Metabolomics Reveal Key Metabolic Pathway Responses to Anxiety State Regulated by Serotonin in Portunus trituberculatus
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Drug Treatments
2.3. Open Field-like Test
2.4. Liquid Chromatography-Mass Spectrometry Analysis
2.5. Statistical Analysis
2.6. Ethical Note
3. Results
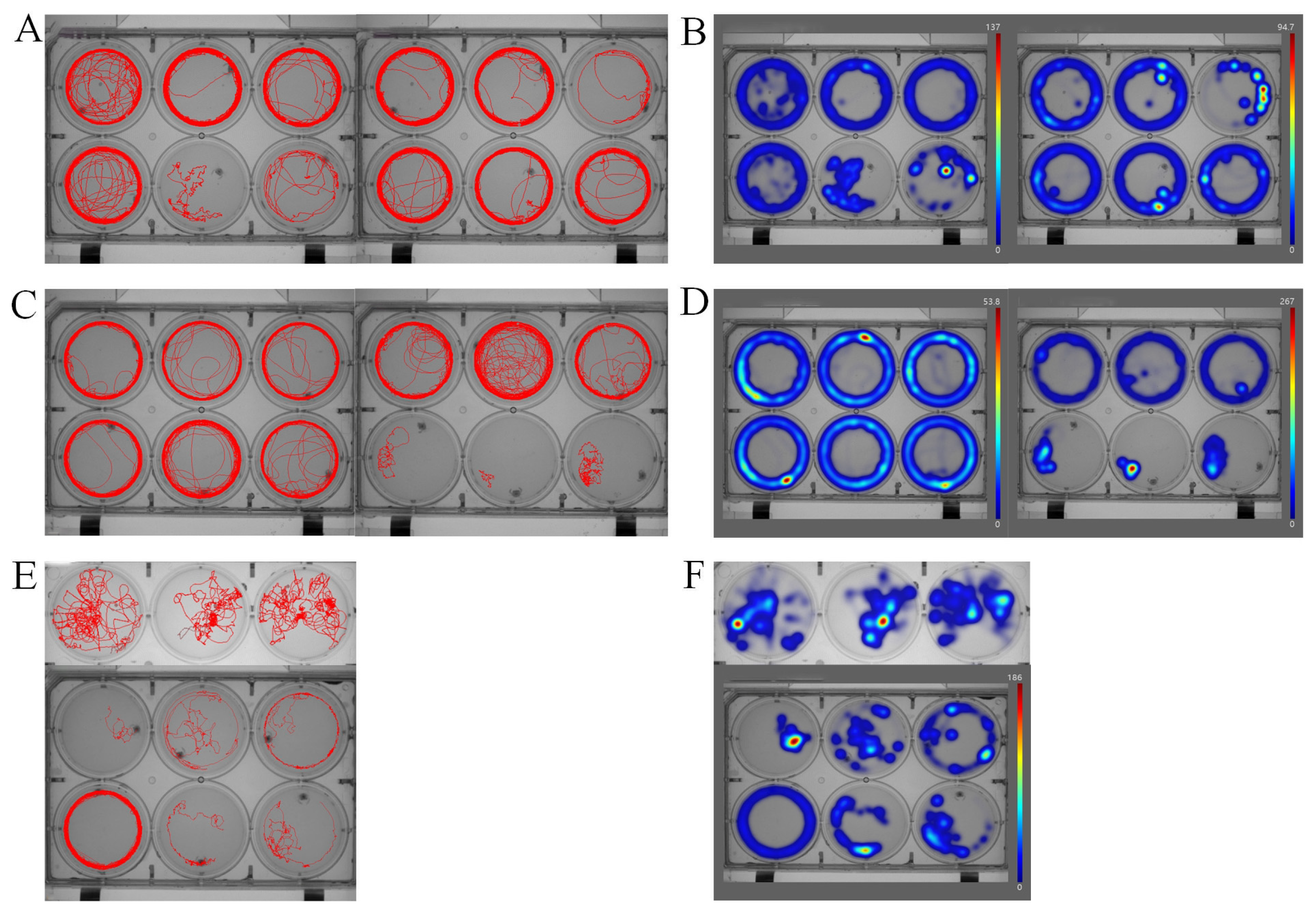
3.1. Anxiety-like Behavior Assays
3.2. Metabolomic Multivariate Analysis of Serotonin and Clonazepam Treatment
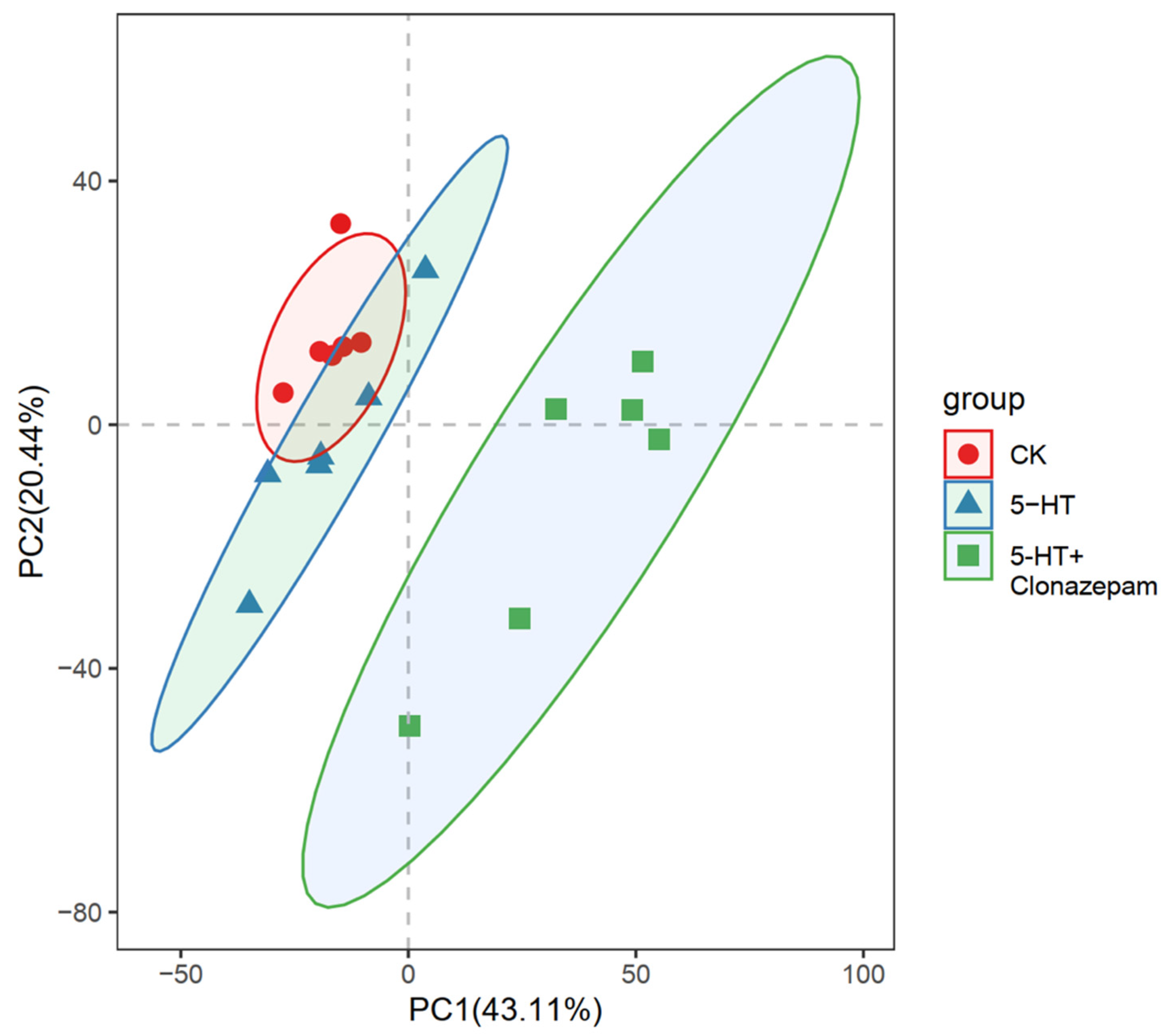
3.3. Principal Component Analysis Model
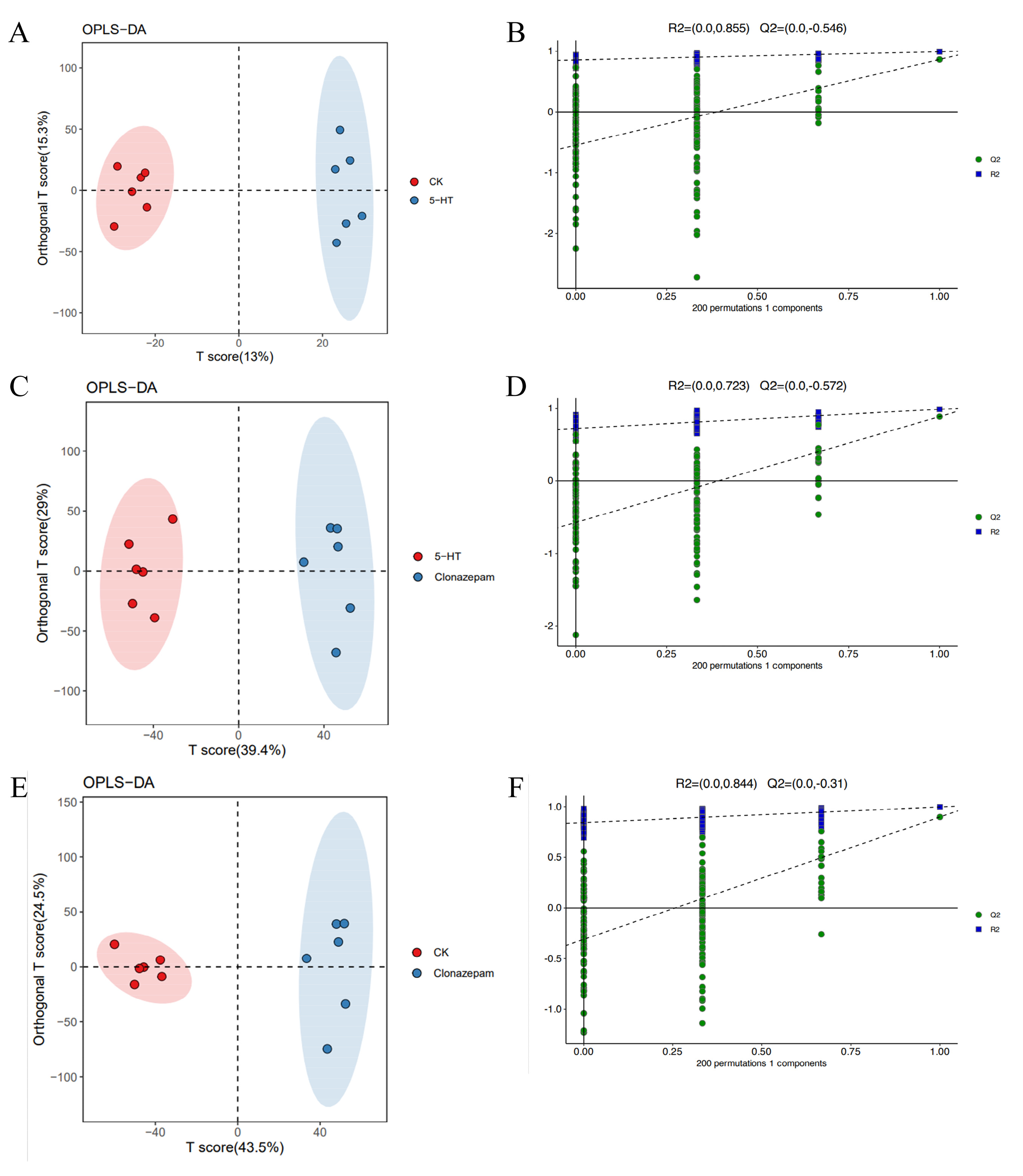
3.4. Partial Least Squares Regression Model
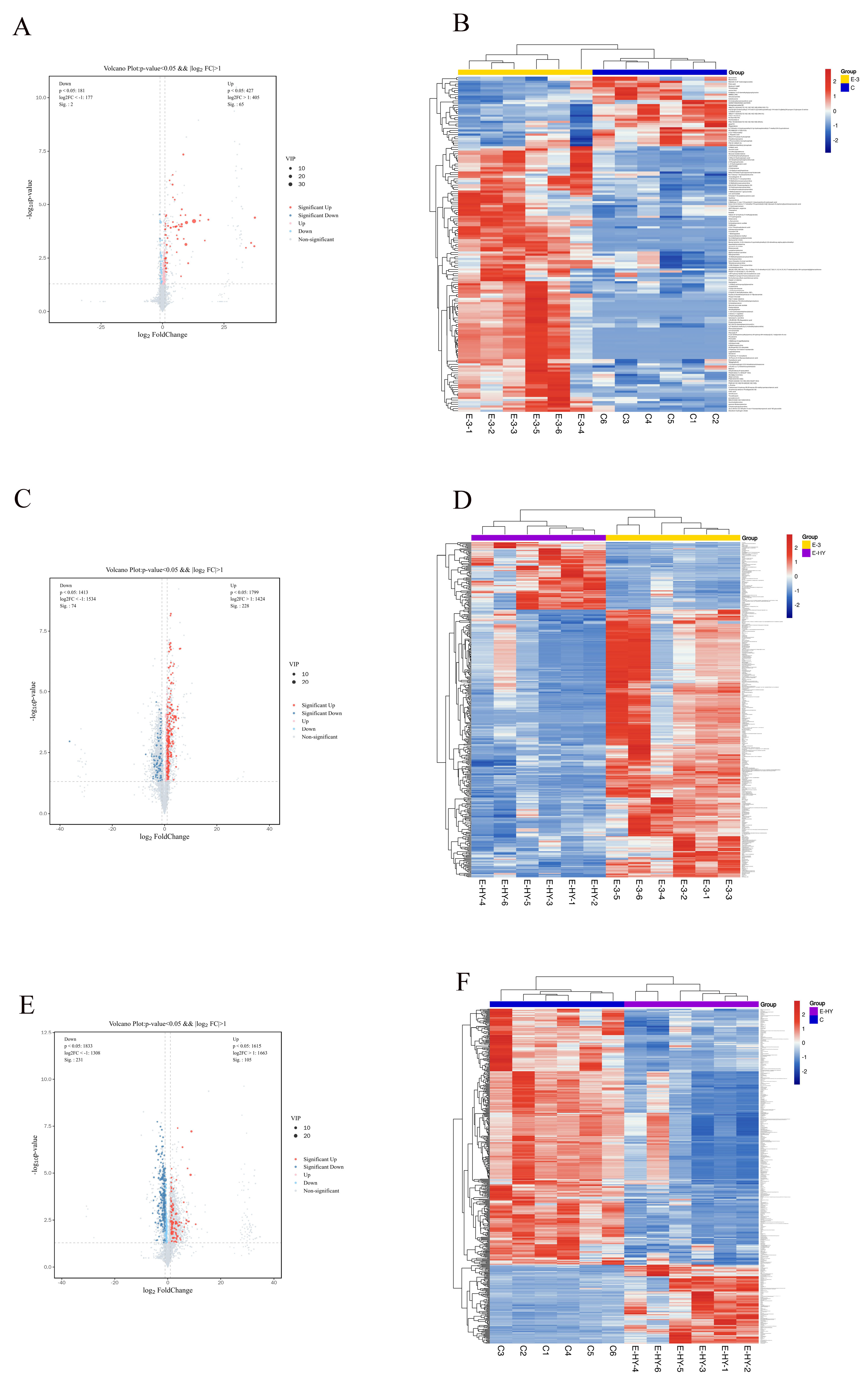
3.5. Screening for Differential Metabolites in P. trituberculatus in the CK, Serotonin, and Clonazepam Groups
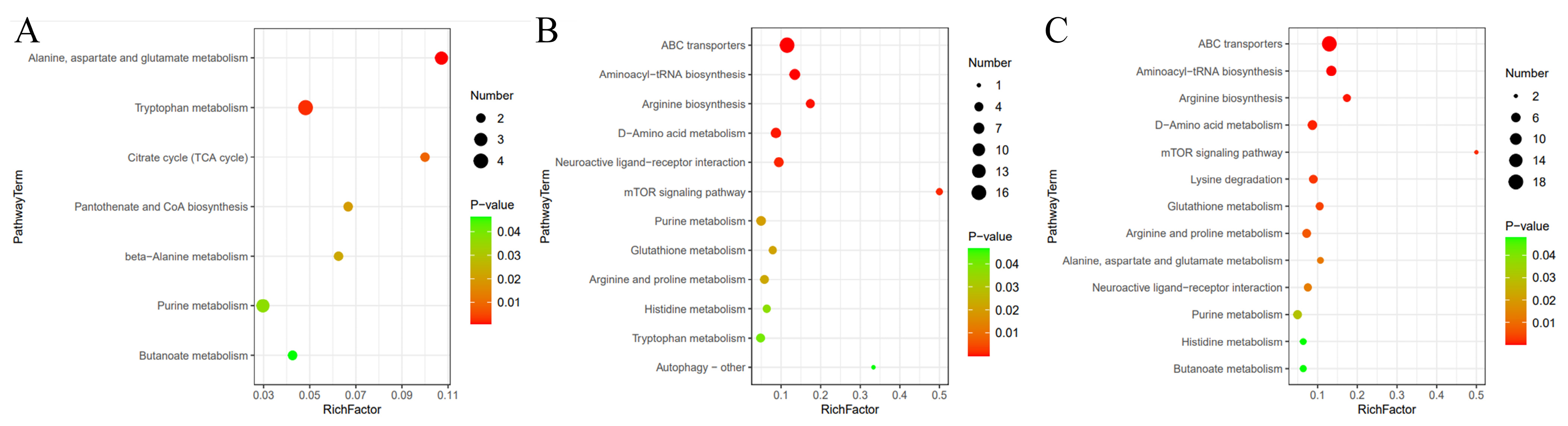
3.6. Metabolic Pathway Enrichment Analysis for Differential Metabolites
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A

References
- Khakpay, R.; Khakpai, F. Modulation of anxiety behavior in gonadectomized animals. Acta Neurobiol. Exp. 2020, 80, 205–216. [Google Scholar] [CrossRef]
- Heisler, L.K.; Zhou, L.; Bajwa, P.; Hsu, J.; Tecott, L.H. Serotonin 5-HT2C receptors regulate anxiety-like behavior. Genes Brain Behav. 2007, 6, 491–496. [Google Scholar] [CrossRef] [PubMed]
- Malan-Muller, S.; Valles-Colomer, M.; Palomo, T.; Leza, J.C. The gut-microbiota-brain axis in a Spanish population in the aftermath of the COVID-19 pandemic: Microbiota composition linked to anxiety, trauma, and depression profiles. Gut Microbes 2023, 15, 2162306. [Google Scholar] [CrossRef]
- Choueiry, N.; Salamoun, T.; Jabbour, H.; El Osta, N.; Hajj, A.; Rabbaa Khabbaz, L. Insomnia and relationship with anxiety in university students: A cross-sectional designed study. PLoS ONE 2016, 11, e0149643. [Google Scholar] [CrossRef]
- Du, M.; Peng, Y.; Li, Y.; Zhu, Y.; Yang, S.; Li, J.; Zou, F.; Wang, Y.; Wu, X.; Zhang, Y.; et al. Effect of trait anxiety on cognitive flexibility: Evidence from event-related potentials and resting-state EEG. Biol. Psychol. 2022, 170, 108319. [Google Scholar] [CrossRef]
- Lutz, J.; Mashal, N.; Kramer, A.; Suresh, M.; Gould, C.; Jordan, J.T.; Wetherell, J.L.; Beaudreau, S.A. A case report of problem solving therapy for reducing suicide risk in older adults with anxiety disorders. Clin. Gerontol. 2020, 43, 110–117. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, H.; Martinez de Toda, I.; Sanz-San Miguel, L.; De la Fuente, M. Sex-related differences in behavioural markers in adult mice for the prediction of lifespan. Biogerontology 2021, 22, 49–62. [Google Scholar] [CrossRef]
- Rangassamy, M.; Dalmas, M.; Féron, C.; Gouat, P.; Rödel, H.G. Similarity of personalities speeds up reproduction in pairs of a monogamous rodent. Anim. Behav. 2015, 103, 7–15. [Google Scholar] [CrossRef]
- Meyer, N.; Richter, S.H.; Schreiber, R.S.; Kloke, V.; Kaiser, S.; Lesch, K.P.; Sachser, N. The unexpected effects of beneficial and adverse social experiences during adolescence on anxiety and aggression and their modulation by genotype. Front. Behav. Neurosci. 2016, 10, 97. [Google Scholar] [CrossRef]
- Patki, G.; Atrooz, F.; Alkadhi, I.; Solanki, N.; Salim, S. High aggression in rats is associated with elevated stress, anxiety-like behavior, and altered catecholamine content in the brain. Neurosci. Lett. 2015, 584, 308–313. [Google Scholar] [CrossRef]
- Heinz, D.E.; Schottle, V.A.; Nemcova, P.; Binder, F.P.; Ebert, T.; Domschke, K.; Wotjak, C.T. Exploratory drive, fear, and anxiety are dissociable and independent components in foraging mice. Transl. Psychiatry 2021, 11, 318. [Google Scholar] [CrossRef] [PubMed]
- Mohammad, F.; Aryal, S.; Ho, J.; Stewart, J.C.; Norman, N.A.; Tan, T.L.; Eisaka, A.; Claridge-Chang, A. Ancient anxiety pathways influence Drosophila defense behaviors. Curr. Biol. 2016, 26, 981–986. [Google Scholar] [CrossRef] [PubMed]
- Kompagne, H.; Bardos, G.; Szenasi, G.; Gacsalyi, I.; Harsing, L.G.; Levay, G. Chronic mild stress generates clear depressive but ambiguous anxiety-like behaviour in rats. Behav. Brain Res. 2008, 193, 311–314. [Google Scholar] [CrossRef] [PubMed]
- Ohl, F.; Roedel, A.; Binder, E.; Holsboer, F. Impact of high and low anxiety on cognitive performance in a modified hole board test in C57BL/6 and DBA/2 mice. Eur. J. Neurosci. 2003, 17, 128–136. [Google Scholar] [CrossRef]
- Sparling, J.E.; Baker, S.L.; Bielajew, C. Effects of combined pre- and post-natal enrichment on anxiety-like, social, and cognitive behaviours in juvenile and adult rat offspring. Behav. Brain Res. 2018, 353, 40–50. [Google Scholar] [CrossRef]
- Lalonde, R.; Strazielle, C. Relations between open-field, elevated plus-maze, and emergence tests as displayed by C57/BL6J and BALB/c mice. J. Neurosci. Methods 2008, 171, 48–52. [Google Scholar] [CrossRef]
- Hope, B.V.; Hamilton, T.J.; Hurd, P.L. Submerged plus maze: A novel test for studying anxiety-like behaviour in fish. Behav. Brain Res. 2019, 362, 332–337. [Google Scholar] [CrossRef]
- Lim, L.W.; Temel, Y.; Visser-Vandewalle, V.; Steinbusch, H.; Schruers, K.; Hameleers, R.; Esquivel, G.; Griez, E.; Blokland, A. Effect of buspirone on the behavioral regulation of rats in low versus high anxiety conditions. Arzneimittelforschung 2008, 58, 269–276. [Google Scholar] [CrossRef]
- Maldonado, E.; Navarro, J.F. Effects of 3,4-methylenedioxy-methamphetamine (MDMA) on anxiety in mice tested in the light-dark box. Prog. Neuropsychopharmacol. Biol. Psychiatry 2000, 24, 463–472. [Google Scholar] [CrossRef]
- Li, X.L.; Aou, S.; Hori, T.; Oomura, Y. Spatial memory deficit and emotional abnormality in OLETF rats. Physiol. Behav. 2002, 75, 15–23. [Google Scholar] [CrossRef]
- Lu, T.; Shen, Y.; Cui, G.X.; Yin, F.W.; Yu, Z.L.; Zhou, D.Y. Detailed analysis of lipids in edible viscera and muscles of cooked crabs Portunus trituberculatus and Portunus pelagicus. J. Aquat. Food Prod. Technol. 2020, 29, 391–406. [Google Scholar] [CrossRef]
- He, J.; Wan, L.; Yu, H.; Peng, Y.; Zhang, D.; Xu, W. Effect of water temperature on embryonic development of Protunus trituberculatus in an off-season breeding mode. Front. Mar. Sci. 2022, 9, 1066151. [Google Scholar] [CrossRef]
- Liu, B.; Zhang, X.; Wang, Z.; Li, W.; Zhang, Q.; Liu, Q.; Liu, W.; Zhang, L.; Liu, Y.; Wang, C. Genetic pattern fluctuations in wild swimming crab populations, under the influence of continuous mass stock enhancement. Fish. Res. 2021, 243, 106075. [Google Scholar] [CrossRef]
- Chen, J.; Chen, X.; Mu, C.; Wang, C.; Ye, Y.; Li, R.; Song, W.; Shi, C.; Liu, L.; Wang, H. Comparative transcriptome analysis reveals the growth and development in larval stages of the swimming crab Portunus trituberculatus. Front. Mar. Sci. 2023, 10, 1172214. [Google Scholar] [CrossRef]
- Liang, Q.; Zhu, B.; Liu, D.; Lu, Y.; Zhang, H.; Wang, F. Serotonin and dopamine regulate the aggressiveness of swimming crabs (Portunus trituberculatus) in different ways. Physiol. Behav. 2023, 263, 114135. [Google Scholar] [CrossRef]
- Liu, D.; Wang, F.; Yang, C.; Hu, N.; Sun, Y. Starvation and a conspecific competitor influence multiple predator effects in a swimming crab (Portunus trituberculatus)-Manila clam (Ruditapes philippinarum) foraging system. J. Exp. Mar. Biol. Ecol. 2017, 495, 35–42. [Google Scholar] [CrossRef]
- Liu, D.; Wang, F.; Lu, Y.; Zhu, B.; Zhang, H. Effects of stocking density on a typical crab-clam polyculture system: Behavioral mechanisms of predation and competition in swimming crab (Portunus trituberculatus). Aquaculture 2022, 547, 737467. [Google Scholar] [CrossRef]
- Zhu, B.; Zhang, H.; Lu, Y.; Wang, F.; Liu, D. The effect of intruder density on territoriality and dominance in male swimming crab (Portunus trituberculatus). Animals 2022, 12, 314. [Google Scholar] [CrossRef]
- Kudryavtseva, N.N.; Bondar, N.P.; Avgustinovich, D.F. Association between experience of aggression and anxiety in male mice. Behav. Brain Res. 2002, 133, 83–93. [Google Scholar] [CrossRef]
- Podhorna, J.; Krsiak, M. Behavioural effects of a benzodiazepine receptor partial agonist, Ro 19-8022, in the social conflict test in mice. Behav. Pharmacol. 2000, 11, 143–151. [Google Scholar] [CrossRef]
- Yu, W.S.; Guan, L.; Kai Tan, S.Z.; Shrestha, S.; Or, Y.Z.; Lufkin, T.; Lin, V.C.; Lim, L.W. Tetratricopeptide repeat domain 9A knockout induces social anxiety and impairs offense behaviors in female mice. Iran. J. Basic Med. Sci. 2022, 25, 698–703. [Google Scholar] [CrossRef] [PubMed]
- Fossat, P.; Bacque-Cazenave, J.; De Deurwaerdere, P.; Delbecque, J.P.; Cattaert, D. Comparative behavior. Anxiety-like behavior in crayfish is controlled by serotonin. Science 2014, 344, 1293–1297. [Google Scholar] [CrossRef] [PubMed]
- Xu, P.; He, Y.; Cao, X.; Valencia-Torres, L.; Yan, X.; Saito, K.; Wang, C.; Yang, Y.; Hinton, A., Jr.; Zhu, L.; et al. Activation of serotonin 2C receptors in dopamine neurons inhibits binge-like eating in mice. Biol. Psychiatry 2017, 81, 737–747. [Google Scholar] [CrossRef]
- Li, X.; He, C.; Shen, M.; Wang, M.; Zhou, J.; Chen, D.; Zhang, T.; Pu, Y. Effects of aqueous extracts and volatile oils prepared from Huaxiang Anshen decoction on p-chlorophenylalanine-induced insomnia mice. J. Ethnopharmacol. 2024, 319 Pt 3, 117331. [Google Scholar] [CrossRef]
- Lin, D.; Parsons, L.H. Anxiogenic-like effect of serotonin1B receptor stimulation in the rat elevated plus-maze. Pharmacol. Biochem. Behav. 2002, 71, 581–587. [Google Scholar] [CrossRef] [PubMed]
- Yu, W.; Yen, Y.C.; Lee, Y.H.; Tan, S.; Xiao, Y.; Lokman, H.; Ting, A.K.T.; Ganegala, H.; Kwon, T.; Ho, W.K.; et al. Prenatal selective serotonin reuptake inhibitor (SSRI) exposure induces working memory and social recognition deficits by disrupting inhibitory synaptic networks in male mice. Mol. Brain 2019, 12, 29. [Google Scholar] [CrossRef]
- Bano, S.; Sharif, H.; Sajid, F.; Hamid, S.B.; Badawy, A.A. Liver tryptophan 2,3-dioxygenase: A determinant of anxiety-like behaviour—Studies with chronic nicotine administration in rats. Behav. Pharmacol. 2023, 34, 307–317. [Google Scholar] [CrossRef]
- Ma, M.; Quan, H.; Chen, S.; Fu, X.; Zang, L.; Dong, L. The anxiolytic effect of polysaccharides from stellariae radix through monoamine neurotransmitters, HPA axis, and ECS/ERK/CREB/BDNF signaling pathway in stress-induced male rats. Brain Res. Bull. 2023, 203, 110768. [Google Scholar] [CrossRef]
- Tao, Y.; Zhou, H.; Li, Z.; Wu, H.; Wu, F.; Miao, Z.; Shi, H.; Huang, F.; Wu, X. TGR5 deficiency-induced anxiety and depression-like behaviors: The role of gut microbiota dysbiosis. J. Affect. Disord. 2024, 344, 219–232. [Google Scholar] [CrossRef]
- Naslund, J.; Studer, E.; Johansson, E.; Eriksson, E. Effects of gonadectomy and serotonin depletion on inter-individual differences in anxiety-like behaviour in male Wistar rats. Behav. Brain Res. 2016, 308, 160–165. [Google Scholar] [CrossRef]
- Echeverri, N.; Govendir, M. Does the selective serotonin reuptake inhibitor (SSRI) fluoxetine modify canine anxiety related behaviour? Vet. Evid. 2022, 7, 1–12. [Google Scholar] [CrossRef]
- Glover, M.E.; Clinton, S.M. Of rodents and humans: A comparative review of the neurobehavioral effects of early life SSRI exposure in preclinical and clinical research. Int. J. Dev. Neurosci. 2016, 51, 50–72. [Google Scholar] [CrossRef] [PubMed]
- Stahl, S.M. Mechanism of action of serotonin selective reuptake inhibitors: Serotonin receptors and pathways mediate therapeutic effects and side effects. J. Affect. Disord. 1998, 51, 215–235. [Google Scholar] [CrossRef] [PubMed]
- Willadsen, M.; Schwarting, R.K.W.; Wohr, M. Acute anxiogenic effects of escitalopram are associated with mild alterations in D-amphetamine-induced behavior and social approach evoked by playback of 50-kHz ultrasonic vocalizations in rats. Neuropharmacology 2023, 241, 109734. [Google Scholar] [CrossRef]
- Martin, J.M.; Bertram, M.G.; Saaristo, M.; Fursdon, J.B.; Hannington, S.L.; Brooks, B.W.; Burket, S.R.; Mole, R.A.; Deal, N.D.S.; Wong, B.B.M. Antidepressants in surface waters: Fluoxetine influences mosquitofish anxiety-related behavior at environmentally relevant levels. Environ. Sci. Technol. 2019, 53, 6035–6043. [Google Scholar] [CrossRef]
- Kim, S.; Kim, J.; Yun, E.J.; Kim, K.H. Food metabolomics: From farm to human. Curr. Opin. Biotechnol. 2016, 37, 16–23. [Google Scholar] [CrossRef]
- Tran, H.; McConville, M.; Loukopoulos, P. Metabolomics in the study of spontaneous animal diseases. J. Vet. Diagn. Investig. 2020, 32, 635–647. [Google Scholar] [CrossRef]
- Wu, W.; Zhang, L.; Zheng, X.; Huang, Q.; Farag, M.A.; Zhu, R.; Zhao, C. Emerging applications of metabolomics in food science and future trends. Food Chem. X 2022, 16, 100500. [Google Scholar] [CrossRef]
- McGee, E.E.; Kiblawi, R.; Playdon, M.C.; Eliassen, A.H. Nutritional metabolomics in cancer epidemiology: Current trends, challenges, and future directions. Curr. Nutr. Rep. 2019, 8, 187–201. [Google Scholar] [CrossRef]
- Qin, N.; Qin, M.; Shi, W.; Kong, L.; Wang, L.; Xu, G.; Guo, Y.; Zhang, J.; Ma, Q. Investigation of pathogenesis of hyperuricemia based on untargeted and targeted metabolomics. Sci. Rep. 2022, 12, 13980. [Google Scholar] [CrossRef]
- Fukusaki, E.; Kobayashi, A. Plant metabolomics: Potential for practical operation. J. Biosci. Bioeng. 2005, 100, 347–354. [Google Scholar] [CrossRef] [PubMed]
- Ramirez, T.; Daneshian, M.; Kamp, H.; Bois, F.Y.; Clench, M.R.; Coen, M.; Donley, B.; Fischer, S.M.; Ekman, D.R.; Fabian, E.; et al. Metabolomics in toxicology and preclinical research. ALTEX 2013, 30, 209–225. [Google Scholar] [CrossRef] [PubMed]
- Filiou, M.D.; Asara, J.M.; Nussbaumer, M.; Teplytska, L.; Landgraf, R.; Turck, C.W. Behavioral extremes of trait anxiety in mice are characterized by distinct metabolic profiles. J. Psychiatr. Res. 2014, 58, 115–122. [Google Scholar] [CrossRef]
- Zhang, Y.; Filiou, M.D.; Reckow, S.; Gormanns, P.; Maccarrone, G.; Kessler, M.S.; Frank, E.; Hambsch, B.; Holsboer, F.; Landgraf, R.; et al. Proteomic and metabolomic profiling of a trait anxiety mouse model implicate affected pathways. Mol. Cell. Proteom. 2011, 10, M111.008110. [Google Scholar] [CrossRef]
- Liu, Y.; Zhao, W.; Lu, Y.; Zhao, Y.; Zhang, Y.; Dai, M.; Hai, S.; Ge, N.; Zhang, S.; Huang, M.; et al. Systematic metabolic characterization of mental disorders reveals age-related metabolic disturbances as potential risk factors for depression in older adults. MedComm 2022, 3, e165. [Google Scholar] [CrossRef]
- Piszczek, L.; Schlax, K.; Wyrzykowska, A.; Piszczek, A.; Audero, E.; Thilo Gross, C. Serotonin 1A auto-receptors are not sufficient to modulate anxiety in mice. Eur. J. Neurosci. 2013, 38, 2621–2627. [Google Scholar] [CrossRef]
- Louiset, E.; Valentijn, J.A.; Vaudry, H.; Cazin, L. Central-type benzodiazepines modulate GABAA receptor chloride channels in cultured pituitary melanotrophs. Mol. Brain Res. 1992, 12, 1–6. [Google Scholar] [CrossRef]
- Ochoa, J.G.; Kilgo, W.A. The role of benzodiazepines in the treatment of epilepsy. Curr. Treat. Options Neurol. 2016, 18, 18. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Wang, B.; Zhong, Z.; Chen, H.; Ding, W.; Hoi, M.P.M. Clonazepam attenuates neurobehavioral abnormalities in offspring exposed to maternal immune activation by enhancing GABAergic neurotransmission. Biochem. Pharmacol. 2021, 192, 114711. [Google Scholar] [CrossRef]
- Gusso, D.; Altenhofen, S.; Fritsch, P.M.; Rubensam, G.; Bonan, C.D. Oxytetracycline induces anxiety-like behavior in adult zebrafish. Toxicol. Appl. Pharmacol. 2021, 426, 115616. [Google Scholar] [CrossRef]
- Li, G.; Jian, T.; Liu, X.; Lv, Q.; Zhang, G.; Ling, J. Application of metabolomics in fungal research. Molecules 2022, 27, 7365. [Google Scholar] [CrossRef] [PubMed]
- Demianchuk, O.; Vatashchuk, M.; Gospodaryov, D.; Hurza, V.; Ivanochko, M.; Derkachov, V.; Berezovskyi, V.; Lushchak, O.; Storey, K.B.; Bayliak, M.; et al. High-fat high-fructose diet and alpha-ketoglutarate affect mouse behavior that is accompanied by changes in oxidative stress response and energy metabolism in the cerebral cortex. Biochim. Biophys. Acta Gen. Subj. 2024, 1868, 130521. [Google Scholar] [CrossRef] [PubMed]
- Yin, X.; Deng, Y.; Guo, C.; Ding, C.; Xu, J.; Wu, F. Behavioral changes and metabolic responses of adult zebrafish (Danio Rerio) exposed to methamphetamine. ACS ES&T Water 2023, 3, 2551–2559. [Google Scholar] [CrossRef]
- Lettieri, G.; Marinaro, C.; Notariale, R.; Perrone, P.; Lombardi, M.; Trotta, A.; Troisi, J.; Piscopo, M. Impact of Heavy Metal Exposure on Mytilus galloprovincialis Spermatozoa: A Metabolomic Investigation. Metabolites 2023, 13, 943. [Google Scholar] [CrossRef] [PubMed]
- Custodio, R.J.P.; Hobloss, Z.; Myllys, M.; Hassan, R.; Gonzalez, D.; Reinders, J.; Bornhorst, J.; Weishaupt, A.K.; Seddek, A.L.; Abbas, T.; et al. Cognitive functions, neurotransmitter alterations, and hippocampal microstructural changes in mice caused by feeding on western diet. Cells 2023, 12, 2331. [Google Scholar] [CrossRef]
- Puurunen, J.; Tiira, K.; Lehtonen, M.; Hanhineva, K.; Lohi, H. Non-targeted metabolite profiling reveals changes in oxidative stress, tryptophan and lipid metabolisms in fearful dogs. Behav. Brain Funct. 2016, 12, 7. [Google Scholar] [CrossRef]
- Fang, Y.; Li, Y.; Liao, X.; Deng, J.; Wang, Q.; Liang, J.; Yan, B. Corydalis yanhusuo polysaccharides ameliorate chronic stress-induced depression in mice through gut microbiota-derived short-chain fatty acid activation of 5-hydroxytryptamine signaling. J. Med. Food 2023, 26, 890–901. [Google Scholar] [CrossRef] [PubMed]
- Ye, F.; Dong, M.C.; Xu, C.X.; Jiang, N.; Chang, Q.; Liu, X.M.; Pan, R.L. Effects of different chronic restraint stress periods on anxiety- and depression-like behaviors and tryptophan-kynurenine metabolism along the brain-gut axis in C57BL/6N mice. Eur. J. Pharmacol. 2023, 965, 176301. [Google Scholar] [CrossRef]
- Alizadeh Pahlavani, H. Possible role of exercise therapy on depression: Effector neurotransmitters as key players. Behav. Brain Res. 2024, 459, 114791. [Google Scholar] [CrossRef]
- Gao, K.; Farzi, A.; Ke, X.; Yu, Y.; Chen, C.; Chen, S.; Yu, T.; Wang, H.; Li, Y. Oral administration of Lactococcus lactis WHH2078 alleviates depressive and anxiety symptoms in mice with induced chronic stress. Food Funct. 2022, 13, 957–969. [Google Scholar] [CrossRef]
- Badawy, A.A. The kynurenine pathway of tryptophan metabolism: A neglected therapeutic target of COVID-19 pathophysiology and immunotherapy. Biosci. Rep. 2023, 43, BSR20230595. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.; Fan, Y.; Xu, L.; Yu, Z.; Wang, S.; Xu, H.; Zhang, J.; Zhang, L.; Liu, W.; Wu, L.; et al. Microbiome and tryptophan metabolomics analysis in adolescent depression: Roles of the gut microbiota in the regulation of tryptophan-derived neurotransmitters and behaviors in human and mice. Microbiome 2023, 11, 145. [Google Scholar] [CrossRef] [PubMed]
- Jang, S.W.; Liu, X.; Pradoldej, S.; Tosini, G.; Chang, Q.; Iuvone, P.M.; Ye, K. N-acetylserotonin activates TrkB receptor in a circadian rhythm. Proc. Natl. Acad. Sci. USA 2010, 107, 3876–3881. [Google Scholar] [CrossRef] [PubMed]
- Kopp, C.; Vogel, E.; Rettori, M.; Delagrange, P.; Misslin, R. Anxiolytic-like properties of melatonin receptor agonists in mice: Involvement of mt1 and/or MT2 receptors in the regulation of emotional responsiveness. Neuropharmacology 2000, 39, 1865–1871. [Google Scholar] [CrossRef]
- Orabona, C.; Puccetti, P.; Vacca, C.; Bicciato, S.; Luchini, A.; Fallarino, F.; Bianchi, R.; Velardi, E.; Perruccio, K.; Velardi, A.; et al. Toward the identification of a tolerogenic signature in IDO-competent dendritic cells. Blood 2006, 107, 2846–2854. [Google Scholar] [CrossRef]
- O’Connor, J.C.; Lawson, M.A.; Andre, C.; Moreau, M.; Lestage, J.; Castanon, N.; Kelley, K.W.; Dantzer, R. Lipopolysaccharide-induced depressive-like behavior is mediated by indoleamine 2,3-dioxygenase activation in mice. Mol. Psychiatry 2009, 14, 511–522. [Google Scholar] [CrossRef] [PubMed]
- Salazar, A.; Gonzalez-Rivera, B.L.; Redus, L.; Parrott, J.M.; O’Connor, J.C. Indoleamine 2,3-dioxygenase mediates anhedonia and anxiety-like behaviors caused by peripheral lipopolysaccharide immune challenge. Horm. Behav. 2012, 62, 202–209. [Google Scholar] [CrossRef]
- Bercik, P.; Verdu, E.F.; Foster, J.A.; Macri, J.; Potter, M.; Huang, X.; Malinowski, P.; Jackson, W.; Blennerhassett, P.; Neufeld, K.A.; et al. Chronic gastrointestinal inflammation induces anxiety-like behavior and alters central nervous system biochemistry in mice. Gastroenterology 2010, 139, 2102–2112.e1. [Google Scholar] [CrossRef]
- Ibos, K.E.; Bodnar, E.; Dinh, H.; Kis, M.; Marvanykovi, F.; Kovacs, Z.Z.A.; Siska, A.; Foldesi, I.; Galla, Z.; Monostori, P.; et al. Chronic kidney disease may evoke anxiety by altering CRH expression in the amygdala and tryptophan metabolism in rats. Pflug. Arch. 2023, 476, 179–196. [Google Scholar] [CrossRef]
- Varga, D.; Heredi, J.; Kanvasi, Z.; Ruszka, M.; Kis, Z.; Ono, E.; Iwamori, N.; Iwamori, T.; Takakuwa, H.; Vecsei, L.; et al. Systemic L-Kynurenine sulfate administration disrupts object recognition memory, alters open field behavior and decreases c-Fos immunopositivity in C57Bl/6 mice. Front. Behav. Neurosci. 2015, 9, 157. [Google Scholar] [CrossRef]
- Ono, T.; Hino, K.; Kimura, T.; Uchimura, Y.; Ashihara, T.; Higa, T.; Kojima, H.; Murakami, T.; Udagawa, J. Excessive folic acid intake combined with undernutrition during gestation alters offspring behavior and brain monoamine profiles. Congenit. Anom. 2022, 62, 169–180. [Google Scholar] [CrossRef] [PubMed]
- Sahara, Y.; Matsuzawa, D.; Ishii, D.; Fuchida, T.; Goto, T.; Sutoh, C.; Shimizu, E. Paternal methyl donor deficient diets during development affect male offspring behavior and memory-related gene expression in mice. Dev. Psychobiol. 2019, 61, 17–28. [Google Scholar] [CrossRef] [PubMed]
- Tuo, L.J.; Song, X.Y.; Zhu, Y.Y.; He, H.N.; Song, Y.P.; Chen, D.Z.; Zheng, X.M.; Zhang, H.; Xu, D.X. Gestational folic acid supplement prevents vitamin D deficiency-induced depression-like behavior by reversing cortical DNA hypomethylation in adult offspring. J. Steroid Biochem. Mol. Biol. 2023, 231, 106313. [Google Scholar] [CrossRef] [PubMed]
- Onaolapo, O.J.; Olofinnade, A.T.; Ojo, F.O.; Falade, J.; Onaolapo, A.Y. Prepubertal continuous dietary folate fortification enhances the brain function of adult mice by modulating antioxidant status, inflammation, and brain neurotransmitter levels. Antinflammatory Antiallergy Agents Med. Chem. 2023, 22, 198–209. [Google Scholar] [CrossRef]
- Hosseini, S.H.; Khabbazhosseini, Z.S.; Khatibi, S.; Yahosseini, A.; Borhaninejad, N.; Beheshti, F.; Kakhki, S. Folic acid supplementation improved nicotine withdrawal-induced of memory loss via affecting oxidative status, inflammatory response, cholinergic activity, BDNF and amyloid-B in adolescent male rat. Neurosci. Lett. 2023, 815, 137489. [Google Scholar] [CrossRef]
- Shemirani, F.; Titcomb, T.J.; Saxby, S.M.; Eyck, P.T.; Rubenstein, L.M.; Hoth, K.F.; Snetselaar, L.G.; Wahls, T.L. Association of serum homocysteine, folate, and vitamin B12 and mood following the Swank and Wahls elimination dietary interventions in relapsing-remitting multiple sclerosis: Secondary analysis of the WAVES trial. Mult. Scler. Relat. Disord. 2023, 75, 104743. [Google Scholar] [CrossRef]
- Bult, C.J.; Blake, J.A.; Smith, C.L.; Kadin, J.A.; Richardson, J.E. Mouse Genome Database (MGD) 2019. Nucleic Acids Res. 2019, 47, D801–D806. [Google Scholar] [CrossRef]
- Perkins, R.; Boal, C.; Rollins, D.; Perez, R.M. Northern bobwhite predator avoidance behavior in response to varying types of threat. J. Wildl. Manag. 2014, 78, 1272–1281. [Google Scholar] [CrossRef]
- Quah, S.K.L.; Cockcroft, G.J.; McIver, L.; Santangelo, A.M.; Roberts, A.C. Avoidant coping style to high imminence threat is linked to higher anxiety-like behavior. Front. Behav. Neurosci. 2020, 14, 34. [Google Scholar] [CrossRef]
- Paçal, E.; Gümüş, A.B.; Günal, A.Ç.; Erkmen, B.; Arslan, P.; Yıldırım, Z.; Erkoç, F. Oxidative stress response as biomarker of exposure of a freshwater invertebrate model organism (Unio mancus Lamarck, 1819) to antifouling copper pyrithione. Pestic. Phytomedicine/Pestic. Fitomedicina 2022, 37, 63–76. [Google Scholar] [CrossRef]
- Greisberg, J.; Gorroochurn, P.; Greisberg, J.K. Sustained anxiety-like behavior in crayfish exposed to thermal burn. J. Stud. Res. 2022, 11, 1–6. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhai, W.; Fu, Y.; Liu, L.; Huang, X.; Wang, S. Metabolomics Reveal Key Metabolic Pathway Responses to Anxiety State Regulated by Serotonin in Portunus trituberculatus. Metabolites 2024, 14, 568. https://doi.org/10.3390/metabo14100568
Zhai W, Fu Y, Liu L, Huang X, Wang S. Metabolomics Reveal Key Metabolic Pathway Responses to Anxiety State Regulated by Serotonin in Portunus trituberculatus. Metabolites. 2024; 14(10):568. https://doi.org/10.3390/metabo14100568
Chicago/Turabian StyleZhai, Wei, Yuanyuan Fu, Lei Liu, Xinlian Huang, and Sixiang Wang. 2024. "Metabolomics Reveal Key Metabolic Pathway Responses to Anxiety State Regulated by Serotonin in Portunus trituberculatus" Metabolites 14, no. 10: 568. https://doi.org/10.3390/metabo14100568
APA StyleZhai, W., Fu, Y., Liu, L., Huang, X., & Wang, S. (2024). Metabolomics Reveal Key Metabolic Pathway Responses to Anxiety State Regulated by Serotonin in Portunus trituberculatus. Metabolites, 14(10), 568. https://doi.org/10.3390/metabo14100568







