Comparative Evaluation of the Chemical Components and Anti-Inflammatory Potential of Yellow- and Blue-Flowered Meconopsis Species: M. integrifolia and M. betonicifolia
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material and Extraction
2.2. Cell Culture and ME Treatment
2.3. Determination of mRNA Transcription Levels
2.4. Composition Identification and Cell Metabolomics Based on LC-MS
2.5. Data Processing and Compound Identification
2.6. Comprehensive Metabolomics Analysis: From Multivariate Statistics to Pathway Enrichment and Network Visualization
2.7. Gene Ontology (GO) Analysis Methodology
2.8. Statistical Analysis
3. Results
3.1. Evaluation of Safety and Efficacy: MIE and MBE’s Impact on Cell Viability and NO Production in Inflammatory Conditions
3.2. Dose-Dependent Antioxidative and Anti-Inflammatory Effects of MIE and MBE
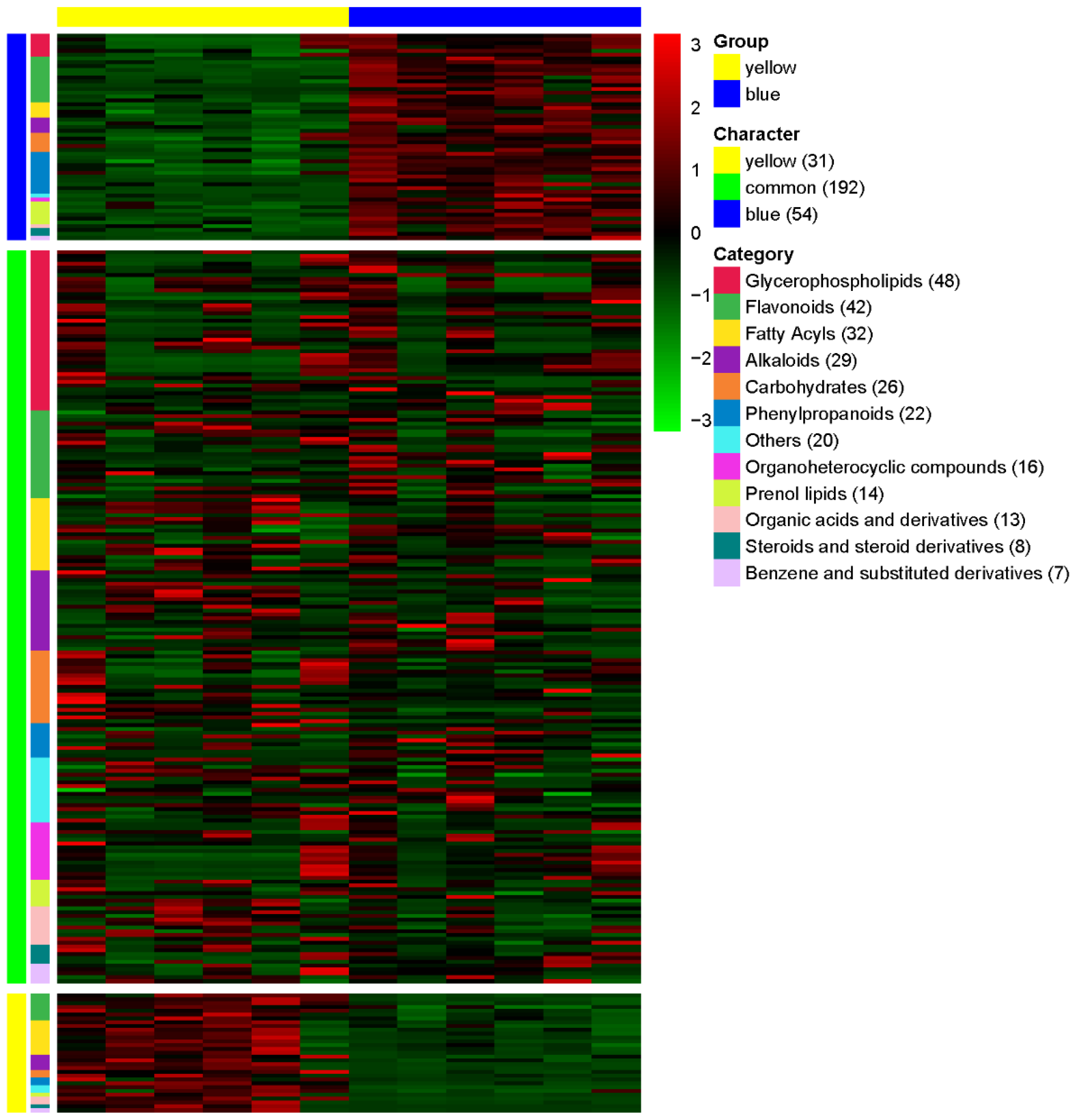
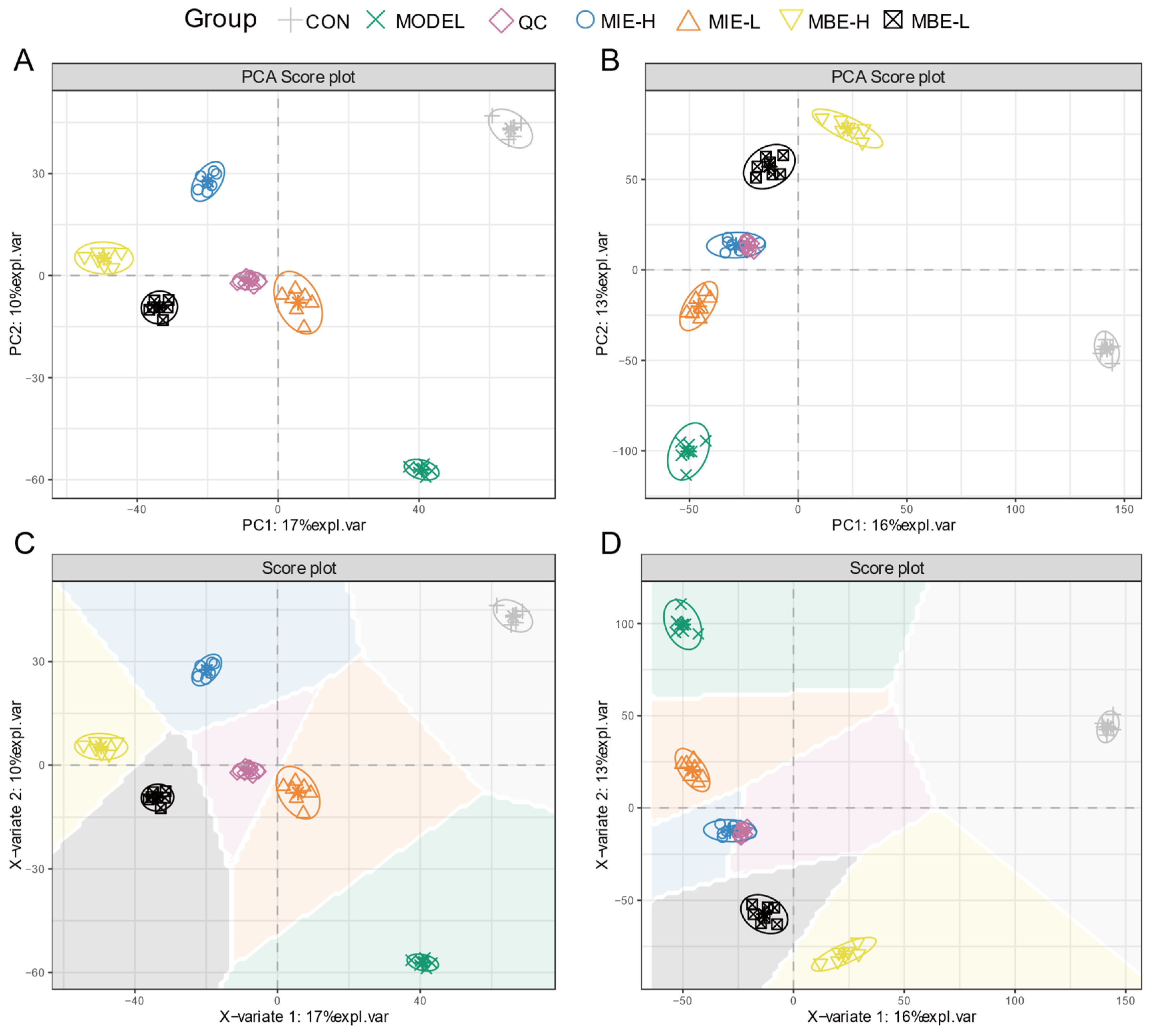
3.3. Comparative Metabolomic Profiling of Yellow and Blue Meconopsis Species: MIE and MBE Shared About 70% Composition
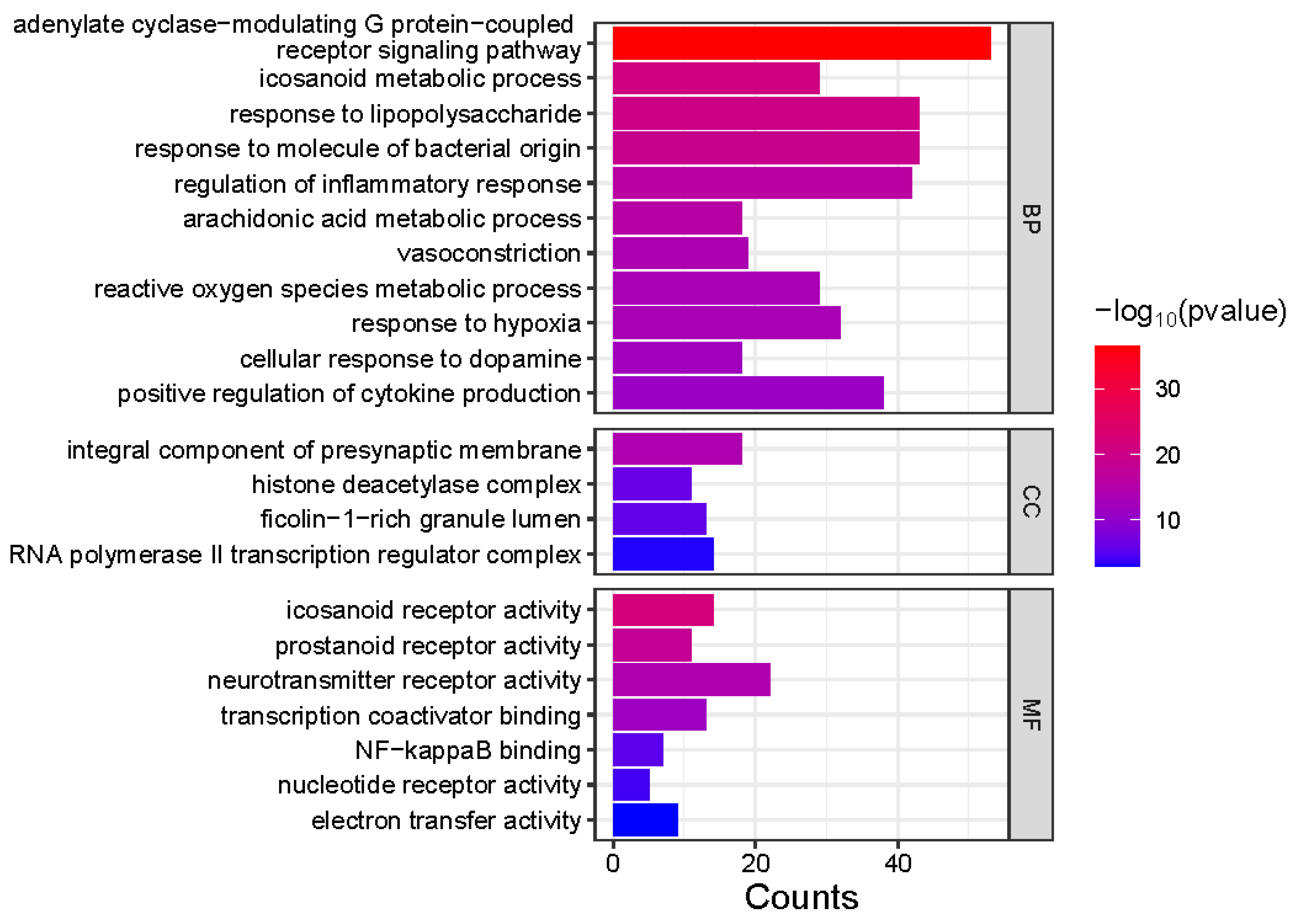
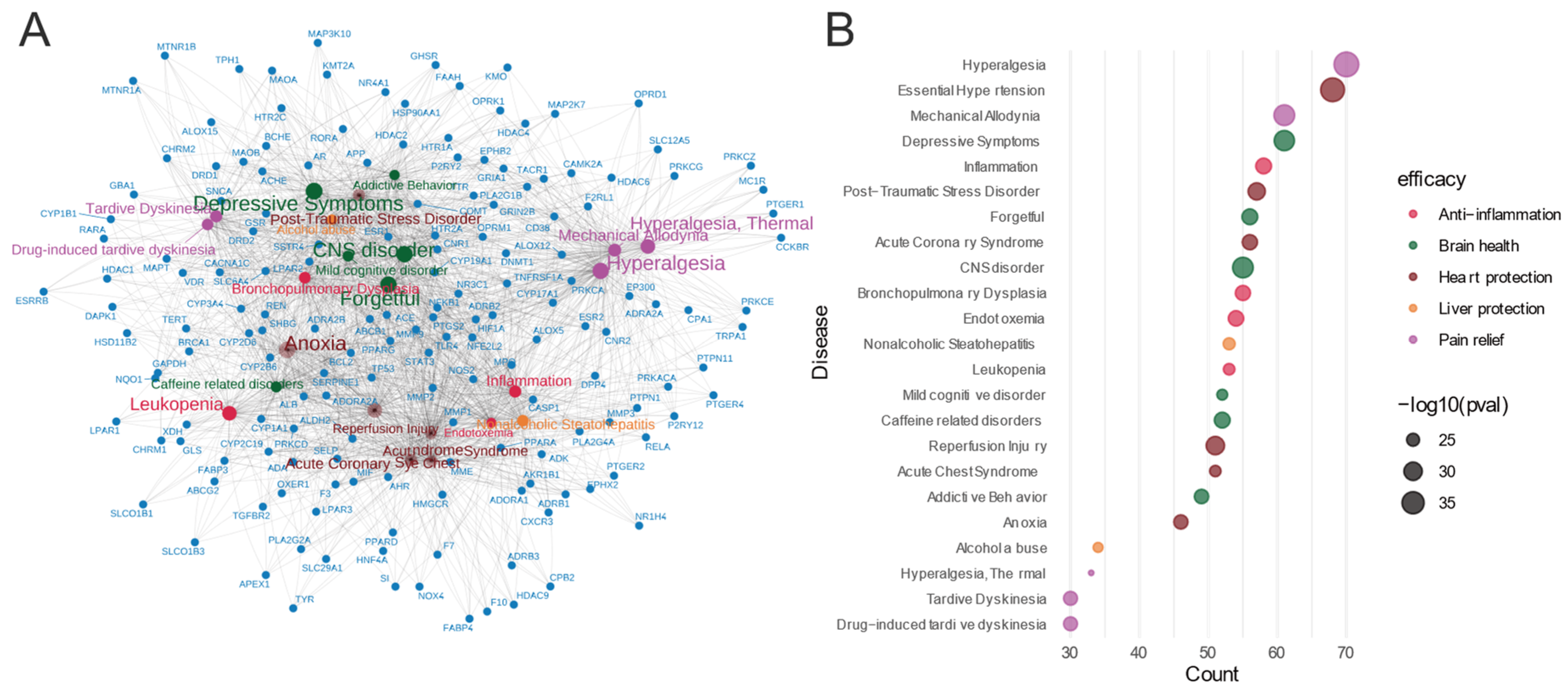
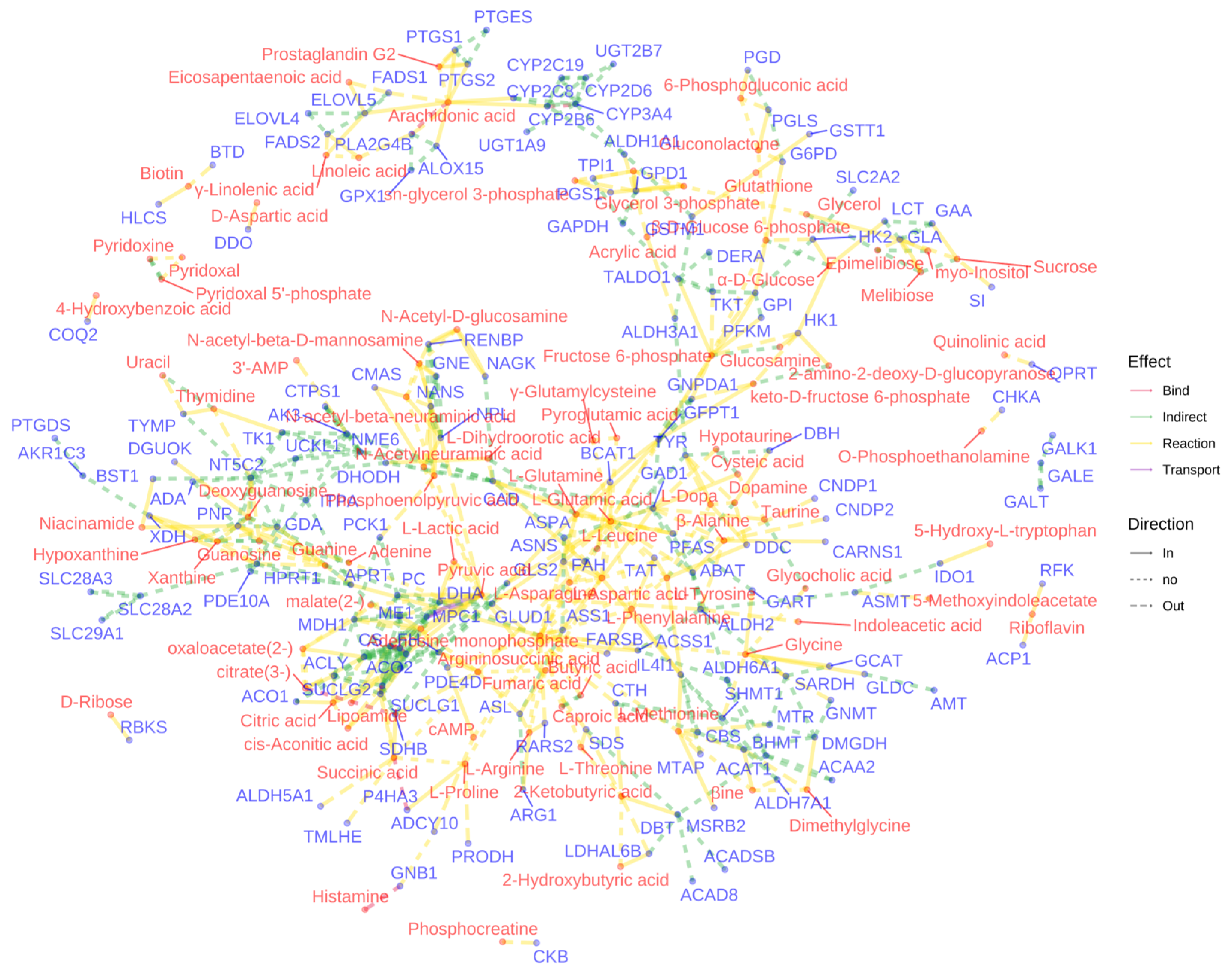
3.4. Molecular Insights into Meconopsis: GO Enrichment and Disease Network Analysis of Common Feature Targets
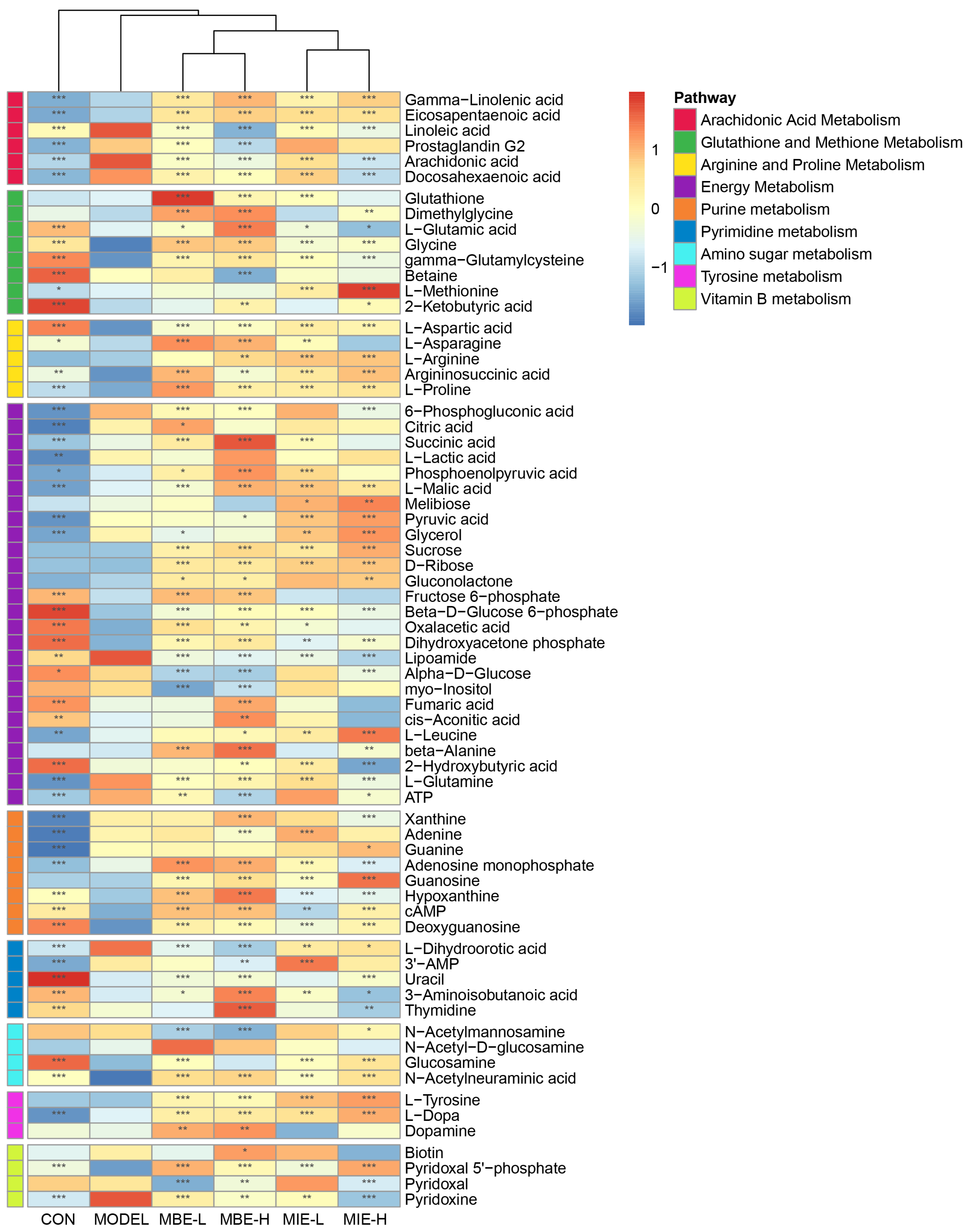
3.5. Metabolomic Profiling Reveals Anti-Inflammatory Effects of Meconopsis Species in LPS-Stimulated RAW264.7 Cells
4. Discussion
4.1. Arachidonic Acid Metabolism
4.2. Glutathione and Methionine Metabolism
4.3. Arginine and Proline Metabolism
4.4. Energy Metabolism
4.5. Other Pathways
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Guo, Q.; Bai, R.; Zhao, B.; Feng, X.; Zhao, Y.; Tu, P.; Chai, X. An Ethnopharmacological, Phytochemical and Pharmacological Review of the Genus Meconopsis. Am. J. Chin. Med. 2016, 44, 439–462. [Google Scholar] [CrossRef] [PubMed]
- Shi, N.; Wang, C.; Wang, J.; Wu, N.; Naudiyal, N.; Zhang, L.; Wang, L.; Sun, J.; Du, W.; Wei, Y.; et al. Biogeographic Patterns and Richness of the Meconopsis Species and Their Influence Factors across the Pan-Himalaya and Adjacent Regions. Diversity 2022, 14, 661. [Google Scholar] [CrossRef]
- Wang, W.-T.; Guo, W.-Y.; Jarvie, S.; Svenning, J.-C. The fate of Meconopsis species in the Tibeto-Himalayan region under future climate change. Ecol. Evol. 2021, 11, 887–899. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Burgess, K.S.; Yang, X.-F.; Ahrends, A.; Gao, L.-M.; Li, D.-Z. Upward elevation and northwest range shifts for alpine Meconopsis species in the Himalaya–Hengduan Mountains region. Ecol. Evol. 2019, 9, 4055–4064. [Google Scholar] [CrossRef]
- Brenna, J.T.; Salem, N., Jr.; Sinclair, A.J.; Cunnane, S.C.; the International Society for the Study of Fatty Acids and Lipids, ISSFAL. Alpha-Linolenic acid supplementation and conversion to n-3 long-chain polyunsaturated fatty acids in humans. Prostaglandins Leukot. Essent. Fat. Acids 2009, 80, 85–91. [Google Scholar] [CrossRef]
- Huang, Y.; Feng, X.; Wang, K.; Li, B.; Chen, K.; Huang, B.; Liu, Y. Authentication of Common Tibetan Medical Species of Meconopsis. J. Southwest Univ. (Nat. Sci. Ed.) 2016, 38, 8–16. [Google Scholar] [CrossRef]
- Zhao, F.; Bai, R.; Li, J.; Feng, X.; Jiao, S.; Wuken, S.; Ge, F.; Zhang, Q.; Zhou, X.; Tu, P.; et al. Meconopsis horridula Hook. f. & Thomson extract and its alkaloid oleracein E exert cardioprotective effects against acute myocardial ischaemic injury in mice. J. Ethnopharmacol. 2020, 258, 112893. [Google Scholar] [CrossRef]
- Yang, F.-S.; Qin, A.-L.; Li, Y.-F.; Wang, X.-Q. Great Genetic Differentiation among Populations of Meconopsis integrifolia and Its Implication for Plant Speciation in the Qinghai-Tibetan Plateau. PLoS ONE 2012, 7, e37196. [Google Scholar] [CrossRef]
- Sergeant, S.; Rahbar, E.; Chilton, F.H. Gamma-linolenic acid, Dihommo-gamma linolenic, Eicosanoids and Inflammatory Processes. Eur. J. Pharmacol. 2016, 785, 77–86. [Google Scholar] [CrossRef]
- Kikut, J.; Komorniak, N.; Ziętek, M.; Palma, J.; Szczuko, M. Inflammation with the participation of arachidonic (AA) and linoleic acid (LA) derivatives (HETEs and HODEs) is necessary in the course of a normal reproductive cycle and pregnancy. J. Reprod. Immunol. 2020, 141, 103177. [Google Scholar] [CrossRef]
- Sul, O.-J.; Ra, S.W. Quercetin Prevents LPS-Induced Oxidative Stress and Inflammation by Modulating NOX2/ROS/NF-kB in Lung Epithelial Cells. Molecules 2021, 26, 6949. [Google Scholar] [CrossRef] [PubMed]
- Jin, J.; Xiao, Y.; Hu, H.; Zou, Q.; Li, Y.; Gao, Y.; Ge, W.; Cheng, X.; Sun, S.C. Proinflammatory TLR signalling is regulated by a TRAF2-dependent proteolysis mechanism in macrophages. Nat. Commun. 2015, 6, 5930. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi, A.; Sharifi, A.; Pourpaknia, R.; Mohammadian, S.; Sahebkar, A. Manipulating macrophage polarization and function using classical HDAC inhibitors: Implications for autoimmunity and inflammation. Crit. Rev. Oncol. Hematol. 2018, 128, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Zhang, A.; Sun, H.; Yan, G.; Wang, P.; Wang, X. Mass spectrometry-based metabolomics: Applications to biomarker and metabolic pathway research. Biomed. Chromatogr. 2016, 30, 7–12. [Google Scholar] [CrossRef] [PubMed]
- Sazonova, E.V.; Chesnokov, M.S.; Zhivotovsky, B.; Kopeina, G.S. Drug toxicity assessment: Cell proliferation versus cell death. Cell Death Discov. 2022, 8, 417. [Google Scholar] [CrossRef]
- Wang, F.; Liigand, J.; Tian, S.; Arndt, D.; Greiner, R.; Wishart, D.S. CFM-ID 4.0: More Accurate ESI-MS/MS Spectral Prediction and Compound Identification. Anal. Chem. 2021, 93, 11692–11700. [Google Scholar] [CrossRef]
- Lê Cao, K.-A.; Boitard, S.; Besse, P. Sparse PLS discriminant analysis: Biologically relevant feature selection and graphical displays for multiclass problems. BMC Bioinform. 2011, 12, 253. [Google Scholar] [CrossRef]
- Shen, H.; Huang, J.Z. Sparse principal component analysis via regularized low rank matrix approximation. J. Multivar. Anal. 2008, 99, 1015–1034. [Google Scholar] [CrossRef]
- Hanna, V.S.; Hafez, E.A.A. Synopsis of arachidonic acid metabolism: A review. J. Adv. Res. 2018, 11, 23–32. [Google Scholar] [CrossRef]
- Aderem, A.A.; Cohen, D.S.; Wright, S.D.; Cohn, Z.A. Bacterial lipopolysaccharides prime macrophages for enhanced release of arachidonic acid metabolites. J. Exp. Med. 1986, 164, 165–179. [Google Scholar] [CrossRef]
- Herschman, H.R.; Xie, W.; Reddy, S. Function and Regulation of Prostaglandin Synthase 2. In Eicosanoids and Other Bioactive Lipids in Cancer, Inflammation, and Radiation Injury, 4; Honn, K.V., Marnett, L.J., Nigam, S., Dennis, E.A., Eds.; Springer: Boston, MA, USA, 1999; pp. 3–8. [Google Scholar]
- Granström, E. The arachidonic acid cascade. Inflammation 1984, 8, S15–S25. [Google Scholar] [CrossRef] [PubMed]
- Averill-Bates, D.A. The antioxidant glutathione. Vitam. Horm. 2023, 121, 109–141. [Google Scholar] [PubMed]
- Lever, M.; McEntyre, C.J.; George, P.M.; Chambers, S.T. Is N,N-dimethylglycine N-oxide a choline and betaine metabolite? Biol. Chem. 2017, 398, 775–784. [Google Scholar] [CrossRef] [PubMed]
- Magnusson, M.; Wang, T.J.; Clish, C.; Engström, G.; Nilsson, P.; Gerszten, R.E.; Melander, O. Dimethylglycine Deficiency and the Development of Diabetes. Diabetes 2015, 64, 3010–3016. [Google Scholar] [CrossRef]
- Sutter, B.M.; Wu, X.; Laxman, S.; Tu, B.P. Methionine inhibits autophagy and promotes growth by inducing the SAM-responsive methylation of PP2A. Cell 2013, 154, 403–415. [Google Scholar] [CrossRef]
- Gupta, N.; Gresser, M.J.; Ford-Hutchinson, A.W. Kinetic mechanism of glutathione conjugation to leukotriene A4 by leukotriene C4 synthase. Biochim. Biophys. Acta (BBA) Lipids Lipid Metab. 1998, 1391, 157–168. [Google Scholar] [CrossRef]
- Wijnands, K.A.P.; Castermans, T.M.R.; Hommen, M.P.J.; Meesters, D.M.; Poeze, M. Arginine and Citrulline and the Immune Response in Sepsis. Nutrients 2015, 7, 1426–1463. [Google Scholar] [CrossRef]
- Moncada, S.; Higgs, A. The L-Arginine-Nitric Oxide Pathway. N. Engl. J. Med. 1993, 329, 2002–2012. [Google Scholar] [CrossRef]
- Martí i Líndez, A.-A.; Reith, W. Arginine-dependent immune responses. Cell. Mol. Life Sci. 2021, 78, 5303–5324. [Google Scholar] [CrossRef]
- Tsai, W.-B.; Long, Y.; Savaraj, N.; Feun, L.G.; Kuo, M.T. Mechanisms of l-Arginine-Auxotrophic Response and Their Cancer Therapeutic Implications. In L-Arginine in Clinical Nutrition; Patel, V.B., Preedy, V.R., Rajendram, R., Eds.; Springer International Publishing: Cham, Switzerland, 2017; pp. 563–575. [Google Scholar]
- Wang, X.; Liu, Y.; Wang, S.; Pi, D.; Leng, W.; Zhu, H.; Zhang, J.; Shi, H.; Li, S.; Lin, X.; et al. Asparagine reduces the mRNA expression of muscle atrophy markers via regulating protein kinase B (Akt), AMP-activated protein kinase α, toll-like receptor 4 and nucleotide-binding oligomerisation domain protein signalling in weaning piglets after lipopolysaccharide challenge. Br. J. Nutr. 2016, 116, 1188–1198. [Google Scholar] [CrossRef]
- Chen, S.; Liu, Y.; Wang, X.; Wang, H.; Li, S.; Shi, H.; Zhu, H.; Zhang, J.; Pi, D.; Hu, C.-A.A.; et al. Asparagine improves intestinal integrity, inhibits TLR4 and NOD signaling, and differently regulates p38 and ERK1/2 signaling in weanling piglets after LPS challenge. Innate Immun. 2016, 22, 577–587. [Google Scholar] [CrossRef] [PubMed]
- Kieler, M.; Hofmann, M.; Schabbauer, G. More than just protein building blocks: How amino acids and related metabolic pathways fuel macrophage polarization. FEBS J. 2021, 288, 3694–3714. [Google Scholar] [CrossRef] [PubMed]
- Vettore, L.A.; Westbrook, R.L.; Tennant, D.A. Proline metabolism and redox; maintaining a balance in health and disease. Amino Acids 2021, 53, 1779–1788. [Google Scholar] [CrossRef] [PubMed]
- Ji, D.; Yin, J.-y.; Li, D.-f.; Zhu, C.-t.; Ye, J.-p.; Pan, Y.-q. Effects of inflammatory and anti-inflammatory environments on the macrophage mitochondrial function. Sci. Rep. 2020, 10, 20324. [Google Scholar] [CrossRef]
- Soto-Heredero, G.; Gómez de las Heras, M.M.; Gabandé-Rodríguez, E.; Oller, J.; Mittelbrunn, M. Glycolysis – a key player in the inflammatory response. FEBS J. 2020, 287, 3350–3369. [Google Scholar] [CrossRef]
- Chiba, S.; Hisamatsu, T.; Suzuki, H.; Mori, K.; Kitazume, M.T.; Shimamura, K.; Mizuno, S.; Nakamoto, N.; Matsuoka, K.; Naganuma, M.; et al. Glycolysis regulates LPS-induced cytokine production in M2 polarized human macrophages. Immunol. Lett. 2017, 183, 17–23. [Google Scholar] [CrossRef]
- Boscá, L.; González-Ramos, S.; Prieto, P.; Fernández-Velasco, M.; Mojena, M.; Martín-Sanz, P.; Alemany, S. Metabolic signatures linked to macrophage polarization: From glucose metabolism to oxidative phosphorylation. Biochem. Soc. Trans. 2015, 43, 740–744. [Google Scholar] [CrossRef]
- Palmieri, E.M.; Holewinski, R.; McGinity, C.L.; Pierri, C.L.; Maio, N.; Weiss, J.M.; Tragni, V.; Miranda, K.M.; Rouault, T.A.; Andresson, T.; et al. Pyruvate dehydrogenase operates as an intramolecular nitroxyl generator during macrophage metabolic reprogramming. Nat. Commun. 2023, 14, 5114. [Google Scholar] [CrossRef]
- Bonvini, A.; Rogero, M.M.; Coqueiro, A.Y.; Raizel, R.; Bella, L.M.; Fock, R.A.; Borelli, P.; Tirapegui, J. Effects of different branched-chain amino acids supplementation protocols on the inflammatory response of LPS-stimulated RAW 264.7 macrophages. Amino Acids 2021, 53, 597–607. [Google Scholar] [CrossRef]
- Ye, Z.; Wang, S.; Zhang, C.; Zhao, Y. Coordinated Modulation of Energy Metabolism and Inflammation by Branched-Chain Amino Acids and Fatty Acids. Front. Endocrinol. 2020, 11, 617. [Google Scholar] [CrossRef]
- Liu, Y.; Xu, R.; Gu, H.; Zhang, E.; Qu, J.; Cao, W.; Huang, X.; Yan, H.; He, J.; Cai, Z. Metabolic reprogramming in macrophage responses. Biomark. Res. 2021, 9, 1. [Google Scholar] [CrossRef] [PubMed]
- Ham, M.; Lee, J.-W.; Choi, A.H.; Jang, H.; Choi, G.; Park, J.; Kozuka, C.; Sears, D.D.; Masuzaki, H.; Kim, J.B. Macrophage Glucose-6-Phosphate Dehydrogenase Stimulates Proinflammatory Responses with Oxidative Stress. Mol. Cell. Biol. 2013, 33, 2425–2435. [Google Scholar] [CrossRef] [PubMed]
- Linden, J.; Koch-Nolte, F.; Dahl, G. Purine Release, Metabolism, and Signaling in the Inflammatory Response. Annu. Rev. Immunol. 2019, 37, 325–347. [Google Scholar] [CrossRef] [PubMed]
- John, S.V.; Seim, G.L.; Erazo-Flores, B.J.; Steill, J.; Freeman, J.; Votava, J.A.; Arp, N.L.; Qing, X.; Stewart, R.; Knoll, L.J.; et al. Macrophages undergo functionally significant reprograming of nucleotide metabolism upon classical activation. bioRxiv 2023. [Google Scholar] [CrossRef]
- Haskó, G.; Pacher, P. Regulation of Macrophage Function by Adenosine. Arterioscler. Thromb. Vasc. Biol. 2012, 32, 865–869. [Google Scholar] [CrossRef]
- Yanaka, N.; Koyama, T.-A.; Komatsu, S.-I.; Nakamura, E.; Kanda, M.; Kato, N. Vitamin B6 suppresses NF-κB activation in LPS-stimulated mouse macrophages. Int. J. Mol. Med. 2005, 16, 1071–1075. [Google Scholar] [CrossRef]
- Shan, M.-R.; Zhou, S.-N.; Fu, C.-N.; Song, J.-W.; Wang, X.-Q.; Bai, W.-W.; Li, P.; Song, P.; Zhu, M.-L.; Ma, Z.-M.; et al. Vitamin B6 inhibits macrophage activation to prevent lipopolysaccharide-induced acute pneumonia in mice. J. Cell. Mol. Med. 2020, 24, 3139–3148. [Google Scholar] [CrossRef]




| Gene | Forward Primer (5′-3′) | Reverse Primer (3′-5′) |
|---|---|---|
| IL-1B | GAAATGCCACCTTTTGACAGTG | TGGATGCTCTCATCAGGACAG |
| IL-1A | TCTCAGATTCACAACTGTTCGTG | AGAAAATGAGGTCGGTCTCACTA |
| TNF | GTAGCCCACGTCGTAGCAAA | ACAAGGTACAACCCATCGGC |
| IL-6 | TGGAGTACCATAGCTACCTGGA | TGGAAATTGGGGTAGGAAGGAC |
| β-actin | GATATCGCTGCGCTGGTCG | CATTCCCACCATCACACCCT |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cheng, P.; Gan, R.; Wang, C.; Xu, Q.; Norbu, K.; Zhou, F.; Kong, S.; Jia, Z.; Jiabu, D.; Feng, X.; et al. Comparative Evaluation of the Chemical Components and Anti-Inflammatory Potential of Yellow- and Blue-Flowered Meconopsis Species: M. integrifolia and M. betonicifolia. Metabolites 2024, 14, 563. https://doi.org/10.3390/metabo14100563
Cheng P, Gan R, Wang C, Xu Q, Norbu K, Zhou F, Kong S, Jia Z, Jiabu D, Feng X, et al. Comparative Evaluation of the Chemical Components and Anti-Inflammatory Potential of Yellow- and Blue-Flowered Meconopsis Species: M. integrifolia and M. betonicifolia. Metabolites. 2024; 14(10):563. https://doi.org/10.3390/metabo14100563
Chicago/Turabian StyleCheng, Peizhao, Ruixi Gan, Cong Wang, Qian Xu, Kelsang Norbu, Feng Zhou, Sixin Kong, Zhuoma Jia, Dawa Jiabu, Xin Feng, and et al. 2024. "Comparative Evaluation of the Chemical Components and Anti-Inflammatory Potential of Yellow- and Blue-Flowered Meconopsis Species: M. integrifolia and M. betonicifolia" Metabolites 14, no. 10: 563. https://doi.org/10.3390/metabo14100563
APA StyleCheng, P., Gan, R., Wang, C., Xu, Q., Norbu, K., Zhou, F., Kong, S., Jia, Z., Jiabu, D., Feng, X., & Wang, J. (2024). Comparative Evaluation of the Chemical Components and Anti-Inflammatory Potential of Yellow- and Blue-Flowered Meconopsis Species: M. integrifolia and M. betonicifolia. Metabolites, 14(10), 563. https://doi.org/10.3390/metabo14100563






