Exploring the In Vitro Effects of Zingerone on Differentiation and Signalling Pathways in Bone Cell Lines
Abstract
:1. Introduction
2. Materials and Methods
2.1. Zingerone Sample Preparation
2.2. Cell Lines, Culture, and Treatment
2.2.1. SAOS-2 Cells
2.2.2. RAW264.7 Cells
2.3. Cell Viability and Proliferation: Resazurin Assay
2.4. Alizarin Red S Staining and Alkaline Phosphatase (ALP) Activity Assay
2.5. Quantitative Polymerase Chain Reaction (q-PCR)
2.6. Tartrate-Resistant Acid Phosphatase (TRAP) Staining and Activity
2.7. Western Blotting
2.8. Statistical Analysis
3. Results
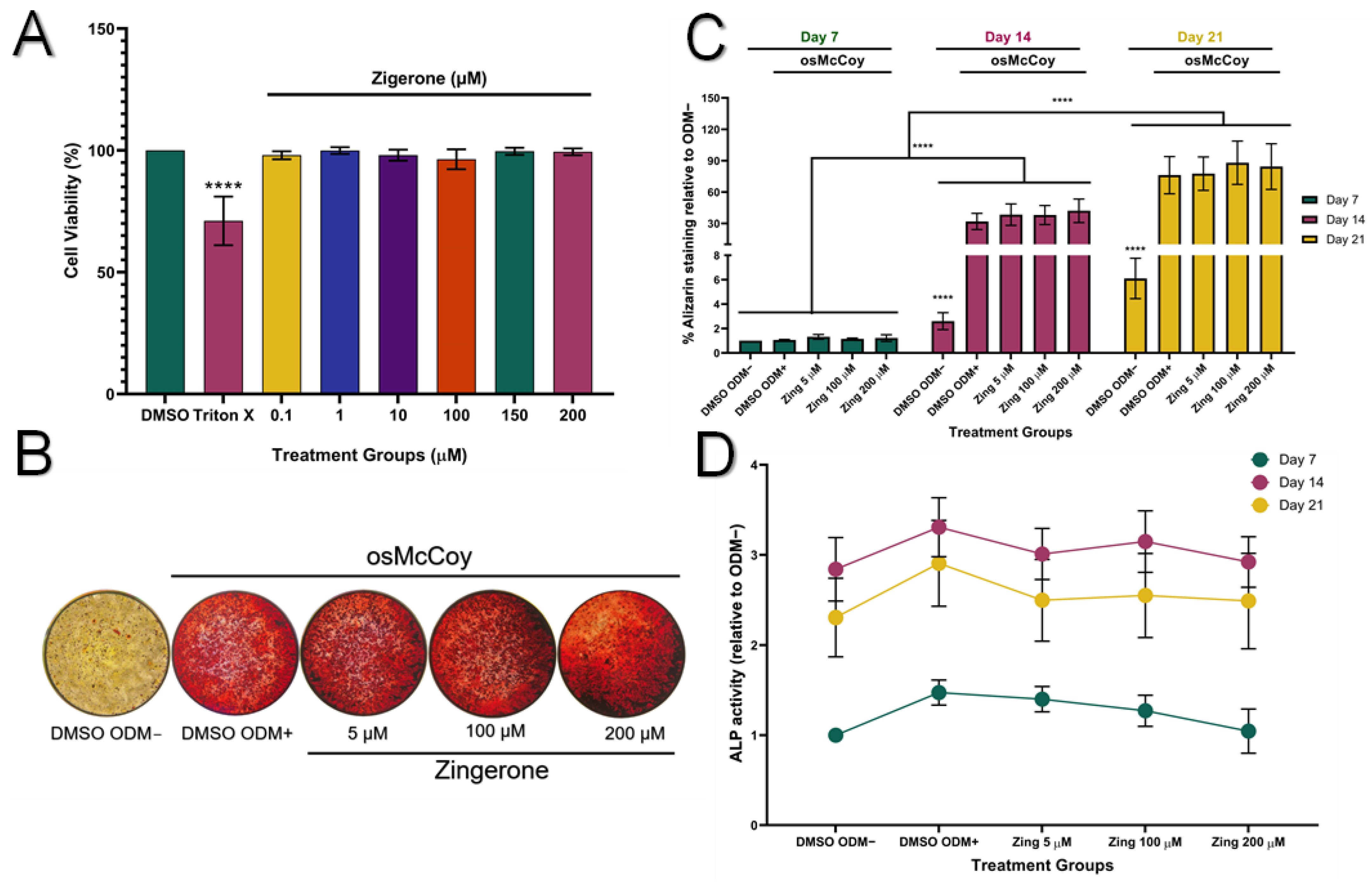
3.1. Zingerone Does Not Affect Mineralisation or ALP Protein Activity in SAOS-2 Cells
3.2. Zingerone Stimulates Runx2 and ALP Expression
3.3. Zingerone Inhibits Osteoclast Formation and Activity in RAW264.7 Macrophages
3.4. Zingerone Did Not Significantly Activate the Expression of MAPK Proteins
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pouresmaeili, F.; Kamalidehghan, B.; Kamarehei, M.; Goh, Y.M. A comprehensive overview on osteoporosis and its risk factors. Ther. Clin. Risk Manag. 2018, 14, 2029–2049. [Google Scholar] [CrossRef] [PubMed]
- Sozen, T.; Ozisik, L.; Basaran, N.C. An overview and management of osteoporosis. Eur. J. Rheumatol. 2017, 4, 46–56. [Google Scholar] [CrossRef] [PubMed]
- Coughlan, T.; Dockery, F. Osteoporosis and fracture risk in older people. Clin. Med. 2014, 14, 187–191. [Google Scholar] [CrossRef] [PubMed]
- Foger-Samwald, U.; Dovjak, P.; Azizi-Semrad, U.; Kerschan-Schindl, K.; Pietschmann, P. Osteoporosis: Pathophysiology and therapeutic options. EXCLI J. 2020, 19, 1017–1037. [Google Scholar] [CrossRef] [PubMed]
- Kasonga, A.E.; Deepak, V.; Kruger, M.C.; Coetzee, M. Arachidonic acid and docosahexaenoic acid suppress osteoclast formation and activity in human CD14+ monocytes, in vitro. PLoS ONE 2015, 10, e0125145. [Google Scholar] [CrossRef] [PubMed]
- Shyu, J.F.; Liu, W.C.; Zheng, C.M.; Fang, T.C.; Hou, Y.C.; Chang, C.T.; Liao, T.Y.; Chen, Y.C.; Lu, K.C. Toxic effects of indoxyl sulfate on osteoclastogenesis and osteoblastogenesis. Int. J. Mol. Sci. 2021, 22, 11265. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.; Seo, I.; Choi, M.H.; Jeong, D. Roles of mitogen-activated protein kinases in osteoclast biology. Int. J. Mol. Sci. 2018, 19, 3004. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.; Lee, S.H.; Ha Kim, J.; Choi, Y.; Kim, N. NFATc1 induces osteoclast fusion via up-regulation of Atp6v0d2 and the dendritic cell-specific transmembrane protein (DC-STAMP). Mol. Endocrinol. 2008, 22, 176–185. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Sapkota, M.; Gao, M.; Choi, H.; Soh, Y. Macrolactin F inhibits RANKL-mediated osteoclastogenesis by suppressing Akt, MAPK and NFATc1 pathways and promotes osteoblastogenesis through a BMP-2/smad/Akt/Runx2 signaling pathway. Eur. J. Pharmacol. 2017, 815, 202–209. [Google Scholar] [CrossRef]
- Park, E.; Lee, C.G.; Lim, E.; Hwang, S.; Yun, S.H.; Kim, J.; Jeong, H.; Yong, Y.; Yun, S.-H.; Choi, C.W.; et al. Osteoprotective effects of loganic acid on osteoblastic and osteoclastic cells and osteoporosis-induced mice. Int. J. Mol. Sci. 2020, 22, 233. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.M.; Lee, E.H. Transcriptional regulatory cascades in Runx2 dependent bone development. Tissue Eng. Part B Rev. 2013, 19, 254–263. [Google Scholar] [CrossRef] [PubMed]
- Maruyama, Z.; Yoshida, C.A.; Furuichi, T.; Amizuka, N.; Ito, M.; Fukuyama, R.; Miyazaki, T.; Kitaura, H.; Nakamura, K.; Fujita, T.; et al. Runx2 determines bone maturity and turnover rate in postnatal bone development and is involved in bone loss in estrogen deficiency. Dev. Dyn. 2007, 236, 1876–1890. [Google Scholar] [CrossRef] [PubMed]
- Ansari, S.; Ito, K.; Hofmann, S. Alkaline phosphatase activity of serum affects osteogenic differentiation cultures. ACS Omega 2022, 7, 12724–12733. [Google Scholar] [CrossRef] [PubMed]
- Tsao, Y.T.; Huang, Y.J.; Wu, H.H.; Liu, Y.A.; Liu, Y.S.; Lee, O.K. Osteocalcin mediates biomineralization during osteogenic maturation in human mesenchymal stromal cells. Int. J. Mol. Sci. 2017, 18, 159. [Google Scholar] [CrossRef] [PubMed]
- Florencio-Silva, R.; Sasso, G.R.d.S.; Sasso-Cerri, E.; Simões, M.J.; Cerri, P.S. Biology of bone tissue: Structure, function, and factors that influence bone cells. BioMed Res. Int. 2015, 2015, 421746. [Google Scholar] [CrossRef]
- Mani, V.; Arivalagan, S.; Siddique, A.I.; Namasivayam, N. Antioxidant and anti-inflammatory role of zingerone in ethanol-induced hepatotoxicity. Mol. Cell Biochem. 2016, 421, 169–181. [Google Scholar] [CrossRef]
- Ahmad, B.; Rehman, M.U.; Amin, I.; Mir, M.U.R.; Ahmad, S.B.; Farooq, A.; Muzamil, S.; Hussain, I.; Masoodi, M.; Fatima, B. Zingerone (4-(4-hydroxy-3-methylphenyl) butan-2-one) protects against alloxan-induced diabetes via alleviation of oxidative stress and inflammation: Probable role of NF-kB activation. Saudi Pharm. J. 2018, 26, 1137–1145. [Google Scholar] [CrossRef] [PubMed]
- Iantomasi, T.; Romagnoli, C.; Palmini, G.; Donati, S.; Falsetti, I.; Miglietta, F.; Aurilia, C.; Marini, F.; Giusti, F.; Brandi, M.L. Oxidative stress and inflammation in osteoporosis: Molecular mechanisms involved and the relationship with micrornas. Int. J. Mol. Sci. 2023, 24, 3772. [Google Scholar] [CrossRef]
- Domazetovic, V.; Marcucci, G.; Iantomasi, T.; Brandi, M.L.; Vincenzini, M.T. Oxidative stress in bone remodeling: Role of antioxidants. Clin. Cases Miner. Bone Metab. 2017, 14, 209–216. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Mou, R.; Li, Y.; Yang, T. Zingerone promotes osteoblast differentiation via MiR-200c-3p/smad7 regulatory axis in human bone mesenchymal stem cells. Med. Sci. Monit. 2020, 26, e919309. [Google Scholar] [CrossRef]
- Yang, D.; Tan, Y.; Xie, X.; Xiao, W.; Kang, J. Zingerone attenuates Ti particle-induced inflammatory osteolysis by suppressing the NF-κB signalling pathway in osteoclasts. Int. Immunopharmacol. 2023, 115, 109720. [Google Scholar] [CrossRef] [PubMed]
- Srinaath, N.; Balagangadharan, K.; Pooja, V.; Paarkavi, U.; Trishla, A.; Selvamurugan, N. Osteogenic potential of zingerone, a phenolic compound in mouse mesenchymal stem cells. Biofactors 2019, 45, 575–582. [Google Scholar] [CrossRef] [PubMed]
- Shamsabadi, S.; Nazer, Y.; Ghasemi, J.; Mahzoon, E.; Baradaran Rahimi, V.; Ajiboye, B.O.; Askari, V.R. Promising influences of zingerone against natural and chemical toxins: A comprehensive and mechanistic review. Toxicon 2023, 233, 107247. [Google Scholar] [CrossRef] [PubMed]
- Ginaldi, L.; Di Benedetto, M.C.; De Martinis, M. Osteoporosis, inflammation and ageing. Immun. Ageing 2005, 2, 14. [Google Scholar] [CrossRef] [PubMed]
- Rehman, M.U.; Rashid, S.M.; Rasool, S.; Shakeel, S.; Ahmad, B.; Ahmad, S.B.; Madkhali, H.; Ganaie, M.A.; Majid, S.; Bhat, S.A. Zingerone (4-(4-hydroxy-3-methylphenyl)butan-2-one) ameliorates renal function via controlling oxidative burst and inflammation in experimental diabetic nephropathy. Arch. Physiol. Biochem. 2019, 125, 201–209. [Google Scholar] [CrossRef] [PubMed]
- Rodan, S.B.; Imai, Y.; Thiede, M.A.; Wesolowski, G.; Thompson, D.; Bar-Shavit, Z.; Shull, S.; Mann, K.; Rodan, G. Characterization of a human osteosarcoma cell line (SAOS-2) with osteoblastic properties. Cancer Res. 1987, 47, 4961–4966. [Google Scholar]
- Czekanska, E.; Stoddart, M.; Richards, R.; Hayes, J. In search of an osteoblast cell model for in vitro research. Eur. Cells Mater. 2012, 24, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Prideaux, M.; Wijenayaka, A.R.; Kumarasinghe, D.D.; Ormsby, R.T.; Evdokiou, A.; Findlay, D.M.; Atkins, G.J. SAOS-2 osteosarcoma cells as an in vitro model for studying the transition of human osteoblasts to osteocytes. Calcif. Tissue Int. 2014, 95, 183–193. [Google Scholar] [CrossRef] [PubMed]
- Dvorakova, J.; Wiesnerova, L.; Chocholata, P.; Kulda, V.; Landsmann, L.; Cedikova, M.; Kripnerova, M.; Eberlova, L.; Babuska, V. Human cells with osteogenic potential in bone tissue research. Biomed. Eng. Online 2023, 22, 33. [Google Scholar] [CrossRef]
- Louis, K.S.; Siegel, A.C. Cell viability analysis using trypan blue: Manual and automated methods. Methods Mol. Biol. 2011, 740, 7–12. [Google Scholar] [CrossRef]
- Strober, W. Trypan blue exclusion test of cell viability. Curr. Protoc. Immunol. 2015, 111, A3B1–A3B3. [Google Scholar] [CrossRef]
- Taciak, B.; Białasek, M.; Braniewska, A.; Sas, Z.; Sawicka, P.; Kiraga, Ł.; Rygiel, T.; Król, M. Evaluation of phenotypic and functional stability of RAW 264.7 cell line through serial passages. PLoS ONE 2018, 13, e0198943. [Google Scholar] [CrossRef] [PubMed]
- Kong, L.; Smith, W.; Hao, D. Overview of RAW264.7 for osteoclastogensis study: Phenotype and stimuli. J. Cell Mol. Med. 2019, 23, 3077–3087. [Google Scholar] [CrossRef] [PubMed]
- Lampiasi, N.; Russo, R.; Kireev, I.; Strelkova, O.; Zhironkina, O.; Zito, F. Osteoclasts differentiation from murine RAW 264.7 cells stimulated by RANKL: Timing and behavior. Biology 2021, 10, 117. [Google Scholar] [CrossRef] [PubMed]
- Kasonga, A.; Kruger, M.C.; Coetzee, M. Activation of PPARs modulates signalling pathways and expression of regulatory genes in osteoclasts derived from human CD14+ monocytes. Int. J. Mol. Sci. 2019, 20, 1798. Available online: https://www.mdpi.com/1422-0067/20/7/1798 (accessed on 5 January 2023). [CrossRef] [PubMed]
- Al-Nasiry, S.; Geusens, N.; Hanssens, M.; Luyten, C.; Pijnenborg, R. The use of Alamar Blue assay for quantitative analysis of viability, migration and invasion of choriocarcinoma cells. Hum. Reprod. 2007, 22, 1304–1309. [Google Scholar] [CrossRef] [PubMed]
- Sagar, T.; Kasonga, A.; Baschant, U.; Rauner, M.; Moosa, S.; Marais, S.; Kruger, M.; Coetzee, M. Aspalathin from Aspalathus linearis (rooibos) reduces osteoclast activity and increases osteoblast activity in vitro. J. Funct. Foods 2020, 64, 103616. [Google Scholar] [CrossRef]
- Akchurin, T.; Aissiou, T.; Kemeny, N.; Prosk, E.; Nigam, N.; Komarova, S.V. Complex dynamics of osteoclast formation and death in long-term cultures. PLoS ONE 2008, 3, e2104. [Google Scholar] [CrossRef]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef] [PubMed]
- Mao, Q.Q.; Xu, X.Y.; Cao, S.Y.; Gan, R.Y.; Corke, H.; Beta, T.; Li, H.-B. Bioactive compounds and bioactivities of ginger (Zingiber officinale Roscoe). Foods 2019, 8, 185. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, B.; Rehman, M.U.; Amin, I.; Arif, A.; Rasool, S.; Bhat, S.A.; Afzal, I.; Hussain, I.; Bilal, S.; Mir, M.U.R. A review on pharmacological properties of zingerone (4-(4-Hydroxy-3-methoxyphenyl)-2-butanone). Sci. World J. 2015, 2015, 816364. [Google Scholar] [CrossRef] [PubMed]
- Nakatani, N. Phenolic antioxidants from herbs and spices. Biofactors 2000, 13, 141–146. [Google Scholar] [CrossRef] [PubMed]
- Domazetovic, V.; Marcucci, G.; Falsetti, I.; Bilia, A.R.; Vincenzini, M.T.; Brandi, M.L.; Iantomasi, T. Blueberry juice antioxidants protect osteogenic activity against oxidative stress and improve long-term activation of the mineralization process in human osteoblast-like SAOS-2 cells: Involvement of SIRT1. Antioxidants 2020, 9, 125. [Google Scholar] [CrossRef] [PubMed]
- Kyeyune-Nyombi, E.; Nicolas, V.; Strong, D.D.; Farley, J. Paradoxical effects of phosphate to directly regulate the level of skeletal alkaline phosphatase activity in human osteosarcoma (SaOS-2) cells and inversely regulate the level of skeletal alkaline phosphatase mRNA. Calcif. Tissue Int. 1995, 56, 154–159. [Google Scholar] [CrossRef]
- Gomathi, K.; Akshaya, N.; Srinaath, N.; Moorthi, A.; Selvamurugan, N. Regulation of Runx2 by post-translational modifications in osteoblast differentiation. Life Sci. 2020, 245, 117389. [Google Scholar] [CrossRef] [PubMed]
- Al-Bari, A.A.; Al Mamun, A. Current advances in regulation of bone homeostasis. FASEB BioAdv. 2020, 2, 668–679. [Google Scholar] [CrossRef] [PubMed]
- Neve, A.; Corrado, A.; Cantatore, F.P. Osteocalcin: Skeletal and extra-skeletal effects. J. Cell Physiol. 2013, 228, 1149–1153. [Google Scholar] [CrossRef] [PubMed]
- Borciani, G.; Montalbano, G.; Baldini, N.; Cerqueni, G.; Vitale-Brovarone, C.; Ciapetti, G. Co-culture systems of osteoblasts and osteoclasts: Simulating in vitro bone remodeling in regenerative approaches. Acta Biomater. 2020, 108, 22–45. [Google Scholar] [CrossRef] [PubMed]
- Skubica, P.; Husakova, M.; Dankova, P. In vitro osteoclastogenesis in autoimmune diseases—Strengths and pitfalls of a tool for studying pathological bone resorption and other disease characteristics. Heliyon 2023, 9, e21925. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Liu, H.; Li, J.; Ma, Y.; Song, C.; Wang, Y.; Li, P.; Chen, Y.; Zhang, Z. Evaluation of culture conditions for osteoclastogenesis in RAW264.7 cells. PLoS ONE 2022, 17, e0277871. [Google Scholar] [CrossRef]
- Ryu, A.H.; Eckalbar, W.L.; Kreimer, A.; Yosef, N.; Ahituv, N. Use antibiotics in cell culture with caution: Genome-wide identification of antibiotic-induced changes in gene expression and regulation. Sci. Rep. 2017, 7, 7533. [Google Scholar] [CrossRef]
- Hirsch, C.; Schildknecht, S. In vitro research reproducibility: Keeping up high standards. Front. Pharmacol. 2019, 10, 1484. [Google Scholar] [CrossRef] [PubMed]
- Ruangsuriya, J.; Budprom, P.; Viriyakhasem, N.; Kongdang, P.; Chokchaitaweesuk, C.; Sirikaew, N.; Chomdej, S.; Nganvongpanit, K.; Ongchai, S. Suppression of cartilage degradation by zingerone involving the p38 and JNK MAPK signaling pathway. Planta Medica 2017, 83, 268–276. [Google Scholar] [CrossRef]
- Qayoom, I.; Raina, D.B.; Sirka, A.; Tarasevicius, S.; Tagil, M.; Kumar, A.; Lidgren, L. Anabolic and antiresorptive actions of locally delivered bisphosphonates for bone repair: A review. Bone Jt. Res. 2018, 7, 548–560. [Google Scholar] [CrossRef] [PubMed]
- Roseti, L.; Borciani, G.; Grassi, F.; Desando, G.; Gambari, L.; Grigolo, B. Nutraceuticals in osteoporosis prevention. Front. Nutr. 2024, 11, 1445955. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.; Huang, Y.; Liu, F.; Wang, Q.; Yao, Y. How aging affects bone health via the intestinal micro-environment. Biocell 2024, 48, 353–362. [Google Scholar] [CrossRef]
- Ge, Z.; Wang, Q.; Zhu, Q.; Yusif, M.; Yu, J.; Xu, X. Improved oral bioavailability, cellular uptake, and cytotoxic activity of zingerone via nano-micelles drug delivery system. J. Microencapsul. 2021, 38, 394–404. [Google Scholar] [CrossRef] [PubMed]
- Oliviero, S.; Millard, E.; Chen, Z.; Rayson, A.; Roberts, B.C.; Ismail, H.M.S.; Bellantuono, I.; Dall’ara, E. Accuracy of in vivo micro-CT imaging in assessing the microstructural properties of the mouse tibia subchondral bone. Front. Endocrinol. 2022, 13, 1016321. [Google Scholar] [CrossRef]




| Gene | Forward Primer Sequence (5′—3′) | Reverse Primer Sequence (5′—3′) |
|---|---|---|
| RPLP0 (housekeeping) | GAAACTGTTTAACTTCGCTTCC | GACTCGTTTGTACCCGTTGATG |
| Runx2 | GCTGTTATGAAAAACCAAGT | GGGAGGATTTGTGAAGAC |
| ALP | ACGTGGCTAAGAATGTCATC | CTGGTAGGCGATGTCCTTA |
| OC | ATGAGAGCCCTCACACTCCTC | CCGTAGAAGCGCCGATAGGC |
| Cycles | Temperature | Duration | Step |
|---|---|---|---|
| 1 | 95 °C | 1 min | Initial denaturing |
| 45 | 95 °C | 15 s | Denaturing |
| 60 °C | 30 s | Annealing | |
| 72 °C | 15 s | Extension | |
| 1 | 72 °C | 1 min | Melting curve |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
De Vos, B.; Kasonga, A.E.; Joubert, A.M.; Nyakudya, T.T. Exploring the In Vitro Effects of Zingerone on Differentiation and Signalling Pathways in Bone Cell Lines. Metabolites 2024, 14, 693. https://doi.org/10.3390/metabo14120693
De Vos B, Kasonga AE, Joubert AM, Nyakudya TT. Exploring the In Vitro Effects of Zingerone on Differentiation and Signalling Pathways in Bone Cell Lines. Metabolites. 2024; 14(12):693. https://doi.org/10.3390/metabo14120693
Chicago/Turabian StyleDe Vos, Brunhildé, Abe E. Kasonga, Anna M. Joubert, and Trevor T. Nyakudya. 2024. "Exploring the In Vitro Effects of Zingerone on Differentiation and Signalling Pathways in Bone Cell Lines" Metabolites 14, no. 12: 693. https://doi.org/10.3390/metabo14120693
APA StyleDe Vos, B., Kasonga, A. E., Joubert, A. M., & Nyakudya, T. T. (2024). Exploring the In Vitro Effects of Zingerone on Differentiation and Signalling Pathways in Bone Cell Lines. Metabolites, 14(12), 693. https://doi.org/10.3390/metabo14120693







