Hepatic Transcriptomics of Broilers with Low and High Feed Conversion in Response to Caloric Restriction
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Animals, Housing, and Tissue Sample Collection
2.2. RNA Isolation and Purification
2.3. Microarray Processing and Data Analysis
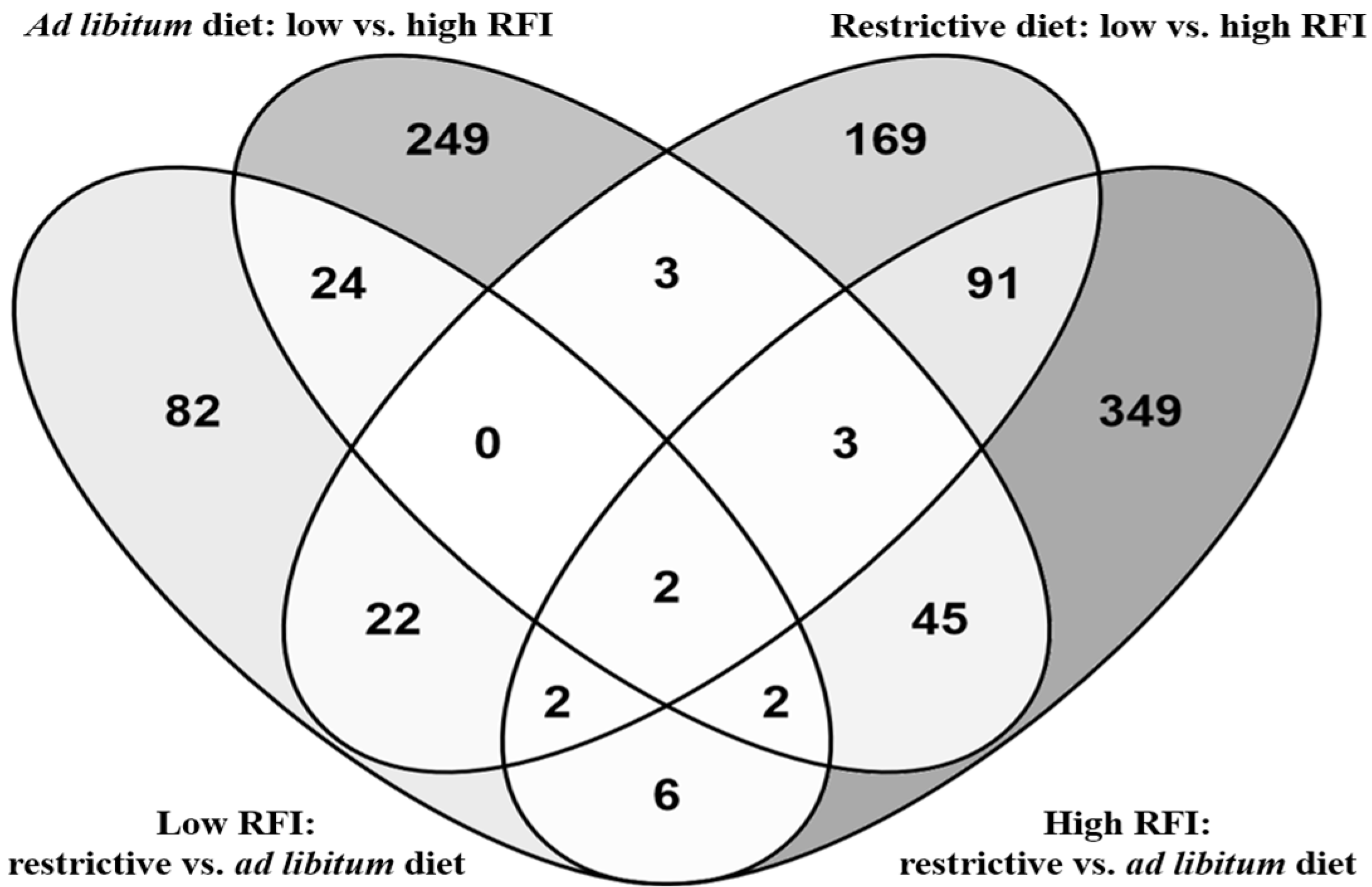
3. Results
4. Discussion
4.1. Low RFI vs. High RFI Under Ad Libitum Feeding
4.2. Low RFI vs. High RFI Under Restricted Feeding
4.3. Restrictive vs. Ad Libitum Feeding of Low-RFI Phenotypes
4.4. Restrictive vs. Ad Libitum Feeding of High-RFI Phenotypes
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Willems, O.; Miller, S.; Wood, B. Aspects of selection for feed efficiency in meat producing poultry. World’s Poult. Sci. J. 2013, 69, 77–88. [Google Scholar] [CrossRef]
- Kennedy, B.; van der Werf, J.; Meuwissen, T. Genetic and statistical properties of residual feed intake. J. Anim. Sci. 1993, 71, 3239–3250. [Google Scholar] [CrossRef]
- Mebratie, W.; Reyer, H.; Wimmers, K.; Bovenhuis, H.; Jensen, J. Genome wide association study of body weight and feed efficiency traits in a commercial broiler chicken population, a re-visitation. Sci. Rep. 2019, 9, 922. [Google Scholar] [CrossRef]
- Aggrey, S.E.; Karnuah, A.B.; Sebastian, B.; Anthony, N.B. Genetic properties of feed efficiency parameters in meat-type chickens. Genet. Sel. Evol. 2010, 42, 25. [Google Scholar] [CrossRef] [PubMed]
- Bebber, J.v.; Mercer, J. Selection for efficiency in broilers: A comparison of residual feed intake with feed conversion ratio. In Proceedings of the 5th World Congress of Genetics Applied to Livestock Production, Guelph, ON, Canada, 7–12 August 1994; pp. 53–56. [Google Scholar]
- Sell-Kubiak, E.; Wimmers, K.; Reyer, H.; Szwaczkowski, T. Genetic aspects of feed efficiency and reduction of environmental footprint in broilers: A review. J. Appl. Genet. 2017, 58, 487–498. [Google Scholar] [CrossRef]
- Mebratie, W.; Madsen, P.; Hawken, R.; Romé, H.; Henshall, J.; Bovenhuis, H.; Jensen, J. Genetic parameters for body weight and different definitions of residual feed intake in broiler chickens. Genet. Sel. Evol. 2019, 51, 53. [Google Scholar] [CrossRef] [PubMed]
- de Freitas, L.F.V.B.; Sakomura, N.K.; de Paula Reis, M.; Mariani, A.B.; Lambert, W.; Andretta, I.; Létourneau-Montminy, M.-P. Coccidiosis infection and growth performance of broilers in experimental trials: Insights from a meta-analysis including modulating factors. Poult. Sci. 2023, 102, 103021. [Google Scholar] [CrossRef] [PubMed]
- Kikusato, M.; Toyomizu, M. Mechanisms underlying the effects of heat stress on intestinal integrity, inflammation, and microbiota in chickens. J. Poult. Sci. 2023, 60, 2023021. [Google Scholar] [CrossRef]
- Sobotik, E.B.; Ramirez, S.; Roth, N.; Tacconi, A.; Pender, C.; Murugesan, R.; Archer, G.S. Evaluating the effects of a dietary synbiotic or synbiotic plus enhanced organic acid on broiler performance and cecal and carcass salmonella load. Poult. Sci. 2021, 100, 101508. [Google Scholar] [CrossRef]
- Gilani, S.; Howarth, G.; Kitessa, S.; Tran, C.; Forder, R.; Hughes, R. New biomarkers for increased intestinal permeability induced by dextran sodium sulphate and fasting in chickens. J. Anim. Physiol. Anim. Nutr. 2016, 101, e237–e245. [Google Scholar] [CrossRef]
- Yamauchi, K.; Kamisoyama, H.; Isshiki, Y. Effects of fasting and refeeding on structures of the intestinal villi and epithelial cells in white leghorn hens. Br. Poult. Sci. 1996, 37, 909–921. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Liu, R.; Zheng, M.; Feng, F.; Liu, D.; Guo, Y.; Zhao, G.; Wen, J. New insights into the associations among feed efficiency, metabolizable efficiency traits and related qtl regions in broiler chickens. J. Anim. Sci. Biotechnol. 2020, 11, 65. [Google Scholar] [CrossRef] [PubMed]
- Metzler-Zebeli, B.U.; Siegerstetter, S.-C.; Magowan, E.; Lawlor, P.G.; O’Connell, N.E.; Zebeli, Q. Feed restriction reveals distinct serum metabolome profiles in chickens divergent in feed efficiency traits. Metabolites 2019, 9, 38. [Google Scholar] [CrossRef] [PubMed]
- Tallentire, C.; Leinonen, I.; Kyriazakis, I. Artificial selection for improved energy efficiency is reaching its limits in broiler chickens. Sci. Rep. 2018, 8, 1168. [Google Scholar] [CrossRef] [PubMed]
- Reyer, H.; Metzler-Zebeli, B.U.; Trakooljul, N.; Oster, M.; Muráni, E.; Ponsuksili, S.; Hadlich, F.; Wimmers, K. Transcriptional shifts account for divergent resource allocation in feed efficient broiler chickens. Sci. Rep. 2018, 8, 12903. [Google Scholar] [CrossRef]
- Ren, Z.; Yan, J.; Hu, Q.; Liu, X.; Pan, C.; Liu, Y.; Zhang, X.; Yang, X.; Yang, X. Phosphorus restriction changes the expression of fibroblast growth factor 23 and its receptors in laying hens. Front. Physiol. 2020, 11, 85. [Google Scholar] [CrossRef]
- Siegerstetter, S.-C.; Petri, R.M.; Magowan, E.; Lawlor, P.G.; Zebeli, Q.; O’Connell, N.E.; Metzler-Zebeli, B.U. Feed restriction modulates the fecal microbiota composition, nutrient retention, and feed efficiency in chickens divergent in residual feed intake. Front. Microbiol. 2018, 9, 2698. [Google Scholar] [CrossRef]
- Bottje, W.; Carstens, G. Association of mitochondrial function and feed efficiency in poultry and livestock species. J. Anim. Sci. 2009, 87, E48–E63. [Google Scholar] [CrossRef]
- van der Klein, S.A.S.; Silva, F.A.; Kwakkel, R.P.; Zuidhof, M.J. The effect of quantitative feed restriction on allometric growth in broilers. Poult. Sci. 2017, 96, 118–126. [Google Scholar] [CrossRef]
- Fondevila, G.; Archs, J.L.; Cámara, L.; de Juan, A.F.; Mateos, G.G. The length of the feed restriction period affects eating behavior, growth performance, and the development of the proximal part of the gastrointestinal tract of young broilers. Poult. Sci. 2020, 99, 1010–1018. [Google Scholar] [CrossRef]
- Amoozmehr, A.; Dastar, B.; Ashayerizadeh, O.; Mirshekar, R.; Abdollahi, M.R. Effect of feed form and nutrient density on growth performance, blood parameters, and intestinal traits in broiler breeder pullets. Poult. Sci. 2023, 102, 102700. [Google Scholar] [CrossRef] [PubMed]
- Metzler-Zebeli, B.U.; Siegerstetter, S.-C.; Magowan, E.; Lawlor, P.G.; Petri, R.M.; O’Connell, N.E.; Zebeli, Q. Feed restriction modifies intestinal microbiota-host mucosal networking in chickens divergent in residual feed intake. mSystems 2019, 4, e00261-18. [Google Scholar] [CrossRef] [PubMed]
- Metzler-Zebeli, B.; Molnár, A.; Hollmann, M.; Magowan, E.; Hawken, R.; Lawlor, P.; Zebeli, Q. Comparison of growth performance and excreta composition in broiler chickens when ranked according to various feed efficiency metrics. J. Anim. Sci. 2016, 94, 2890–2899. [Google Scholar] [CrossRef] [PubMed]
- Hadlich, F.; Reyer, H.; Oster, M.; Trakooljul, N.; Muráni, E.; Ponsuksili, S.; Wimmers, K. Reprobe: Workflow for revised probe assignment and updated probe-set annotation in microarrays. Genom. Proteom. Bioinform. 2021, 19, 1043–1049. [Google Scholar] [CrossRef]
- Tallentire, C.W.; Leinonen, I.; Kyriazakis, I. Breeding for efficiency in the broiler chicken: A review. Agron. Sustain. Dev. 2016, 36, 66. [Google Scholar] [CrossRef]
- Havenstein, G.; Ferket, P.; Qureshi, M. Growth, livability, and feed conversion of 1957 versus 2001 broilers when fed representative 1957 and 2001 broiler diets. Poult. Sci. 2003, 82, 1500–1508. [Google Scholar] [CrossRef]
- Chen, C.; Su, Z.; Li, Y.; Luan, P.; Wang, S.; Zhang, H.; Xiao, F.; Guo, H.; Cao, Z.; Li, H. Estimation of the genetic parameters of traits relevant to feed efficiency: Result from broiler lines divergent for high or low abdominal fat content. Poult. Sci. 2021, 100, 461–466. [Google Scholar] [CrossRef] [PubMed]
- Dadfar, M.-J.; Torshizi, R.V.; Maghsoudi, A.; Ehsani, A.; Masoudi, A.A. Trade-off between feed efficiency and immunity in specialized high-performing chickens. Poult. Sci. 2023, 102, 102703. [Google Scholar] [CrossRef]
- Greene, E.S.; Abdelli, N.; Dridi, J.S.; Dridi, S. Avian neuropeptide y: Beyond feed intake regulation. Vet. Sci. 2022, 9, 171. [Google Scholar] [CrossRef]
- Ye, J.; Jiang, S.; Cheng, Z.; Ding, F.; Fan, Q.; Lin, X.; Wang, Y.; Gou, Z. Feed restriction improves lipid metabolism by changing the structure of the cecal microbial community and enhances the meat quality and flavor of bearded chickens. Animals 2022, 12, 970. [Google Scholar] [CrossRef]
- Anene, D.O.; Akter, Y.; Groves, P.J.; Horadagoda, N.; Liu, S.Y.; Moss, A.; Hutchison, C.; O’Shea, C.J. Association of feed efficiency with organ characteristics and fatty liver haemorrhagic syndrome in laying hens. Sci. Rep. 2023, 13, 5872. [Google Scholar] [CrossRef] [PubMed]
- Lutkewitte, A.; McCommis, K.; Schweitzer, G.; Chambers, K.; Graham, M.; Wang, L.; Patti, G.; Hall, A.; Finck, B. Hepatic monoacylglycerol acyltransferase 1 is induced by prolonged food deprivation to modulate the hepatic fasting response. J. Lipid Res. 2019, 60, 528–538. [Google Scholar] [CrossRef] [PubMed]
- Salmória, L.A.; Ibelli, A.M.G.; Tavernari, F.d.C.; Peixoto, J.d.O.; Morés, M.A.Z.; Marcelino, D.E.P.; Pinto, K.D.S.; Coldebella, A.; Surek, D.; Kawski, V.L. Cyp24a1 and trpc3 gene expression in kidneys and their involvement in calcium and phosphate metabolism in laying hens. Animals 2024, 14, 1407. [Google Scholar] [CrossRef] [PubMed]
- Kajimoto, K.; Suemitsu, E.; Sato, Y.; Sakurai, Y.; Harashima, H. Liver-specific silencing of lipin1 reduces fat mass as well as hepatic triglyceride biosynthesis in mice. Biol. Pharm. Bull. 2016, 39, 1653–1661. [Google Scholar] [CrossRef] [PubMed]
- Zerjal, T.; Härtle, S.; Gourichon, D.; Guillory, V.; Bruneau, N.; Laloë, D.; Pinard-van Der Laan, M.-H.; Trapp, S.; Bed’hom, B.; Quéré, P. Assessment of trade-offs between feed efficiency, growth-related traits, and immune activity in experimental lines of layer chickens. Genet. Sel. Evol. 2021, 53, 44. [Google Scholar] [CrossRef]
- Casey, A.K.; Gray, H.F.; Chimalapati, S.; Hernandez, G.; Moehlman, A.T.; Stewart, N.; Fields, H.A.; Gulen, B.; Servage, K.A.; Stefanius, K. Fic-mediated ampylation tempers the unfolded protein response during physiological stress. Proc. Natl. Acad. Sci. USA 2022, 119, e2208317119. [Google Scholar] [CrossRef]
- Casey, A.K.; Stewart, N.M.; Zaidi, N.; Gray, H.F.; Cox, A.; Fields, H.A.; Orth, K. Ficd regulates adaptation to the unfolded protein response in the murine liver. Biochimie 2024, 225, 114–124. [Google Scholar] [CrossRef]
- Montiel-Castro, A.J.; González-Cervantes, R.M.; Bravo-Ruiseco, G.; Pacheco-López, G. The microbiota-gut-brain axis: Neurobehavioral correlates, health and sociality. Front. Integr. Neurosci. 2013, 7, 70. [Google Scholar] [CrossRef]
- Henkel, A.; Green, R.M. The unfolded protein response in fatty liver disease. Semin. Liver Dis. 2013, 33, 321–329. [Google Scholar]
- Xu, C.; Bailly-Maitre, B.; Reed, J.C. Endoplasmic reticulum stress: Cell life and death decisions. J. Clin. Investig. 2005, 115, 2656–2664. [Google Scholar] [CrossRef]
- Lemmer, I.L.; Willemsen, N.; Hilal, N.; Bartelt, A. A guide to understanding endoplasmic reticulum stress in metabolic disorders. Mol. Metab. 2021, 47, 101169. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, K.P.; Kawai, Y.K.; Ikenaka, Y.; Kawata, M.; Ikushiro, S.-I.; Sakaki, T.; Ishizuka, M. Avian cytochrome p450 (cyp) 1-3 family genes: Isoforms, evolutionary relationships, and mRNA expression in chicken liver. PLoS ONE 2013, 8, e75689. [Google Scholar] [CrossRef]
- Scheer, N.; Kapelyukh, Y.; Chatham, L.; Rode, A.; Buechel, S.; Wolf, C. Generation and characterization of novel cytochrome p450 cyp2c gene cluster knockout and cyp2c9 humanized mouse lines. Mol. Pharmacol. 2012, 82, 1022–1029. [Google Scholar] [CrossRef]
- Yang, X.; Zhang, B.; Molony, C.; Chudin, E.; Hao, K.; Zhu, J.; Gaedigk, A.; Suver, C.; Zhong, H.; Leeder, J. Systematic genetic and genomic analysis of cytochrome p450 enzyme activities in human liver. Genome Res. 2010, 20, 1020–1036. [Google Scholar] [CrossRef]
- Li, M.; Xu, Z.; Lu, W.; Wang, L.; Zhang, Y. Potential pharmacokinetic effect of chicken xenobiotic receptor activator on sulfadiazine: Involvement of p-glycoprotein induction. Antibiotics 2022, 11, 1005. [Google Scholar] [CrossRef]
- Murcia, H.W.; Diaz, G.J. Protective effect of glutathione s-transferase enzyme activity against aflatoxin b1 in poultry species: Relationship between glutathione s-transferase enzyme kinetic parameters, and resistance to aflatoxin b1. Poult. Sci. 2021, 100, 101235. [Google Scholar] [CrossRef] [PubMed]
- Burchell, B.; Coughtrie, M.W.; Jansen, P.L. Function and regulation of UDP-glucuronosyltransferase genes in health and liver disease: Report of the seventh international workshop on glucuronidation. Hepatology 1994, 20, 1622–1630. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.; Hill, C.; Bitto, A.; Kaeberlein, M. Antiaging diets: Separating fact from fiction. Science 2021, 374, eabe7365. [Google Scholar] [CrossRef]
- Millet, L.; Vidal, H.; Andreelli, F.; Larrouy, D.; Riou, J.-P.; Ricquier, D.; Laville, M.; Langin, D. Increased uncoupling protein-2 and-3 mrna expression during fasting in obese and lean humans. J. Clin. Investig. 1997, 100, 2665–2670. [Google Scholar] [CrossRef]
- Lindholm, C.; Altimiras, J. Physiological and behavioural effects of intermittent fasting vs daily caloric restriction in meat-type poultry. Animal 2023, 17, 100849. [Google Scholar] [CrossRef]
- Bokkers, E.A.; Koene, P. Behaviour of fast-and slow growing broilers to 12 weeks of age and the physical consequences. Appl. Anim. Behav. Sci. 2003, 81, 59–72. [Google Scholar] [CrossRef]
- Stan, S.; Delvin, E.; Lambert, M.; Seidman, E.; Levy, E. Apo a-iv: An update on regulation and physiologic functions. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2003, 1631, 177–187. [Google Scholar] [CrossRef] [PubMed]
- Landowski, M.; Bhute, V.J.; Grindel, S.; Haugstad, Z.; Gyening, Y.K.; Tytanic, M.; Brush, R.S.; Moyer, L.J.; Nelson, D.W.; Davis, C.R. Transmembrane protein 135 regulates lipid homeostasis through its role in peroxisomal dha metabolism. Commun. Biol. 2023, 6, 8. [Google Scholar] [CrossRef] [PubMed]
- Lefevre, M.; Redman, L.M.; Heilbronn, L.K.; Smith, J.V.; Martin, C.K.; Rood, J.C.; Greenway, F.L.; Williamson, D.A.; Smith, S.R.; Ravussin, E. Caloric restriction alone and with exercise improves cvd risk in healthy non-obese individuals. Atherosclerosis 2009, 203, 206–213. [Google Scholar] [CrossRef] [PubMed]
- Ponczek, M.B. High molecular weight kininogen: A review of the structural literature. Int. J. Mol. Sci. 2021, 22, 13370. [Google Scholar] [CrossRef]
- Man, K.Y.; Chow, K.L.; Man, Y.B.; Mo, W.Y.; Wong, M.H. Use of biochar as feed supplements for animal farming. Crit. Rev. Environ. Sci. Technol. 2021, 51, 187–217. [Google Scholar] [CrossRef]
- Ridker, P.M.; Brown, N.J.; Vaughan, D.E.; Harrison, D.G.; Mehta, J.L. Established and emerging plasma biomarkers in the prediction of first atherothrombotic events. Circulation 2004, 109, IV-6–IV-19. [Google Scholar] [CrossRef]
- Kamely, M.; He, W.; Wakaruk, J.; Whelan, R.; Naranjo, V.; Barreda, D.R. Impact of reduced dietary crude protein in the starter phase on immune development and response of broilers throughout the growth period. Front. Vet. Sci. 2020, 7, 436. [Google Scholar] [CrossRef]


| Trait | Ad Libitum Feeding | Restricted Feeding 1 | p-Value | ||||
|---|---|---|---|---|---|---|---|
| Low RFI (High FE) | High RFI (Low FE) | Low RFI (High FE) | High RFI (Low FE) | SEM | RFI Group | Restriction | |
| n | 8 | 8 | 8 | 8 | |||
| BW (kg) | 2.47 ab | 2.58 a | 2.49 ab | 2.26 b | 0.12 | 0.516 | 0.094 |
| Metabolizable mid BW (g) | 188 a | 188 a | 175 b | 170 b | 2.95 | 0.349 | <0.001 |
| RFI (g) | −109.1 c | 230.2 a | −74.0 c | 119.3 b | 34.0 | <0.001 | 0.114 |
| FCR (g/g) | 1.35 b | 1.51 a | 1.37 b | 1.51 a | 0.021 | <0.001 | 0.998 |
| Comparison | Term Name (Database) | Adj. p-Value |
|---|---|---|
| Low RFI vs. high RFI (ad libitum feeding) 1 No. of genes: 328 | Positive regulation of cell–substrate adhesion (GO:BP) | 0.001 |
| Positive regulation of cell–matrix adhesion (GO:BP) | 0.013 | |
| Low RFI vs. high RFI (restricted feeding) No. of genes: 292 | Positive regulation of heterotypic cell–cell adhesion (GO:BP) | 0.001 |
| Regulation of heterotypic cell–cell adhesion (GO:BP) | 0.016 | |
| Complement cascade (REAC) | 0.009 | |
| Restrictive vs. ad libitum feeding (low RFI) No. of genes: 140 | Drug metabolism—cytochrome P450 (KEGG) | 0.022 |
| Metabolism of xenobiotics by cytochrome P450 (KEGG) | 0.024 | |
| Restrictive vs. ad libitum feeding (high RFI) No. of genes: 500 | Regulation of coagulation (GO:BP) | 0.006 |
| Regulation of response to external stimulus (GO:BP) | 0.009 | |
| Regulation of wound healing (GO:BP) | 0.014 | |
| Negative regulation of coagulation (GO:BP) | 0.028 | |
| Positive regulation of ERK1 and ERK2 cascade (GO:BP) | 0.032 | |
| Regulation of blood coagulation (GO:BP) | 0.035 | |
| Superoxide metabolic process (GO:BP) | 0.042 | |
| Negative regulation of wound healing (GO:BP) | 0.042 | |
| Regulation of haemostasis (GO:BP) | 0.042 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Omotoso, A.O.; Reyer, H.; Oster, M.; Ponsuksili, S.; Metzler-Zebeli, B.; Wimmers, K. Hepatic Transcriptomics of Broilers with Low and High Feed Conversion in Response to Caloric Restriction. Metabolites 2024, 14, 625. https://doi.org/10.3390/metabo14110625
Omotoso AO, Reyer H, Oster M, Ponsuksili S, Metzler-Zebeli B, Wimmers K. Hepatic Transcriptomics of Broilers with Low and High Feed Conversion in Response to Caloric Restriction. Metabolites. 2024; 14(11):625. https://doi.org/10.3390/metabo14110625
Chicago/Turabian StyleOmotoso, Adewunmi O., Henry Reyer, Michael Oster, Siriluck Ponsuksili, Barbara Metzler-Zebeli, and Klaus Wimmers. 2024. "Hepatic Transcriptomics of Broilers with Low and High Feed Conversion in Response to Caloric Restriction" Metabolites 14, no. 11: 625. https://doi.org/10.3390/metabo14110625
APA StyleOmotoso, A. O., Reyer, H., Oster, M., Ponsuksili, S., Metzler-Zebeli, B., & Wimmers, K. (2024). Hepatic Transcriptomics of Broilers with Low and High Feed Conversion in Response to Caloric Restriction. Metabolites, 14(11), 625. https://doi.org/10.3390/metabo14110625








