Type 1 Diabetes and Cataracts: Investigating Mediating Effects of Serum Metabolites Using Bidirectional Mendelian Randomization
Abstract
1. Introduction
2. Methods
2.1. Study Design
2.2. Data Sources and Selection of Instrumental Variables
2.3. Statistical Analysis
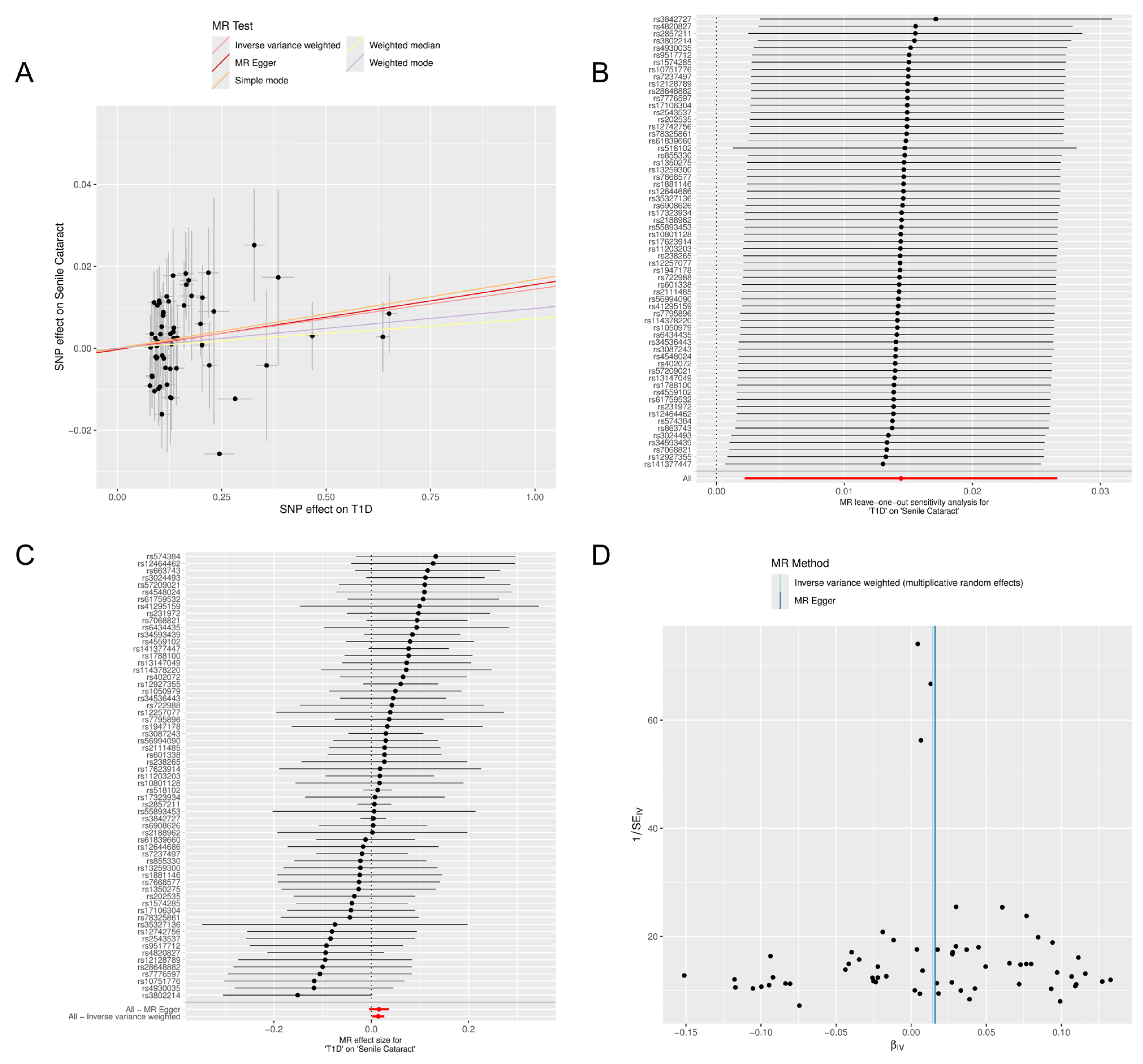
3. Results
3.1. Selection of IVs
3.2. MR Analysis
3.3. Sensitivity Analysis
3.4. Confounding Analysis
3.5. Mediation Analyses of Potential Blood Metabolites
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- DiMeglio, L.A.; Evans-Molina, C.; Oram, R.A. Type 1 diabetes. Lancet 2018, 391, 2449–2462. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Keindl, M.; Fedotkina, O.; du Plessis, E.; Jain, R.; Bergum, B.; Mygind Jensen, T.; Laustrup Møller, C.; Falhammar, H.; Nyström, T.; Catrina, S.B.; et al. Increased Plasma Soluble Interleukin-2 Receptor Alpha Levels in Patients With Long-Term Type 1 Diabetes With Vascular Complications Associated With IL2RA and PTPN2 Gene Polymorphisms. Front. Endocrinol. 2020, 11, 575469. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Šimunović, M.; Paradžik, M.; Škrabić, R.; Unić, I.; Bućan, K.; Škrabić, V. Cataract as Early Ocular Complication in Children and Adolescents with Type 1 Diabetes Mellitus. Int. J. Endocrinol. 2018, 2018, 6763586. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Liu, Y.C.; Wilkins, M.; Kim, T.; Malyugin, B.; Mehta, J.S. Cataracts. Lancet 2017, 390, 600–612. [Google Scholar] [CrossRef] [PubMed]
- Asbell, P.A.; Dualan, I.; Mindel, J.; Brocks, D.; Ahmad, M.; Epstein, S. Age-related cataract. Lancet 2005, 365, 599–609. [Google Scholar] [CrossRef] [PubMed]
- Ighodaro, O.M. Molecular pathways associated with oxidative stress in diabetes mellitus. Biomed. Pharmacother. 2018, 108, 656–662. [Google Scholar] [CrossRef] [PubMed]
- Wojnar, W.; Zych, M.; Borymski, S.; Kaczmarczyk-Sedlak, I. Chrysin Reduces Oxidative Stress but Does Not Affect Polyol Pathway in the Lenses of Type 1 Diabetic Rats. Antioxidants 2020, 9, 160. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chitra, P.S.; Chaki, D.; Boiroju, N.K.; Mokalla, T.R.; Gadde, A.K.; Agraharam, S.G.; Reddy, G.B. Status of oxidative stress markers, advanced glycation index, and polyol pathway in age-related cataract subjects with and without diabetes. Exp. Eye Res. 2020, 200, 108230. [Google Scholar] [CrossRef] [PubMed]
- Pradeep, S.R.; Srinivasan, K. Ameliorative Influence of Dietary Fenugreek (Trigonella foenum-graecum) Seeds and Onion (Allium cepa) on Eye Lens Abnormalities via Modulation of Crystallin Proteins and Polyol Pathway in Experimental Diabetes. Curr. Eye Res. 2018, 43, 1108–1118. [Google Scholar] [CrossRef] [PubMed]
- American Diabetes Association. 11. Microvascular Complications and Foot Care: Standards of Medical Care in Diabetes-2020. Diabetes Care 2020, 43 (Suppl. 1), S135–S151. [Google Scholar] [CrossRef] [PubMed]
- American Diabetes Association. 13. Children and Adolescents: Standards of Medical Care in Diabetes-2020. Diabetes Care 2020, 43 (Suppl. 1), S163–S182. [Google Scholar] [CrossRef] [PubMed]
- Quintos, J.B.; Torga, A.P.; Simon, M.A. Diabetes cataract in a 10-year-old girl with new-onset type 1 diabetes mellitus. BMJ Case Rep. 2019, 12, e227437. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Uspal, N.G.; Schapiro, E.S. Cataracts as the initial manifestation of type 1 diabetes mellitus. Pediatr. Emerg. Care 2011, 27, 132–134. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Zeng, H.; Xuan, R.; Lei, S.; Li, J.; Lai, X.; Liu, J. Bilateral cataracts as the first manifestation of type 1 diabetes mellitus: A case report. Medicine 2018, 97, e12874. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Dicembrini, I.; Cosentino, C.; Monami, M.; Mannucci, E.; Pala, L. Effects of real-time continuous glucose monitoring in type 1 diabetes: A meta-analysis of randomized controlled trials. Acta Diabetol. 2021, 58, 401–410. [Google Scholar] [CrossRef] [PubMed]
- Gubitosi-Klug, R.A.; Braffett, B.H.; Bebu, I.; Johnson, M.L.; Farrell, K.; Kenny, D.; Trapani, V.R.; Meadema-Mayer, L.; Soliman, E.Z.; Pop-Busui, R.; et al. Continuous Glucose Monitoring in Adults With Type 1 Diabetes With 35 Years Duration From the DCCT/EDIC Study. Diabetes Care 2022, 45, 659–665. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hainsworth, D.P.; Bebu, I.; Aiello, L.P.; Sivitz, W.; Gubitosi-Klug, R.; Malone, J.; White, N.H.; Danis, R.; Wallia, A.; Gao, X.; et al. Risk Factors for Retinopathy in Type 1 Diabetes: The DCCT/EDIC Study. Diabetes Care 2019, 42, 875–882. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sekula, P.; Del Greco, M.F.; Pattaro, C.; Köttgen, A. Mendelian Randomization as an Approach to Assess Causality Using Observational Data. J. Am. Soc. Nephrol. 2016, 27, 3253–3265. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Birney, E. Mendelian Randomization. Cold Spring Harb. Perspect. Med. 2022, 12, a041302. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ference, B.A.; Holmes, M.V.; Smith, G.D. Using Mendelian Randomization to Improve the Design of Randomized Trials. Cold Spring Harb. Perspect. Med. 2021, 11, a040980. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Skrivankova, V.W.; Richmond, R.C.; Woolf, B.A.R.; Yarmolinsky, J.; Davies, N.M.; Swanson, S.A.; VanderWeele, T.J.; Higgins, J.P.T.; Timpson, N.J.; Dimou, N.; et al. Strengthening the Reporting of Observational Studies in Epidemiology Using Mendelian Randomization: The STROBE-MR Statement. JAMA 2021, 326, 1614–1621. [Google Scholar] [CrossRef] [PubMed]
- Skrivankova, V.W.; Richmond, R.C.; Woolf, B.A.R.; Davies, N.M.; Swanson, S.A.; VanderWeele, T.J.; Timpson, N.J.; Higgins, J.P.T.; Dimou, N.; Langenberg, C.; et al. Strengthening the reporting of observational studies in epidemiology using mendelian randomisation (STROBE-MR): Explanation and elaboration. BMJ 2021, 375, n2233. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yao, S.; Zhang, M.; Dong, S.S.; Wang, J.H.; Zhang, K.; Guo, J.; Guo, Y.; Yang, T.L. Bidirectional two-sample Mendelian randomization analysis identifies causal associations between relative carbohydrate intake and depression. Nat. Hum. Behav. 2022, 6, 1569–1576. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Chen, Z.; Pärna, K.; van Zon, S.K.R.; Snieder, H.; Thio, C.H.L. Mediators of the association between educational attainment and type 2 diabetes mellitus: A two-step multivariable Mendelian randomisation study. Diabetologia 2022, 65, 1364–1374. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Forgetta, V.; Manousaki, D.; Istomine, R.; Ross, S.; Tessier, M.-C.; Marchand, L.; Li, M.; Qu, H.-Q.; Bradfield, J.P.; Grant, S.F.A.; et al. Rare Genetic Variants of Large Effect Influence Risk of Type 1 Diabetes. Diabetes 2020, 69, 784–795. [Google Scholar] [CrossRef]
- Chiou, J.; Geusz, R.J.; Okino, M.L.; Han, J.Y.; Miller, M.; Melton, R.; Beebe, E.; Benaglio, P.; Huang, S.; Korgaonkar, K.; et al. Interpreting type 1 diabetes risk with genetics and single-cell epigenomics. Nature 2021, 594, 398–402. [Google Scholar] [CrossRef]
- Sakaue, S.; Kanai, M.; Tanigawa, Y.; Karjalainen, J.; Kurki, M.; Koshiba, S.; Narita, A.; Konuma, T.; Yamamoto, K.; Akiyama, M.; et al. A cross-population atlas of genetic associations for 220 human phenotypes. Nat. Genet. 2021, 53, 1415–1424. [Google Scholar] [CrossRef]
- Kurki, M.I.; Karjalainen, J.; Palta, P.; Sipilä, T.P.; Kristiansson, K.; Donner, K.M.; Reeve, M.P.; Laivuori, H.; Aavikko, M.; Kaunisto, M.A.; et al. FinnGen provides genetic insights from a well-phenotyped isolated population. Nature 2023, 613, 508–518. [Google Scholar] [CrossRef]
- Chen, Y.; Lu, T.; Pettersson-Kymmer, U.; Stewart, I.D.; Butler-Laporte, G.; Nakanishi, T.; Cerani, A.; Liang, K.Y.H.; Yoshiji, S.; Willett, J.D.S.; et al. Genomic atlas of the plasma metabolome prioritizes metabolites implicated in human diseases. Nat. Genet. 2023, 55, 44–53. [Google Scholar] [CrossRef]
- Emdin, C.A.; Khera, A.V.; Kathiresan, S. Mendelian Randomization. JAMA 2017, 318, 1925–1926. [Google Scholar] [CrossRef]
- Burgess, S.; Davey Smith, G.; Davies, N.M.; Dudbridge, F.; Gill, D.; Glymour, M.M.; Hartwig, F.P.; Kutalik, Z.; Holmes, M.V.; Minelli, C.; et al. Guidelines for performing Mendelian randomization investigations: Update for summer 2023. Wellcome Open Res. 2023, 4, 186. [Google Scholar] [CrossRef] [PubMed]
- Lu, W.L.; Shen, P.C.; Lee, C.H.; Su, Y.T.; Chen, L.M. High Risk of Early Cataracts in Young Type 1 Diabetes Group: A Nationwide Cohort Study. Int. J. Endocrinol. 2020, 2020, 8160256. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Xia, J.L.; Patnaik, J.L.; Lynch, A.M.; Christopher, K.L. Comparison of cataract surgery outcomes in patients with type 1 vs type 2 diabetes mellitus and patients without diabetes mellitus. J. Cataract. Refract. Surg. 2023, 49, 608–613. [Google Scholar] [CrossRef] [PubMed]
- Marsiglio, J.; McPherson, J.P.; Kovacsovics-Bankowski, M.; Jeter, J.; Vaklavas, C.; Swami, U.; Grossmann, D.; Erickson-Wayman, A.; Soares, H.P.; Kerrigan, K.; et al. A single center case series of immune checkpoint inhibitor-induced type 1 diabetes mellitus, patterns of disease onset and long-term clinical outcome. Front. Immunol. 2023, 14, 1229823. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cook, E.; Stratton, E.; Thornton, M.D. Acute Cataract Development in a Pediatric Patient With Type 1 Diabetes. J. Emerg. Med. 2020, 58, e207–e209. [Google Scholar] [CrossRef] [PubMed]
- Sen, P.; Dickens, A.M.; López-Bascón, M.A.; Lindeman, T.; Kemppainen, E.; Lamichhane, S.; Rönkkö, T.; Ilonen, J.; Toppari, J.; Veijola, R.; et al. Metabolic alterations in immune cells associate with progression to type 1 diabetes. Diabetologia 2020, 63, 1017–1031. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hamed, M.S.; Samy, M.; Mahmoud, H.; Yehia, N. Study of the difficult glycemic control in relation to the presence of diabetes-autoantibodies in a sample of Egyptians with type 1 diabetes. Diabetes Res. Clin. Pract. 2019, 152, 53–57. [Google Scholar] [CrossRef] [PubMed]
- Vangaveti, V.N.; Jansen, H.; Kennedy, R.L.; Malabu, U.H. Hydroxyoctadecadienoic acids: Oxidised derivatives of linoleic acid and their role in inflammation associated with metabolic syndrome and cancer. Eur. J. Pharmacol. 2016, 785, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Mastrogiovanni, M.; Trostchansky, A.; Naya, H.; Dominguez, R.; Marco, C.; Povedano, M.; López-Vales, R.; Rubbo, H. HPLC-MS/MS Oxylipin Analysis of Plasma from Amyotrophic Lateral Sclerosis Patients. Biomedicines 2022, 10, 674. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mrugacz, M.; Pony-Uram, M.; Bryl, A.; Zorena, K. Current Approach to the Pathogenesis of Diabetic Cataracts. Int. J. Mol. Sci. 2023, 24, 6317. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gan, J.; Chen, J.; Ma, R.L.; Deng, Y.; Ding, X.S.; Zhu, S.Y.; Sun, A.J. Action Mechanisms of Metformin Combined with Exenatide and Metformin Only in the Treatment of PCOS in Obese Patients. Int. J. Endocrinol. 2023, 2023, 4288004. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mondul, A.M.; Moore, S.C.; Weinstein, S.J.; Karoly, E.D.; Sampson, J.N.; Albanes, D. Metabolomic analysis of prostate cancer risk in a prospective cohort: The alpha-tocolpherol, beta-carotene cancer prevention (ATBC) study. Int. J. Cancer 2015, 137, 2124–2132. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Padmanabha, S.; Vallikannan, B. Fatty acids modulate the efficacy of lutein in cataract prevention: Assessment of oxidative and inflammatory parameters in rats. Biochem. Biophys. Res. Commun. 2018, 500, 435–442, Erratum in: Biochem. Biophys. Res. Commun. 2020, 530, 611. [Google Scholar] [CrossRef]
- Padmanabha, S.; Vallikannan, B. Fatty acids influence the efficacy of lutein in the modulation of α-crystallin chaperone function: Evidence from selenite induced cataract rat model. Biochem. Biophys. Res. Commun. 2020, 529, 425–431. [Google Scholar] [CrossRef] [PubMed]
- Guan, F.; Du, W.; Zhang, J.; Su, C.; Zhang, B.; Deng, K.; Du, S.; Wang, H. Amino Acids and Lipids Associated with Long-Term and Short-Term Red Meat Consumption in the Chinese Population: An Untargeted Metabolomics Study. Nutrients 2021, 13, 4567. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Holeček, M. Origin and Roles of Alanine and Glutamine in Gluconeogenesis in the Liver, Kidneys, and Small Intestine under Physiological and Pathological Conditions. Int. J. Mol. Sci. 2024, 25, 7037. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sarabhai, T.; Roden, M. Hungry for your alanine: When liver depends on muscle proteolysis. J. Clin. Investig. 2019, 129, 4563–4566. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hatazawa, Y.; Qian, K.; Gong, D.W.; Kamei, Y. PGC-1α regulates alanine metabolism in muscle cells. PLoS ONE 2018, 13, e0190904. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Petersen, K.F.; Dufour, S.; Cline, G.W.; Shulman, G.I. Regulation of hepatic mitochondrial oxidation by glucose-alanine cycling during starvation in humans. J. Clin. Investig. 2019, 129, 4671–4675. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sifuentes-Franco, S.; Padilla-Tejeda, D.E.; Carrillo-Ibarra, S.; Miranda-Díaz, A.G. Oxidative Stress, Apoptosis, and Mitochondrial Function in Diabetic Nephropathy. Int. J. Endocrinol. 2018, 2018, 1875870. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhang, Z.; Huang, Q.; Zhao, D.; Lian, F.; Li, X.; Qi, W. The impact of oxidative stress-induced mitochondrial dysfunction on diabetic microvascular complications. Front. Endocrinol. 2023, 14, 1112363. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wu, M.Y.; Yiang, G.T.; Lai, T.T.; Li, C.J. The Oxidative Stress and Mitochondrial Dysfunction during the Pathogenesis of Diabetic Retinopathy. Oxid. Med. Cell Longev. 2018, 2018, 3420187. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kang, Q.; Yang, C. Oxidative stress and diabetic retinopathy: Molecular mechanisms, pathogenetic role and therapeutic implications. Redox. Biol. 2020, 37, 101799. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kowluru, R.A.; Mohammad, G. Mitochondrial Fragmentation in a High Homocysteine Environment in Diabetic Retinopathy. Antioxidants 2022, 11, 365. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]





| Exposure | MR Methods | nSNP | Beta | OR (95% CI) | p-Value |
|---|---|---|---|---|---|
| T1D (Finn) | MR Egger | 63 | 0.016 | 1.016 (0.996–1.037) | 0.130 |
| Weighted median | 63 | 0.007 | 1.007 (0.990–1.025) | 0.426 | |
| Inverse variance weighted | 63 | 0.014 | 1.015 (1.002–1.027) | 0.021 | |
| Simple mode | 63 | 0.017 | 1.017 (0.976–1.060) | 0.429 | |
| Weighted mode | 63 | 0.010 | 1.010 (0.992–1.028) | 0.283 | |
| Maximum likelihood | 63 | 0.014 | 1.014 (1.002–1.027) | 0.022 | |
| T1D (GCST010681) | MR Egger | 38 | 0.022 | 1.023 (1.013–1.033) | 7.87 × 10−5 |
| Weighted median | 38 | 0.026 | 1.026 (1.016–1.037) | 6.43 × 10−7 | |
| Inverse variance weighted | 38 | 0.015 | 1.015 (1.008–1.023) | 1.30 × 10−5 | |
| Simple mode | 38 | −0.005 | 0.995 (0.971–1.019) | 0.675 | |
| Weighted mode | 38 | 0.022 | 1.022 (1.013–1.032) | 2.17 × 10−5 | |
| Maximum likelihood | 38 | 0.015 | 1.015 (1.009–1.022) | 5.37 × 10−6 | |
| T1D (GCST90018925) | MR Egger | 17 | 0.032 | 1.032 (1.000–1.066) | 0.072 |
| Weighted median | 17 | 0.046 | 1.047 (1.030–1.064) | 3.05 × 10−8 | |
| Inverse variance weighted | 17 | 0.046 | 1.047 (1.026–1.069) | 1.20 × 10−5 | |
| Simple mode | 17 | 0.058 | 1.060 (1.008–1.114) | 0.037 | |
| Weighted mode | 17 | 0.045 | 1.046 (1.029–1.063) | 6.27 × 10−5 | |
| Maximum likelihood | 17 | 0.047 | 1.048 (1.033–1.062) | 3.92 × 10−11 | |
| T1D with coma | MR Egger | 7 | 0.010 | 1.010 (0.990–1.031) | 0.358 |
| Weighted median | 7 | 0.014 | 1.014 (1.004–1.024) | 0.004 | |
| Inverse variance weighted | 7 | 0.015 | 1.015 (1.005–1.026) | 0.004 | |
| Simple mode | 7 | 0.022 | 1.022 (1.003–1.042) | 0.060 | |
| Weighted mode | 7 | 0.015 | 1.014 (1.005–1.025) | 0.028 | |
| T1D with ketoacidosis | MR Egger | 8 | 0.015 | 1.015 (1.000–1.031) | 0.104 |
| Weighted median | 8 | 0.012 | 1.012 (1.003–1.022) | 0.007 | |
| Inverse variance weighted | 8 | 0.012 | 1.012 (1.002–1.021) | 0.014 | |
| Simple mode | 8 | −0.004 | 0.996 (0.974–1.019) | 0.738 | |
| Weighted mode | 8 | 0.012 | 1.012 (1.002–1.022) | 0.048 | |
| T1D with neurological complications | MR Egger | 4 | 0.031 | 1.032 (0.988–1.078) | 0.296 |
| Weighted median | 4 | 0.021 | 1.021 (1.012–1.030) | 3.65 × 10−6 | |
| Inverse variance weighted | 4 | 0.020 | 1.020 (1.009–1.032) | 0.001 | |
| Simple mode | 4 | 0.020 | 1.021 (1.007–1.034) | 0.060 | |
| Weighted mode | 4 | 0.021 | 1.021 (1.012–1.030) | 0.020 | |
| T1D with ophthalmic complications | MR Egger | 12 | 0.024 | 1.024 (1.005–1.043) | 0.032 |
| Weighted median | 12 | 0.016 | 1.016 (1.005–1.027) | 0.004 | |
| Inverse variance weighted | 12 | 0.015 | 1.015 (1.004–1.026) | 0.006 | |
| Simple mode | 12 | 0.007 | 1.007 (0.989–1.026) | 0.441 | |
| Weighted mode | 12 | 0.017 | 1.017 (1.006–1.028) | 0.014 | |
| T1D with other specified multiple unspecified complications | MR Egger | 11 | 0.012 | 1.012 (0.996–1.028) | 0.165 |
| Weighted median | 11 | 0.011 | 1.011 (1.001–1.022) | 0.039 | |
| Inverse variance weighted | 11 | 0.011 | 1.011 (1.002–1.021) | 0.023 | |
| Simple mode | 11 | 0.006 | 1.006 (0.984–1.029) | 0.603 | |
| Weighted mode | 11 | 0.010 | 1.010 (1.000–1.021) | 0.112 | |
| T1D without complications | MR Egger | 13 | 0.026 | 1.026 (0.999–1.054) | 0.085 |
| Weighted median | 13 | 0.026 | 1.026 (1.012–1.042) | 4.57 × 10−4 | |
| Inverse variance weighted | 13 | 0.016 | 1.016 (1.000–1.033) | 0.057 | |
| Simple mode | 13 | 0.020 | 1.021 (0.989–1.053) | 0.223 | |
| Weighted mode | 13 | 0.019 | 1.019 (1.004–1.033) | 0.025 | |
| T1D with renal complications | MR Egger | 6 | 0.016 | 1.015 (0.994–1.038) | 0.226 |
| Weighted median | 6 | 0.013 | 1.013 (1.003–1.024) | 0.010 | |
| Inverse variance weighted | 6 | 0.012 | 1.012 (0.999–1.026) | 0.070 | |
| Simple mode | 6 | −0.017 | 0.984 (0.952–1.016) | 0.364 | |
| Weighted mode | 6 | 0.013 | 1.013 (1.004–1.024) | 0.039 |
| Exposure | Outcome | Heterogeneity | Pleiotropy | |
|---|---|---|---|---|
| MR-EggerQ (p-Value) | IVW Q (p-Value) | Egger_Intercept (p-Value) | ||
| T1D (Finn) | Cataract | 53.056 (0.756) | 53.087 (0.783) | −0.000 (0.861) |
| T1D (GCST010681) | 36.573 (0.442) | 40.217 (0.330) | −0.004 (0.066) | |
| T1D (GCST90018925) | 33.757 (0.004) | 36.763 (0.002) | 0.006 (0.266) | |
| T1D with coma | 8.575 (0.127) | 9.191 (0.163) | 0.005 (0.575) | |
| T1D with ketoacidosis | 8.538 (0.201) | 8.987 (0.254) | −0.004 (0.595) | |
| T1D with neurological complications | 5.467 (0.065) | 6.217 (0.102) | −0.011 (0.653) | |
| T1D with ophthalmic complications | 14.701 (0.143) | 16.454 (0.125) | −0.006 (0.301) | |
| T1D with other specified multiple unspecified complications | 11.632 (0.235) | 11.656 (0.309) | −0.001 (0.894) | |
| T1D without complications | 30.158 (0.001) | 32.476 (0.001) | −0.006 (0.378) | |
| T1D with renal complications | 9.977 (0.041) | 10.407 (0.064) | −0.004 (0.699) |
| Exposure | Outcome | MR Method | nSNP | Beta | Se | p-Value |
|---|---|---|---|---|---|---|
| T1D | Cataract (Finn) | IVW | 63 | 0.014 | 0.006 | 0.012 |
| T1D | N-lactoyl valine levels | 77 | −0.023 | 0.009 | 0.010 | |
| T1D | Trans-urocanate levels | 74 | −0.021 | 0.009 | 0.023 | |
| T1D | Alanine to pyruvate ratio | 71 | 0.021 | 0.008 | 0.006 | |
| T1D | Adenosine 5′-diphosphate (ADP) to EDTA ratio | 74 | 0.025 | 0.013 | 0.050 | |
| N-lactoyl valine levels | Cataract (Finn) | 8 | −0.028 | 0.013 | 0.033 | |
| Trans-urocanate levels | Cataract (Finn) | 11 | −0.033 | 0.015 | 0.032 | |
| Alanine to pyruvate ratio | Cataract (Finn) | 9 | 0.065 | 0.032 | 0.039 | |
| Adenosine 5′-diphosphate (ADP) to EDTA ratio | Cataract (Finn) | 9 | −0.044 | 0.017 | 0.008 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shi, Y.; Qin, J.; Li, Y.; Yang, J.; Lu, Y. Type 1 Diabetes and Cataracts: Investigating Mediating Effects of Serum Metabolites Using Bidirectional Mendelian Randomization. Metabolites 2024, 14, 644. https://doi.org/10.3390/metabo14110644
Shi Y, Qin J, Li Y, Yang J, Lu Y. Type 1 Diabetes and Cataracts: Investigating Mediating Effects of Serum Metabolites Using Bidirectional Mendelian Randomization. Metabolites. 2024; 14(11):644. https://doi.org/10.3390/metabo14110644
Chicago/Turabian StyleShi, Yumeng, Jingxi Qin, Yankai Li, Jin Yang, and Yi Lu. 2024. "Type 1 Diabetes and Cataracts: Investigating Mediating Effects of Serum Metabolites Using Bidirectional Mendelian Randomization" Metabolites 14, no. 11: 644. https://doi.org/10.3390/metabo14110644
APA StyleShi, Y., Qin, J., Li, Y., Yang, J., & Lu, Y. (2024). Type 1 Diabetes and Cataracts: Investigating Mediating Effects of Serum Metabolites Using Bidirectional Mendelian Randomization. Metabolites, 14(11), 644. https://doi.org/10.3390/metabo14110644








