysis in metabolomics. Curr. Metabolomics 2013, 1, 92–107. [Google Scholar]
- Worley, B.; Powers, R. PCA as a practical indicator of OPLS-DA model reliability. Curr. Metabolomics 2016, 4, 97–103. [Google Scholar] [CrossRef]
- Chen, Y.; Li, E.-M.; Xu, L.-Y. Guide to Metabolomics Analysis: A Bioinformatics Workflow. Metabolites 2022, 12, 357. [Google Scholar] [CrossRef]
- Szymańska, E.; Saccenti, E.; Smilde, A.K.; Westerhuis, J.A. Double-check: Validation of diagnostic statistics for PLS-DA models in metabolomics studies. Metabolomics 2012, 8, 3–16. [Google Scholar] [CrossRef]
- Philp, A.; Macdonald, A.L.; Watt, P.W. Lactate–a signal coordinating cell and systemic function. J. Exp. Biol. 2005, 208, 4561–4575. [Google Scholar] [CrossRef]
- Yang, W.-H.; Park, H.; Grau, M.; Heine, O. Decreased Blood Glucose and Lactate: Is a Useful Indicator of Recovery Ability in Athletes? Int. J. Environ. Res. Public Health 2020, 17, 5470. [Google Scholar] [CrossRef]
- Colberg, S.R.; Hernandez, M.J.; Shahzad, F. Blood glucose responses to type, intensity, duration, and timing of exercise. Diabetes Care 2013, 36, e177. [Google Scholar] [CrossRef] [PubMed]
- Beneke, R.; Leithäuser, R.M.; Ochentel, O. Blood lactate diagnostics in exercise testing and training. Int. J. Sports Physiol. Perform. 2011, 6, 8–24. [Google Scholar] [CrossRef] [PubMed]
- Cintineo, H.P.; Arent, M.A.; Antonio, J.; Arent, S.M. Effects of protein supplementation on performance and recovery in resistance and endurance training. Front. Nutr. 2018, 5, 83. [Google Scholar] [CrossRef] [PubMed]
- Kloby Nielsen, L.L.; Tandrup Lambert, M.N.; Jeppesen, P.B. The effect of ingesting carbohydrate and proteins on athletic performance: A systematic review and meta-analysis of randomized controlled trials. Nutrients 2020, 12, 1483. [Google Scholar] [CrossRef]
- Hussein, N.; Ah-Sing, E.; Wilkinson, P.; Leach, C.; Griffin, B.A.; Millward, D.J. Long-chain conversion of [13C]linoleic acid and alpha-linolenic acid in response to marked changes in their dietary intake in men. J. Lipid Res. 2005, 46, 269–280. [Google Scholar] [CrossRef]
- Zárate, R.; el Jaber-Vazdekis, N.; Tejera, N.; Pérez, J.A.; Rodríguez, C. Significance of long chain polyunsaturated fatty acids in human health. Clin. Transl. Med. 2017, 6, 25. [Google Scholar] [CrossRef]
- Burdge, G. Metabolism of α-linolenic acid in humans. Prostaglandins Leukot. Essent. Fat. Acids 2006, 75, 161–168. [Google Scholar] [CrossRef]
- Fukao, T.; Lopaschuk, G.D.; Mitchell, G.A. Pathways and control of ketone body metabolism: On the fringe of lipid biochemistry. Prostaglandins Leukot. Essent. Fat. Acids 2004, 70, 243–251. [Google Scholar] [CrossRef]
- Cox, P.J.; Clarke, K. Acute nutritional ketosis: Implications for exercise performance and metabolism. Extrem. Physiol. Med. 2014, 3, 17. [Google Scholar] [CrossRef]
- Sansone, M.; Sansone, A.; Borrione, P.; Romanelli, F.; Di Luigi, L.; Sgrò, P. Effects of ketone bodies on endurance exercise. Curr. Sports Med. Rep. 2018, 17, 444–453. [Google Scholar] [CrossRef]
- Tambasco-Studart, M.; Titiz, O.; Raschle, T.; Forster, G.; Amrhein, N.; Fitzpatrick, T.B. Vitamin B6 biosynthesis in higher plants. Proc. Natl. Acad. Sci. 2005, 102, 13687–13692. [Google Scholar] [CrossRef] [PubMed]
- Ramos, R.J.; Albersen, M.; Vringer, E.; Bosma, M.; Zwakenberg, S.; Zwartkruis, F.; Jans, J.J.; Verhoeven-Duif, N.M. Discovery of pyridoxal reductase activity as part of human vitamin B6 metabolism. Biochim. Et Biophys. Acta (BBA)-Gen. Subj. 2019, 1863, 1088–1097. [Google Scholar] [CrossRef] [PubMed]
- Hanna, M.C.; Turner, A.J.; Kirkness, E.F. Human pyridoxal kinase: cDNA cloning, expression, and modulation by ligands of the benzodiazepine receptor. J. Biol. Chem. 1997, 272, 10756–10760. [Google Scholar] [CrossRef]
- Parra, M.; Stahl, S.; Hellmann, H. Vitamin B₆ and Its Role in Cell Metabolism and Physiology. Cells 2018, 7, 84. [Google Scholar] [CrossRef]
- Combs, G.F., Jr.; McClung, J.P. The Vitamins: Fundamental Aspects in Nutrition and Health; Academic Press: Cambridge, MA, USA, 2016. [Google Scholar]



| Variable | PRO Group (n = 10) | PEP Group (n = 9) | p-Value |
|---|---|---|---|
| Age (years) | 16.8 ± 1.32 | 16.78 ± 1.3 | 0.971 |
| Height (cm) | 175.6 ± 4.97 | 176.67 ± 5.61 | 0.666 |
| Weight (kg) | 63.77 ± 7.92 | 61.33 ± 4.47 | 0.428 |
| Training experience (years) | 3.20 ± 1.03 | 3.17 ± 1.27 | 0.743 |
| BMI (kg/m2) | 20.53 ± 1.56 | 19.48 ± 1.04 | 0.106 |
| Body fat (%) | 7.99 ± 2.64 | 6.51 ± 2.31 | 0.213 |
| 1500 m run (s) | 280.2 ± 14.76 | 286.44 ± 14.12 | 0.171 |
| VO2max (mL/kg/min) | 64 ± 3.4 | 62.72 ± 2.89 | 0.136 |
| 30 m sprint (s) | 4.28 ± 0.28 | 4.18 ± 0.12 | 0.537 |
| Peak power (W/kg) | 10.67 ± 1.46 | 10.96 ± 0.76 | 0.661 |
| Variable | PRO Group (n = 10) | PEP Group (n = 9) | ||||
|---|---|---|---|---|---|---|
| Baseline | Post-Intervention | Δ | Baseline | Post-Intervention | Δ | |
| Body weight (kg) | 63.77 ± 7.92 | 64.43 ± 7.93 | 1.10% | 61.33 ± 4.47 | 62.76 ± 4.96 *** | 2.28% |
| BMI(kg/m2) | 20.53 ± 1.56 | 20.68 ± 1.63 | 0.74% | 19.48 ± 1.04 | 19.92 ± 1.23 *** | 2.24% |
| Body fat (%) | 7.99 ± 2.64 | 9.4 ± 1.69 * | 25.13% | 6.51 ± 2.31 | 9.77 ± 2.57 *** | 56.27% † |
| Skeletal muscle mass (kg) | 33.13 ± 4.29 | 33.06 ± 4.03 | −0.08% | 32.33 ± 2.72 | 32.08 ± 2.95 | −0.85% |
| Lung capacity (L) | 4.6 ± 0.65 | 4.84 ± 0.51 ** | 5.84% | 4.21 ± 0.56 | 4.77 ± 0.49 *** | 13.76% † |
| Variable | PRO Group (n = 10) | PEP Group (n = 9) | ||||
|---|---|---|---|---|---|---|
| Baseline | Post-Intervention | Δ | Baseline | Post-Intervention | Δ | |
| 1500 m run (s) | 280.2 ± 14.76 | 285.1 ± 15.68 | 1.94% | 289.67 ± 14.02 | 286.44 ± 14.12 | −0.94% |
| 10 km roller skating (s) | 1607.1 ± 99.54 | 1602.2 ± 100.32 | −0.30% | 1608.89 ±117.71 | 1590.44 ±111.85 * | −1.12% |
| VO2max (mL/kg/min) | 64 ± 3.4 | 63.86 ± 3.53 | −0.16% | 61.56 ± 3.4 | 62.72 ± 2.89 | 2% |
| Lactate threshold | 3.12 ± 0.4 | 3.13 ± 0.54 | 0.23% | 2.81 ± 0.44 | 3.1 ± 0.42 | 11.77% |
| Individual lactate threshold | 49.4 ± 3.6 | 50 ± 6.63 | 1.04% | 46.11 ± 6.45 | 50.57 ± 5.69 * | 10.91% |
| RPE post-exhaustion | 16.5 ± 1.43 | 16.9 ± 1.1 | 3.29% | 15.78 ± 0.97 | 16.89 ± 0.6 * | 7.41% |
| Blood glucose (mmol/L) | ||||||
| Resting | 6.6 ± 0.49 | 5.37 ± 0.55 *** | −18.34% | 6.09 ± 1.02 | 5.34 ± 0.59 | −9.03% |
| Immediately a | 7.17 ± 0.56 | 6.36 ± 1.05 ** | −11.70% | 7.09 ± 1.07 | 5.34 ± 0.5 ** # | −23.27% † |
| Variable | PRO Group (n = 10) | PEP Group (n = 9) | ||||
|---|---|---|---|---|---|---|
| Baseline | Post-Intervention | Δ | Baseline | Post-Intervention | Δ | |
| 30 m sprint (s) | 4.28 ± 0.28 | 4.15 ± 0.21 * | −2.86% | 4.21 ± 0.15 | 4.18 ± 0.12 | −0.81% |
| Hexagon test of agility (s) | 9.12 ± 0.48 | 8.81 ± 0.41 * | −3.30% | 9.05 ± 0.38 | 8.83 ± 0.37 | −2.33% |
| Bench press relative strength | 1.13 ± 0.2 | 1.1 ± 0.16 | −1.59% | 1.05 ± 0.22 | 1.01 ± 0.18 | −2.33% |
| CMJ (m) | 0.38 ± 0.06 | 0.34 ± 0.04 | −10.25% | 0.37 ± 0.07 | 0.34 ± 0.07 | −4.14% |
| SJ (m) | 0.32 ± 0.05 | 0.3 ± 0.05 | −5.13% | 0.29 ± 0.07 | 0.3 ± 0.05 | 8.01% |
| Average power (W/kg) | 8.58 ± 0.83 | 8.67 ± 0.73 | 1.29% | 8.35 ± 0.85 | 8.7 ± 0.42 | 4.87% |
| Peak power (W/kg) | 10.67 ± 1.46 | 10.77 ± 1.39 | 1.15% | 10.4 ± 1.11 | 10.96 ± 0.76 | 6.31% |
| Fatigue index | 0.28 ± 0.08 | 0.19 ± 0.05 * | −28.37% | 0.29 ± 0.06 | 0.2 ± 0.04 *** | −29.88% |
| Blood glucose (mmol/L) | ||||||
| Resting | 6.76 ± 0.6 | 6.48 ± 0.8 | −3.34% | 6.03 ± 0.69 # | 6.32 ± 0.94 | 5.15% |
| Immediately b | 6.29 ± 0.4 | 6.64 ± 1.23 | 5.85% | 6.67 ± 0.66 | 5.76 ± 0.59 ** | −13.13% † |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xiang, M.; Han, Q.; Chen, Y.; Duan, S.; Han, X.; Sui, X.; Ren, C.; Wang, Q. Wheat Peptides as Catalysts for Athletic Performance Improvement in Cross-Country Skiers: A Randomized Controlled Trial. Metabolites 2024, 14, 538. https://doi.org/10.3390/metabo14100538
Xiang M, Han Q, Chen Y, Duan S, Han X, Sui X, Ren C, Wang Q. Wheat Peptides as Catalysts for Athletic Performance Improvement in Cross-Country Skiers: A Randomized Controlled Trial. Metabolites. 2024; 14(10):538. https://doi.org/10.3390/metabo14100538
Chicago/Turabian StyleXiang, Mai, Qi Han, Yue Chen, Shenglin Duan, Xiaofeng Han, Xuemei Sui, Chaoxue Ren, and Qirong Wang. 2024. "Wheat Peptides as Catalysts for Athletic Performance Improvement in Cross-Country Skiers: A Randomized Controlled Trial" Metabolites 14, no. 10: 538. https://doi.org/10.3390/metabo14100538
APA StyleXiang, M., Han, Q., Chen, Y., Duan, S., Han, X., Sui, X., Ren, C., & Wang, Q. (2024). Wheat Peptides as Catalysts for Athletic Performance Improvement in Cross-Country Skiers: A Randomized Controlled Trial. Metabolites, 14(10), 538. https://doi.org/10.3390/metabo14100538









