Biostimulant-Based Molecular Priming Improves Crop Quality and Enhances Yield of Raspberry and Strawberry Fruits
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material, Growth, Biostimulant Treatment, and Measures of Fruit Weight and Yield
2.2. Metabolome Analysis of Primary Metabolites by LC-MS
2.3. Analysis of Elements by ICP-MS
3. Results
3.1. The Ascophyllum Nodosum-Derived Biostimulant SuperFifty Increases Fruit Size, Weight, and Yield in Raspberry and Strawberry Plants
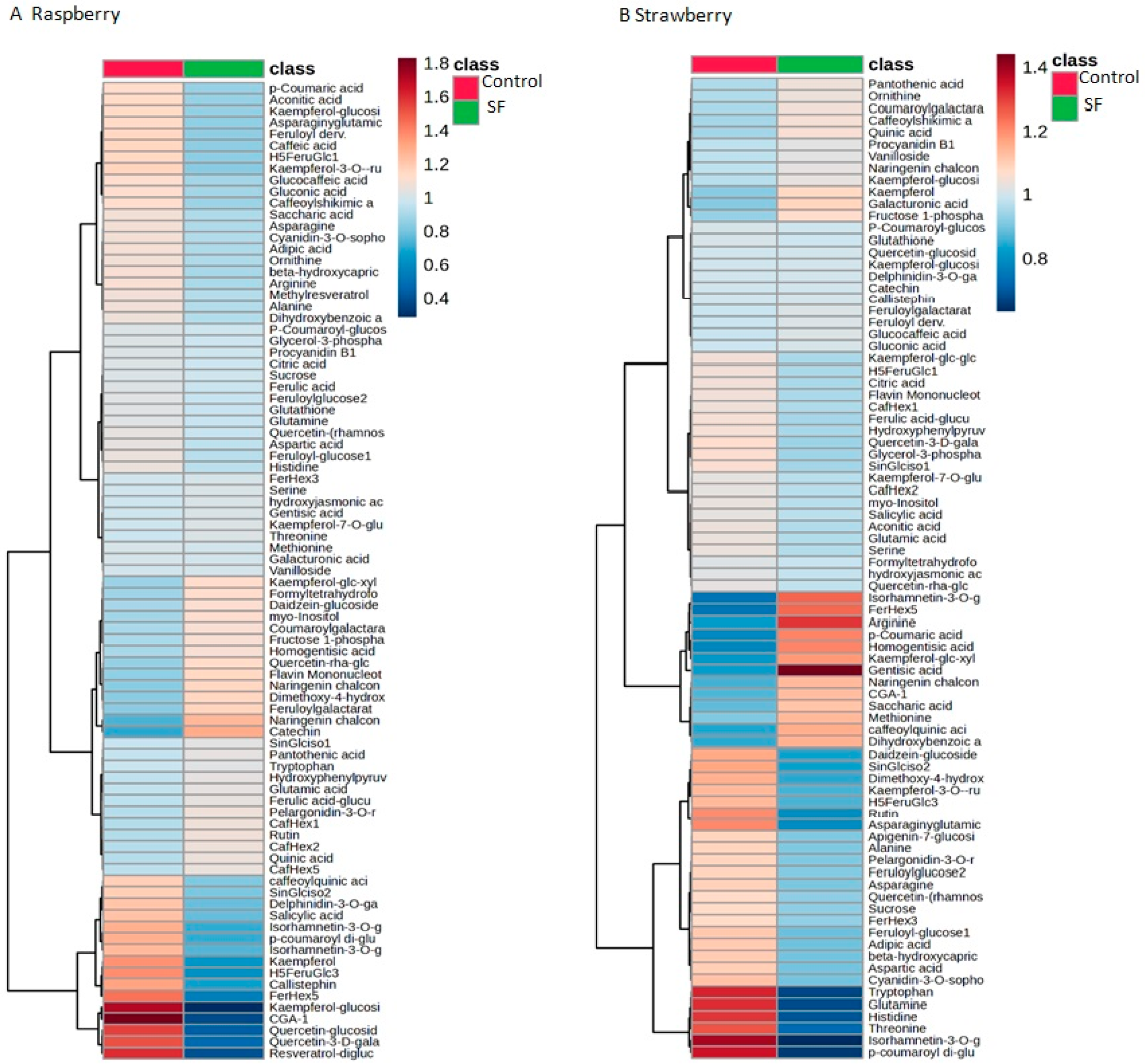
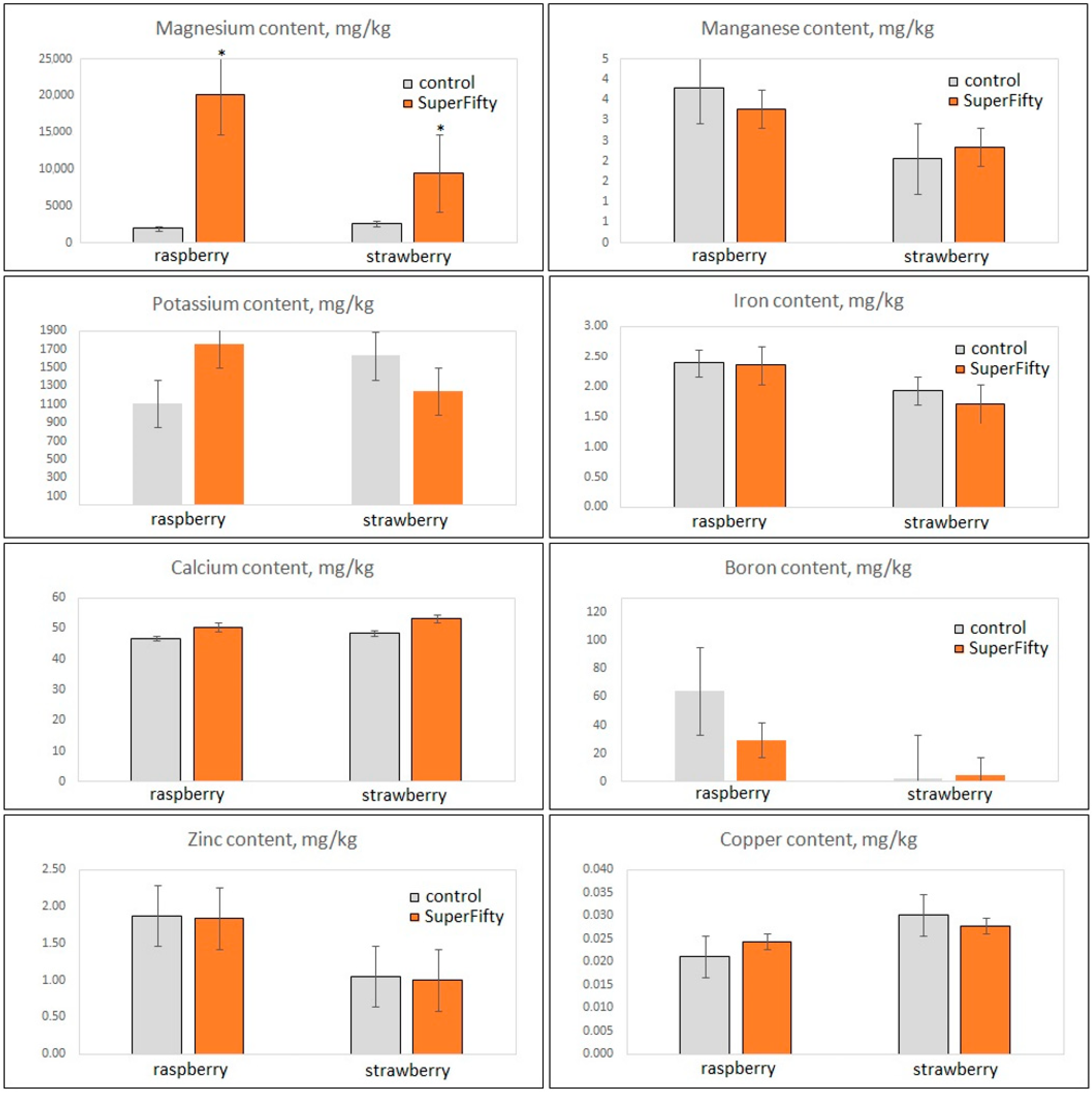
3.2. Nutritional Properties of Berry Fruits, Evaluated by GC-MS and ICP-MS Analyses of Primary Metabolites and Essential Elements, Are Preserved in the Biostimulant-Primed Plants
3.3. Market Benefit and Environmental Sustainability Analyses of the Molecular Priming Technology Applied to Raspberry and Strawberry Crops
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sujeeth, N.; Petrov, V.; Guinan, K.J.; Rasul, F.; O’Sullivan, J.T.; Gechev, T.S. Current insights into the molecular mode of action of seaweed-based biostimulants and the sustainability of seaweeds as raw material resources. Int. J. Mol. Sci. 2022, 23, 7654. [Google Scholar] [CrossRef] [PubMed]
- Du Jardin, P. Plant Biostimulants: Definition, concept, main categories and regulation. Sci. Hortic. 2015, 196, 3–14. [Google Scholar] [CrossRef]
- Kerchev, P.; van der Meer, T.; Sujeeth, N.; Verlee, A.; Stevens, C.; van Breusegem, F.; Gechev, T. Molecular priming as an approach to induce tolerance against abiotic and oxidative stresses in crop plants. Biotechnol. Adv. 2020, 40, 107503. [Google Scholar] [CrossRef] [PubMed]
- Nephali, L.; Piater, L.A.; Dubery, I.A.; Patterson, V.; Huyser, J.; Burgess, K.; Tugizimana, F. Biostimulants for plant growth and mitigation of abiotic stresses: A metabolomics perspective. Metabolites 2020, 10, 505. [Google Scholar] [CrossRef]
- Staykov, N.S.; Angelov, M.; Petrov, V.; Minkov, P.; Kanojia, A.; Guinan, K.J.; Alseekh, S.; Fernie, A.R.; Sujeeth, N.; Gechev, T.S. An Ascophyllum nodosum-derived biostimulant protects model and crop plants from oxidative stress. Metabolites 2020, 11, 24. [Google Scholar] [CrossRef]
- Omidbakhshfard, M.A.; Sujeeth, N.; Gupta, S.; Omranian, N.; Guinan, K.J.; Brotman, Y.; Nikoloski, Z.; Fernie, A.R.; Mueller-Roeber, B.; Gechev, T.S. A biostimulant obtained from the seaweed Ascophyllum nodosum protects Arabidopsis thaliana from severe oxidative stress. Int. J. Mol. Sci. 2020, 21, 474. [Google Scholar] [CrossRef]
- Ali, J.; Jan, I.; Ullah, H.; Ahmed, N.; Alam, M.; Ullah, R.; El-Sharnouby, M.; Kesba, H.; Shukry, M.; Sayed, S.; et al. Influence of Ascophyllum nodosum extract foliar spray on the physiological and biochemical attributes of okra under drought stress. Plants 2022, 11, 790. [Google Scholar] [CrossRef]
- Rayirath, P.; Benkel, B.; Mark Hodges, D.; Allan-Wojtas, P.; Mackinnon, S.; Critchley, A.T.; Prithiviraj, B. Lipophilic components of the brown seaweed, Ascophyllum nodosum, enhance freezing tolerance in Arabidopsis thaliana. Planta 2009, 230, 135–147. [Google Scholar] [CrossRef]
- Shukla, P.S.; Shotton, K.; Norman, E.; Neily, W.; Critchley, A.T.; Prithiviraj, B. Seaweed extract improve drought tolerance of soybean by regulating stress-response genes. AoB Plants 2017, 10, plx051. [Google Scholar] [CrossRef]
- Crespo, P.; Bordonaba, J.G.; Terry, L.A.; Carlen, C. Characterisation of major taste and health-related compounds of four strawberry genotypes grown at different Swiss production sites. Food Chem. 2010, 122, 16–24. [Google Scholar] [CrossRef]
- Bobinaitė, R.; Viškelis, P.; Venskutonis, P.R. Chemical composition of raspberry (Rubus spp.) cultivars. In Nutritional Composition of Fruit Cultivars; Simmonds, M.S.J., Preedy, V.R., Eds.; Academic Press: Cambridge, MA, USA, 2016; pp. 713–731. [Google Scholar]
- Zahedi, S.M.; Hosseini, M.S.; Hoveizeh, N.F.; Kadkhodaei, S.; Vaculik, M. Physiological and biochemical responses of commercial strawberry cultivars under optimal and drought stress conditions. Plants 2023, 12, 496. [Google Scholar] [CrossRef] [PubMed]
- Packer Berry per Capita Availability Growth Shines. 2024. Available online: https://www.thepacker.com/news/produce-crops/berry-capita-availability-growth-shines (accessed on 4 September 2024).
- Mattner, S.W.; Milinkovic, M.; Arioli, T. Increased growth response of strawberry roots to a commercial extract from Durvillaea potatorum and Ascophyllum nodosum. J. Appl. Phycol. 2018, 30, 2943–2951. [Google Scholar] [CrossRef] [PubMed]
- Tohge, T.; Fernie, A.R. Combining genetic diversity, informatics and metabolomics to facilitate annotation of plant gene function. Nat. Protoc. 2010, 5, 1210–1227. [Google Scholar] [CrossRef] [PubMed]
- Tohge, T.; Fernie, A.R. Web-based resources for massspectrometry-based metabolomics: A user’s guide. Phytochemistry 2009, 70, 450–456. [Google Scholar] [CrossRef]
- Miller, O. Microwave digestion of plant tissue in closed vessels. In Handbook of Reference Methods for Plant Analysis, 1st ed.; Kalra, Y.P., Ed.; CRC Press: Boca Raton, FL, USA, 1998; pp. 69–73. ISBN 978-1-57444-124-6. [Google Scholar]
- Sigala Aguilar, N.A.; Gonzales Fuentes, J.A.; Valdez Aguilar, L.A.; López Pérez, M.G.; Macias, J.M.; Benavides-Mendoza, A.; González Morales, S. Effect of elicitors and biostimulants on the content of bioactive compounds in raspberry fruits. Hortic. Sci. 2023, 50, 101–111. [Google Scholar] [CrossRef]
- Tinte, M.M.; Masike, K.; Steenkamp, P.A.; Huyser, J.; van der Hooft, J.J.J.; Tugizimana, F. Computational metabolomics tools reveal metabolic reconfigurations underlying the effects of biostimulant seaweed extracts on maize plants under drought stress conditions. Metabolites 2022, 12, 487. [Google Scholar] [CrossRef]
- Drobek, M.; Cybulska, J.; Zdunek, A.; Sas-Paszt, L.; Frąc, M. Effect of microbial biostimulants on the antioxidant profile, antioxidant capacity and activity of enzymes influencing the quality level of raspberries (Rubus idaeus L.). Food Chem. 2024, 454, 139746. [Google Scholar] [CrossRef]
- Consentino, B.B.; Vultaggio, L.; Iacuzzi, N.; La Bella, S.; De Pasquale, C.; Rouphael, Y.; Ntatsi, G.; Virga, G.; Sabatino, L. Iodine biofortification and seaweed extract-based biostimulant supply interactively drive the yield, quality, and functional traits in strawberry fruits. Plants 2023, 12, 245. [Google Scholar] [CrossRef]
- Bajpai, S.; Shukla, P.S.; Asiedu, S.; Pruski, K.; Prithiviraj, B. A biostimulant preparation of brown seaweed Ascophyllum nodosum suppresses powdery mildew of strawberry. Plant Pathol. J. 2019, 35, 406–416. [Google Scholar] [CrossRef]
- De Baaij, J.H.; Hoenderop, J.G.; Bindels, R.J. Magnesium in man: Implications for health and disease. Physiol. Rev. 2015, 95, 1–46. [Google Scholar] [CrossRef]
- Feeney, K.A.; Hansen, L.L.; Putker, M.; Olivares-Yañez, C.; Day, J.; Eades, L.J.; Larrondo, L.F.; Hoyle, N.P.; O’Neill, J.S.; van Ooijen, G. Daily magnesium fluxes regulate cellular timekeeping and energy balance. Nature 2016, 532, 375–379. [Google Scholar] [CrossRef] [PubMed]
- King, D.E.; Mainous, A.G.; Geesey, M.E.; Woolson, R.F. Dietary magnesium and C-reactive protein levels. J. Am. Coll. Nutr. 2005, 24, 166–171. [Google Scholar] [CrossRef] [PubMed]
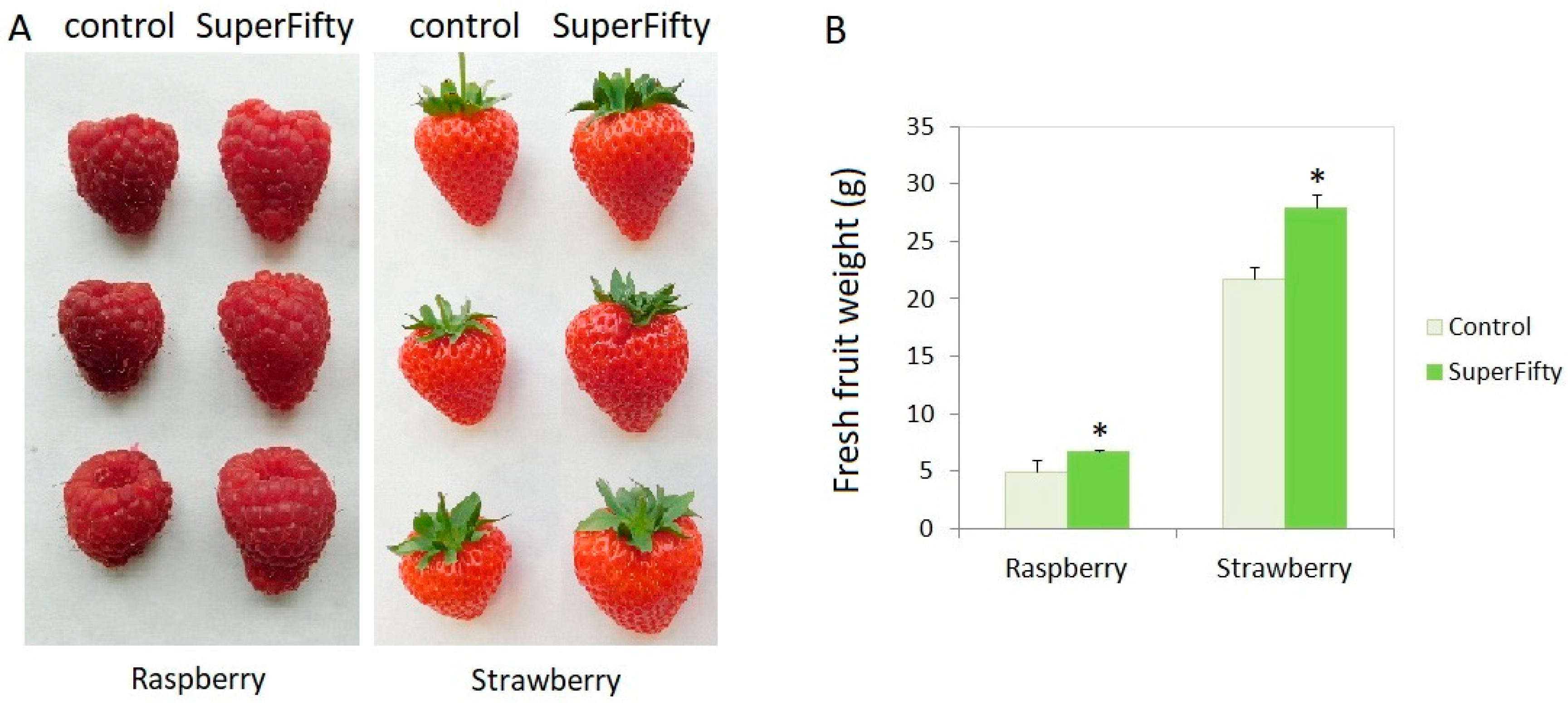
| Crop | Fruit Fresh Weight (g) | % Increase Due to SuperFifty | |
|---|---|---|---|
| Control (Unprimed) | SuperFifty-Primed | ||
| Raspberry | 2742.4 | 3897.2 | 42.1 |
| Strawberry | 16,943.2 | 22,686.92 | 33.9 |
| Raspberry | Strawberry | |||
|---|---|---|---|---|
| Metabolite | Control | SF | Control | SF |
| Sucrose | 57,760.00 | 56,180.00 | 29,040.00 | 24,798.00 |
| Serine | 278.60 | 285.40 | 45.28 | 42.11 |
| Alanine | 596.60 | 510.80 | 344.40 | 287.40 |
| Threonine | 176.20 | 184.20 | 98.25 | 55.97 |
| Asparagine | 8672.00 | 7304.00 | 5256.00 | 4328.00 |
| Ornithine | 134.80 | 112.87 | 5.50 | 5.96 |
| Aspartic acid | 6048.00 | 5466.00 | 3784.00 | 3076.00 |
| Glutamine | 2036.00 | 1906.80 | 2419.40 | 1239.40 |
| Glutamic acid | 10,314.00 | 11,324.00 | 4044.00 | 3744.00 |
| Histidine | 253.60 | 224.80 | 140.88 | 74.51 |
| Glycerol-3-phosphate | 195.60 | 188.00 | 110.47 | 97.88 |
| Aconitic acid | 84.16 | 63.71 | 138.79 | 128.23 |
| Arginine | 530.20 | 436.60 | 2.95 | 4.72 |
| myo-Inositol | 10,970.00 | 13,824.00 | 19,740.00 | 18,520.00 |
| Citric acid | 86,780.00 | 83,140.00 | 53,680.00 | 49,080.00 |
| Quinic acid | 369.20 | 424.60 | 1029.40 | 1149.20 |
| Galacturonic acid | 2124.00 | 2114.00 | 2172.00 | 2596.00 |
| Gluconic acid | 10,422.00 | 8274.00 | 6806.00 | 6898.00 |
| Saccharic acid | 5442.00 | 4588.00 | 1430.00 | 1818.00 |
| Fructose 1-phosphate | 2888.00 | 3500.00 | 3484.00 | 4008.00 |
| Asparaginyglutamic acid | 174.40 | 129.14 | 379.20 | 249.40 |
| Glutathione | 328.20 | 307.80 | 615.80 | 605.20 |
| Caffeic acid | 33.44 | 24.44 | 0.00 | 0.00 |
| Methionine | 7.62 | 7.53 | 0.57 | 0.71 |
| Tryptophan | 274.60 | 291.80 | 824.80 | 415.00 |
| Pantothenic acid | 473.80 | 514.40 | 39.95 | 43.51 |
| Catechin | 225.60 | 416.40 | 5850.00 | 5864.00 |
| Salicylic acid | 173.20 | 109.25 | 304.40 | 287.60 |
| Vanilloside | 381.20 | 383.80 | 4.18 | 4.39 |
| Glucocaffeic acid | 411.60 | 327.00 | 30.08 | 30.96 |
| Resveratrol-diglucoside | 21.55 | 5.39 | 0.00 | 0.00 |
| Procyanidin B1 | 2366.00 | 2282.00 | 418.20 | 439.00 |
| Formyltetrahydrofolate | 9.76 | 12.42 | 37.58 | 36.39 |
| Rutin | 35.55 | 41.62 | 6.69 | 4.43 |
| Ferulic acid | 13.21 | 12.56 | 0.00 | 0.00 |
| Kaempferol | 2.42 | 1.14 | 11.16 | 13.22 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kazakov, P.; Alseekh, S.; Ivanova, V.; Gechev, T. Biostimulant-Based Molecular Priming Improves Crop Quality and Enhances Yield of Raspberry and Strawberry Fruits. Metabolites 2024, 14, 594. https://doi.org/10.3390/metabo14110594
Kazakov P, Alseekh S, Ivanova V, Gechev T. Biostimulant-Based Molecular Priming Improves Crop Quality and Enhances Yield of Raspberry and Strawberry Fruits. Metabolites. 2024; 14(11):594. https://doi.org/10.3390/metabo14110594
Chicago/Turabian StyleKazakov, Petar, Saleh Alseekh, Valentina Ivanova, and Tsanko Gechev. 2024. "Biostimulant-Based Molecular Priming Improves Crop Quality and Enhances Yield of Raspberry and Strawberry Fruits" Metabolites 14, no. 11: 594. https://doi.org/10.3390/metabo14110594
APA StyleKazakov, P., Alseekh, S., Ivanova, V., & Gechev, T. (2024). Biostimulant-Based Molecular Priming Improves Crop Quality and Enhances Yield of Raspberry and Strawberry Fruits. Metabolites, 14(11), 594. https://doi.org/10.3390/metabo14110594








