Longitudinal Profiling of Fasting Plasma Metabolome in Response to Weight-Loss Interventions in Patients with Morbid Obesity
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Participants
2.2. Anthropometric and Clinical Measures
2.3. Study Outcomes
2.4. Metabolomic Data Acquisition, Pre-Processing, and Quality Control
2.5. Statistical Analysis
2.5.1. Prospective Association Analysis
2.5.2. Repeated Measurement Analysis
2.5.3. Differential Metabolic Networks
2.5.4. Pathway-Enrichment Analysis
3. Results
3.1. Baseline Plasma Metabolites Associated with Changes in Glycemic Outcomes, Independent of Weight Loss
3.2. Longitudinal Changes in Plasma Metabolites Associated with Changes in Glycemic Outcomes, Independent of Weight Loss
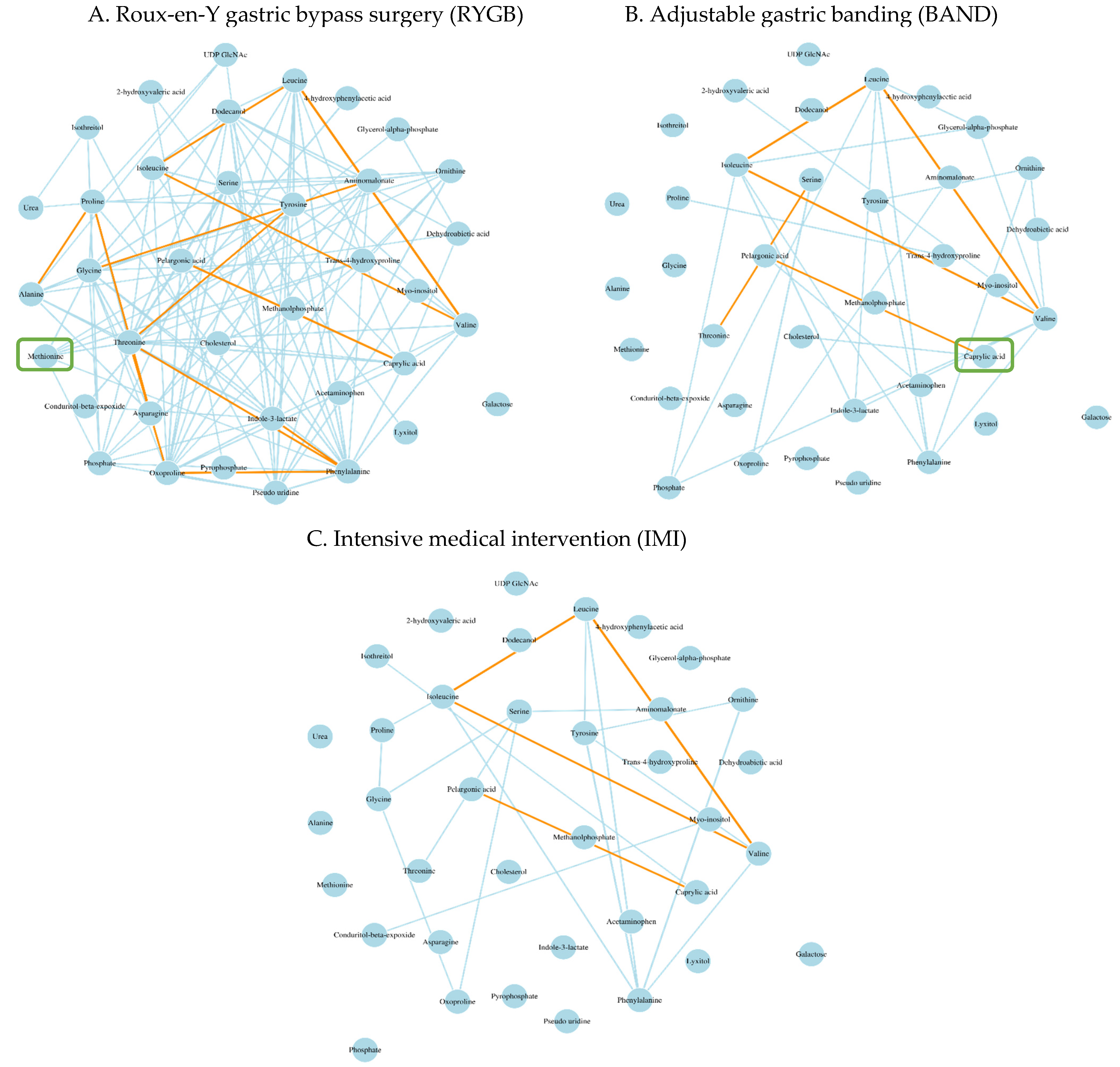
3.3. Differential Metabolic Networks Associated with Different Types of Weight-Loss Intervention
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Semlitsch, T.; Stigler, F.L.; Jeitler, K.; Horvath, K.; Siebenhofer, A. Management of overweight and obesity in primary care-A systematic overview of international evidence-based guidelines. Obes. Rev. 2019, 20, 1218–1230. [Google Scholar] [CrossRef]
- Twig, G.; Zucker, I.; Afek, A.; Cukierman-Yaffe, T.; Bendor, C.D.; Derazne, E.; Lutski, M.; Shohat, T.; Mosenzon, O.; Tzur, D.; et al. Adolescent Obesity and Early-Onset Type 2 Diabetes. Diabetes Care 2020, 43, 1487–1495. [Google Scholar] [CrossRef]
- Jensen, M.D.; Ryan, D.H.; Apovian, C.M.; Ard, J.D.; Comuzzie, A.G.; Donato, K.A.; Hu, F.B.; Hubbard, V.S.; Jakicic, J.M.; Kushner, R.F.; et al. 2013 AHA/ACC/TOS guideline for the management of overweight and obesity in adults: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and The Obesity Society. J. Am. Coll. Cardiol. 2014, 63, 2985–3023. [Google Scholar] [CrossRef]
- Bray, G.A.; Heisel, W.E.; Afshin, A.; Jensen, M.D.; Dietz, W.H.; Long, M.; Kushner, R.F.; Daniels, S.R.; Wadden, T.A.; Tsai, A.G.; et al. The Science of Obesity Management: An Endocrine Society Scientific Statement. Endocr. Rev. 2018, 39, 79–132. [Google Scholar] [CrossRef]
- Wadden, T.A.; Hollander, P.; Klein, S.; Niswender, K.; Woo, V.; Hale, P.M.; Aronne, L. Weight maintenance and additional weight loss with liraglutide after low-calorie-diet-induced weight loss: The SCALE Maintenance randomized study. Int. J. Obes. 2015, 39, 187. [Google Scholar] [CrossRef]
- Chang, S.H.; Stoll, C.R.; Song, J.; Varela, J.E.; Eagon, C.J.; Colditz, G.A. The effectiveness and risks of bariatric surgery: An updated systematic review and meta-analysis, 2003–2012. JAMA Surg. 2014, 149, 275–287. [Google Scholar] [CrossRef]
- Kashyap, S.R.; Bhatt, D.L.; Wolski, K.; Watanabe, R.M.; Abdul-Ghani, M.; Abood, B.; Pothier, C.E.; Brethauer, S.; Nissen, S.; Gupta, M.; et al. Metabolic effects of bariatric surgery in patients with moderate obesity and type 2 diabetes: Analysis of a randomized control trial comparing surgery with intensive medical treatment. Diabetes Care 2013, 36, 2175–2182. [Google Scholar] [CrossRef]
- Dicker, D.; Yahalom, R.; Comaneshter, D.S.; Vinker, S. Long-Term Outcomes of Three Types of Bariatric Surgery on Obesity and Type 2 Diabetes Control and Remission. Obes. Surg. 2016, 26, 1814–1820. [Google Scholar] [CrossRef]
- Cummings, D.E.; Arterburn, D.E.; Westbrook, E.O.; Kuzma, J.N.; Stewart, S.D.; Chan, C.P.; Bock, S.N.; Landers, J.T.; Kratz, M.; Foster-Schubert, K.E.; et al. Gastric bypass surgery vs intensive lifestyle and medical intervention for type 2 diabetes: The CROSSROADS randomised controlled trial. Diabetologia 2016, 59, 945–953. [Google Scholar] [CrossRef]
- Aasbrenn, M.; Schnurr, T.M.; Have, C.T.; Svendstrup, M.; Hansen, D.L.; Worm, D.; Balslev-Harder, M.; Hollensted, M.; Grarup, N.; Burgdorf, K.S.; et al. Genetic Determinants of Weight Loss After Bariatric Surgery. Obes. Surg. 2019, 29, 2554–2561. [Google Scholar] [CrossRef]
- Kirchner, H.; Nylen, C.; Laber, S.; Barres, R.; Yan, J.; Krook, A.; Zierath, J.R.; Naslund, E. Altered promoter methylation of PDK4, IL1 B, IL6, and TNF after Roux-en Y gastric bypass. Surg. Obes. Relat. Dis. 2014, 10, 671–678. [Google Scholar] [CrossRef]
- Nannipieri, M.; Baldi, S.; Mari, A.; Colligiani, D.; Guarino, D.; Camastra, S.; Barsotti, E.; Berta, R.; Moriconi, D.; Bellini, R.; et al. Roux-en-Y gastric bypass and sleeve gastrectomy: Mechanisms of diabetes remission and role of gut hormones. J. Clin. Endocrinol. Metab. 2013, 98, 4391–4399. [Google Scholar] [CrossRef]
- Luo, P.; Yu, H.; Zhao, X.; Bao, Y.; Hong, C.S.; Zhang, P.; Tu, Y.; Yin, P.; Gao, P.; Wei, L.; et al. Metabolomics Study of Roux-en-Y Gastric Bypass Surgery (RYGB) to Treat Type 2 Diabetes Patients Based on Ultraperformance Liquid Chromatography-Mass Spectrometry. J. Proteome Res. 2016, 15, 1288–1299. [Google Scholar] [CrossRef]
- Heffron, S.P.; Singh, A.; Zagzag, J.; Youn, H.A.; Underberg, J.A.; Fielding, G.A.; Ren-Fielding, C.J. Laparoscopic gastric banding resolves the metabolic syndrome and improves lipid profile over five years in obese patients with body mass index 30–40 kg/m2. Atherosclerosis 2014, 237, 183–190. [Google Scholar] [CrossRef]
- Contrepois, K.; Liang, L.; Snyder, M. Can Metabolic Profiles Be Used as a Phenotypic Readout of the Genome to Enhance Precision Medicine? Clin. Chem. 2016, 62, 676–678. [Google Scholar] [CrossRef]
- Zierer, J.; Jackson, M.A.; Kastenmüller, G.; Mangino, M.; Long, T.; Telenti, A.; Mohney, R.P.; Small, K.S.; Bell, J.T.; Steves, C.J.; et al. The fecal metabolome as a functional readout of the gut microbiome. Nat. Genet. 2018, 50, 790–795. [Google Scholar] [CrossRef]
- Laferrère, B.; Reilly, D.; Arias, S.; Swerdlow, N.; Gorroochurn, P.; Bawa, B.; Bose, M.; Teixeira, J.; Stevens, R.D.; Wenner, B.R.; et al. Differential metabolic impact of gastric bypass surgery versus dietary intervention in obese diabetic subjects despite identical weight loss. Sci. Transl. Med. 2011, 3, 80re2. [Google Scholar] [CrossRef]
- Lips, M.A.; Van Klinken, J.B.; van Harmelen, V.; Dharuri, H.K.; t Hoen, P.A.; Laros, J.F.; van Ommen, G.J.; Janssen, I.M.; Van Ramshorst, B.; Van Wagensveld, B.A.; et al. Roux-en-Y gastric bypass surgery, but not calorie restriction, reduces plasma branched-chain amino acids in obese women independent of weight loss or the presence of type 2 diabetes. Diabetes Care 2014, 37, 3150–3156. [Google Scholar] [CrossRef]
- Ashrafian, H.; Li, J.V.; Spagou, K.; Harling, L.; Masson, P.; Darzi, A.; Nicholson, J.K.; Holmes, E.; Athanasiou, T. Bariatric surgery modulates circulating and cardiac metabolites. J. Proteome Res. 2014, 13, 570–580. [Google Scholar] [CrossRef]
- Patti, M.E.; Houten, S.M.; Bianco, A.C.; Bernier, R.; Larsen, P.R.; Holst, J.J.; Badman, M.K.; Maratos-Flier, E.; Mun, E.C.; Pihlajamaki, J.; et al. Serum bile acids are higher in humans with prior gastric bypass: Potential contribution to improved glucose and lipid metabolism. Obesity 2009, 17, 1671–1677. [Google Scholar] [CrossRef]
- Vaz, M.; Pereira, S.S.; Monteiro, M.P. Metabolomic signatures after bariatric surgery—A systematic review. Rev. Endocr. Metab. Disord. 2022, 23, 503–519. [Google Scholar] [CrossRef]
- Pantelis, A.G. Metabolomics in Bariatric and Metabolic Surgery Research and the Potential of Deep Learning in Bridging the Gap. Metabolites 2022, 12, 458. [Google Scholar] [CrossRef] [PubMed]
- Sandoval, D.A.; Patti, M.E. Glucose metabolism after bariatric surgery: Implications for T2DM remission and hypoglycaemia. Nat. Rev. Endocrinol. 2023, 19, 164–176. [Google Scholar] [CrossRef]
- Chondronikola, M.; Harris, L.L.; Klein, S. Bariatric surgery and type 2 diabetes: Are there weight loss-independent therapeutic effects of upper gastrointestinal bypass? J. Intern. Med. 2016, 280, 476–486. [Google Scholar] [CrossRef]
- Brantley, P.J.; Guan, W.; Brock, R.; Zhang, D.; Hu, G. HEADS UP: Design and Methods of a Louisiana State-Funded Surgical and Non-Surgical Weight Loss Program. Int. J. Environ. Res. Public Health 2020, 17, 2999. [Google Scholar] [CrossRef]
- Pickering, T.G.; Hall, J.E.; Appel, L.J.; Falkner, B.E.; Graves, J.; Hill, M.N.; Jones, D.W.; Kurtz, T.; Sheps, S.G.; Roccella, E.J. Recommendations for blood pressure measurement in humans and experimental animals: Part 1: Blood pressure measurement in humans: A statement for professionals from the Subcommittee of Professional and Public Education of the American Heart Association Council on High Blood Pressure Research. Circulation 2005, 111, 697–716. [Google Scholar] [CrossRef]
- Diagnosis and classification of diabetes mellitus. Diabetes Care 2014, 37, S81–S90. [CrossRef]
- Fiehn, O.; Kind, T. Metabolite profiling in blood plasma. Methods Mol. Biol. 2007, 358, 3–17. [Google Scholar] [CrossRef]
- Fiehn, O.; Garvey, W.T.; Newman, J.W.; Lok, K.H.; Hoppel, C.L.; Adams, S.H. Plasma metabolomic profiles reflective of glucose homeostasis in non-diabetic and type 2 diabetic obese African-American women. PLoS ONE 2010, 5, e15234. [Google Scholar] [CrossRef]
- Fiehn, O.; Wohlgemuth, G.; Scholz, M. Setup and annotation of metabolomic experiments by integrating biological and mass spectrometric metadata. In Data Integration in the Life Sciences: Second International Workshop, DILS 2005, San Diego, CA, USA, July 20–22, 2005. Proceedings 2; Springer: Berlin/Heidelberg, Germany, 2005; pp. 224–239. [Google Scholar]
- Scholz, M.; Fiehn, O. SetupX—A public study design database for metabolomic projects. Pac. Symp. Biocomput. 2007, 169–180. [Google Scholar]
- Fan, S.; Kind, T.; Cajka, T.; Hazen, S.L.; Tang, W.H.W.; Kaddurah-Daouk, R.; Irvin, M.R.; Arnett, D.K.; Barupal, D.K.; Fiehn, O. Systematic Error Removal Using Random Forest for Normalizing Large-Scale Untargeted Lipidomics Data. Anal. Chem. 2019, 91, 3590–3596. [Google Scholar] [CrossRef]
- Storey, J.D.; Tibshirani, R. Statistical significance for genomewide studies. Proc. Natl. Acad. Sci. USA 2003, 100, 9440–9445. [Google Scholar] [CrossRef]
- Langfelder, P.; Horvath, S. WGCNA: An R package for weighted correlation network analysis. BMC Bioinform. 2008, 9, 559. [Google Scholar] [CrossRef]
- Horvath, S.; Dong, J. Geometric interpretation of gene coexpression network analysis. PLoS Comput. Biol. 2008, 4, e1000117. [Google Scholar] [CrossRef]
- Peschel, S.; Müller, C.L.; von Mutius, E.; Boulesteix, A.L.; Depner, M. NetCoMi: Network construction and comparison for microbiome data in R. Brief. Bioinform. 2021, 22, bbaa290. [Google Scholar] [CrossRef]
- Pang, Z.; Chong, J.; Zhou, G.; de Lima Morais, D.A.; Chang, L.; Barrette, M.; Gauthier, C.; Jacques, P.; Li, S.; Xia, J. MetaboAnalyst 5.0: Narrowing the gap between raw spectra and functional insights. Nucleic Acids Res. 2021, 49, W388–W396. [Google Scholar] [CrossRef]
- Chong, J.; Wishart, D.S.; Xia, J. Using MetaboAnalyst 4.0 for Comprehensive and Integrative Metabolomics Data Analysis. Curr. Protoc. Bioinform. 2019, 68, e86. [Google Scholar] [CrossRef]
- Jiménez-Sánchez, C.; Mezza, T.; Sinturel, F.; Li, L.; Di Giuseppe, G.; Quero, G.; Jornayvaz, F.R.; Guessous, I.; Dibner, C.; Schrauwen, P.; et al. Circulating 1,5-Anhydroglucitol as a Biomarker of ß-cell Mass Independent of a Diabetes Phenotype in Human Subjects. J. Clin. Endocrinol. Metab. 2022, 107, 2833–2843. [Google Scholar] [CrossRef]
- Yamanouchi, T.; Akanuma, H.; Nakamura, T.; Akaoka, I.; Akanuma, Y. Reduction of plasma 1,5-anhydroglucitol (1-deoxyglucose) concentration in diabetic patients. Diabetologia 1988, 31, 41–45. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Krznar, P.; Erban, A.; Agazzi, A.; Martin-Levilain, J.; Supale, S.; Kopka, J.; Zamboni, N.; Maechler, P. Metabolomics Identifies a Biomarker Revealing In Vivo Loss of Functional β-Cell Mass Before Diabetes Onset. Diabetes 2019, 68, 2272–2286. [Google Scholar] [CrossRef] [PubMed]
- Tanianskii, D.A.; Jarzebska, N.; Birkenfeld, A.L.; O’Sullivan, J.F.; Rodionov, R.N. Beta-Aminoisobutyric Acid as a Novel Regulator of Carbohydrate and Lipid Metabolism. Nutrients 2019, 11, 524. [Google Scholar] [CrossRef]
- Roberts, L.D.; Boström, P.; O’Sullivan, J.F.; Schinzel, R.T.; Lewis, G.D.; Dejam, A.; Lee, Y.K.; Palma, M.J.; Calhoun, S.; Georgiadi, A.; et al. β-Aminoisobutyric acid induces browning of white fat and hepatic β-oxidation and is inversely correlated with cardiometabolic risk factors. Cell Metab. 2014, 19, 96–108. [Google Scholar] [CrossRef]
- Angelidi, A.M.; Kokkinos, A.; Sanoudou, D.; Connelly, M.A.; Alexandrou, A.; Mingrone, G.; Mantzoros, C.S. Early metabolomic, lipid and lipoprotein changes in response to medical and surgical therapeutic approaches to obesity. Metabolism 2023, 138, 155346. [Google Scholar] [CrossRef]
- Rogova, O.; Herzog, K.; Al-Majdoub, M.; Miskelly, M.; Lindqvist, A.; Bennet, L.; Hedenbro, J.L.; Wierup, N.; Spégel, P. Metabolic remission precedes possible weight regain after gastric bypass surgery. Obesity 2023, 31, 2530–2542. [Google Scholar] [CrossRef] [PubMed]
- Thaker, V.V.; Kwee, L.C.; Chen, H.; Bahnson, J.; Ilkayeva, O.; Muehlbauer, M.J.; Wolfe, B.; Purnell, J.Q.; Pi-Sunyer, X.; Newgard, C.B.; et al. Metabolite signature of diabetes remission in individuals with obesity undergoing weight loss interventions. Obesity 2023, 32, 304–314. [Google Scholar] [CrossRef]
- Cheng, C.W.; Biton, M.; Haber, A.L.; Gunduz, N.; Eng, G.; Gaynor, L.T.; Tripathi, S.; Calibasi-Kocal, G.; Rickelt, S.; Butty, V.L.; et al. Ketone Body Signaling Mediates Intestinal Stem Cell Homeostasis and Adaptation to Diet. Cell 2019, 178, 1115–1131.e15. [Google Scholar] [CrossRef] [PubMed]
- Shimazu, T.; Hirschey, M.D.; Newman, J.; He, W.; Shirakawa, K.; Le Moan, N.; Grueter, C.A.; Lim, H.; Saunders, L.R.; Stevens, R.D.; et al. Suppression of oxidative stress by β-hydroxybutyrate, an endogenous histone deacetylase inhibitor. Science 2013, 339, 211–214. [Google Scholar] [CrossRef] [PubMed]
- Koliaki, C.; Liatis, S.; le Roux, C.W.; Kokkinos, A. The role of bariatric surgery to treat diabetes: Current challenges and perspectives. BMC Endocr. Disord. 2017, 17, 50. [Google Scholar] [CrossRef]
- Samczuk, P.; Ciborowski, M.; Kretowski, A. Application of Metabolomics to Study Effects of Bariatric Surgery. J. Diabetes Res. 2018, 2018, 6270875. [Google Scholar] [CrossRef]
- Navik, U.; Sheth, V.G.; Khurana, A.; Jawalekar, S.S.; Allawadhi, P.; Gaddam, R.R.; Bhatti, J.S.; Tikoo, K. Methionine as a double-edged sword in health and disease: Current perspective and future challenges. Ageing Res. Rev. 2021, 72, 101500. [Google Scholar] [CrossRef]
- Furukawa, S.; Fujita, T.; Shimabukuro, M.; Iwaki, M.; Yamada, Y.; Nakajima, Y.; Nakayama, O.; Makishima, M.; Matsuda, M.; Shimomura, I. Increased oxidative stress in obesity and its impact on metabolic syndrome. J. Clin. Investig. 2004, 114, 1752–1761. [Google Scholar] [CrossRef] [PubMed]
- Ighodaro, O.M. Molecular pathways associated with oxidative stress in diabetes mellitus. Biomed. Pharmacother. Biomed. Pharmacother. 2018, 108, 656–662. [Google Scholar] [CrossRef] [PubMed]
- Miller, W.M.; Ziegler, K.M.; Yilmaz, A.; Saiyed, N.; Ustun, I.; Akyol, S.; Idler, J.; Sims, M.D.; Maddens, M.E.; Graham, S.F. Association of Metabolomic Biomarkers with Sleeve Gastrectomy Weight Loss Outcomes. Metabolites 2023, 13, 506. [Google Scholar] [CrossRef] [PubMed]
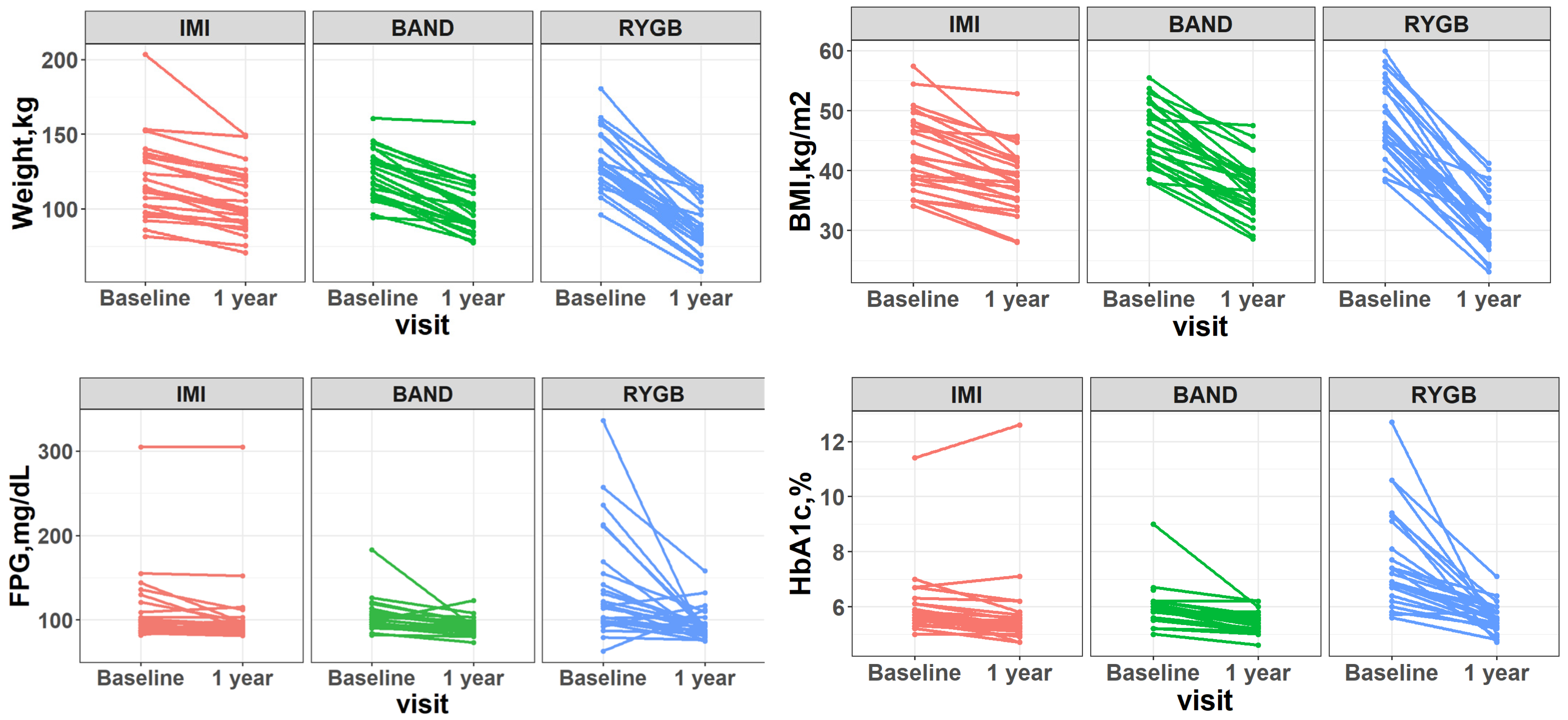

| Characteristics | Intensive Medical Intervention (n = 25) | Adjustable Gastric Banding (n = 25) | Roux-en-Y Gastric Bypass Surgery (n = 25) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Baseline | 1 Year | Change | p-Value | Baseline | 1 Year | Change | p-Value | Baseline | 1 Year | Change | p-Value | |
| Age, (year) | 51 ± 8 | - | - | - | 50 ± 8 | - | - | - | 51 ± 9 | - | - | - |
| Female, n (%) | 21 (84) | - | - | - | 21 (84) | - | - | - | 21 (84) | - | - | |
| White, n (%) | 17 (68) | - | - | - | 19 (76) | - | - | - | 18 (72) | - | - | - |
| BMI, kg/m2 | 43.3 ± 6.5 | 38.3 ± 5.7 | −5.0 ± 3.3 | 5.69 × 10−8 | 45.7 ± 5.3 | 36.9 ± 5.0 | −8.8 ± 3.9 | 3.13 × 10−11 | 48.5 ± 6.2 | 31.2 ± 5.0 | −17.2 ± 4.8 | 2.43 × 10−15 |
| Weight, kg | 120 ± 26 | 106 ± 21 | −14 ± 11 | 5.69 × 10−7 | 123 ± 16 | 100 ± 18 | −24 ± 10 | 1.43 × 10−11 | 133 ± 20 | 86 ± 16 | −47 ± 14 | 4.91 × 10−15 |
| WC, cm | 123 ± 15 | 115 ± 13 | −7 ± 8 | 1.69 × 10−4 | 130 ± 10 | 110 ± 12 | −20 ± 9.3 | 1.39 × 10−10 | 140 ± 11 | 101 ± 13 | −38 ± 12 | 6.51 × 10−14 |
| SBP, mmHg | 126 ± 12 | 120 ± 17 | −6 ± 12 | 3.27 × 10−2 | 129 ± 15 | 115 ± 13 | −14 ± 19 | 1.05 × 10−3 | 129 ± 14 | 120 ± 19 | −10 ± 20 | 2.13 × 10−2 |
| DBP, mmHg | 82 ± 9 | 80 ± 8 | −2 ± 8 | 1.82 × 10−1 | 81 ± 8 | 73 ± 7 | −8 ± 8 | 1.26 × 10−5 | 78 ± 11 | 73 ± 9 | −5 ± 12 | 4.84 × 10−2 |
| Total cholesterol, mg/dL | 188.4 ± 28.8 | 188.8 ± 28.9 | 0.4 ± 30 | 0.95 | 185.4 ± 39.9 | 183.3 ± 40.8 | 1.1 ± 26 | 0.83 | 180 ± 40 | 180 ± 29 | −6.8 ± 36 | 0.36 |
| FPG, mg/dL | 98 [90, 109] | 93 [90, 98] | − 3 [−7, 2] | 3.18 × 10−2 | 103 [94, 110] | 92 [88, 96] | −11 [−15, −4] | 6.33 × 10−4 | 118 [98, 155] | 92 [84, 96] | − 27 [−58, −6] | 6.98 × 10−4 |
| HbA1c, % | 5.7 [5.5, 6.1] | 5.3 [5.2, 5.6] | −0.4 [−0.5, −0.2] | 1.53 × 10−3 | 5.9 [5.8, 6.1] | 5.4 [5.2, 5.6] | −0.5 [−0.6, −0.3] | 4.10 × 10−5 | 7.2 [6.4, 9.1] | 5.5 [5.3, 5.8] | −1.3 [−3.1, −0.8] | 1.45 × 10−5 |
| Metabolites | IMI | BAND | RYGB | RYGB vs. IMI | RYGB vs. BAND | BAND vs. IMI | |||
|---|---|---|---|---|---|---|---|---|---|
| β* (SE) | β* (SE) | β* (SE) | β† (SE) | p Value | β† (SE) | p Value | β† (SE) | p Value | |
| Pyrophosphate | 1.74 (3.15) | 0.01 (0.09) | 17.38 (2.63) | 15.64 (4.16) | 3.76 × 10−4 | 17.37 (2.63) | 9.50 × 10−9 | −1.73 (3.15) | 5.85 × 10−1 |
| Behenic acid | 0.11 (0.12) | −0.46 (0.22) | 1.78 (0.37) | 1.67 (0.38) | 4.70 × 10−5 | 2.24 (0.43) | 2.36 × 10−6 | −0.57 (0.25) | 2.79 × 10−2 |
| 3-aminoisobutyric acid | 0.13 (0.16) | 0.02 (0.14) | 2.09 (0.45) | 1.96 (0.48) | 1.35 × 10−4 | 2.07 (0.47) | 4.63 × 10−5 | −0.11 (0.22) | 6.09 × 10−1 |
| Hydrocinnamic acid | 0.09 (0.27) | −0.12 (0.15) | 0.88 (0.44) | 1.79 (0.51) | 8.37 × 10−4 | 2.00 (0.46) | 4.92 × 10−5 | −0.21 (0.31) | 5.00 × 10−1 |
| Gluconic acid | 0.11 (0.27) | 0.14 (0.19) | −0.89 (0.14) | −1.00 (0.30) | 1.48 × 10−3 | −1.02 (0.24) | 5.27 × 10−5 | 0.03 (0.32) | 9.34 × 10−1 |
| Butane-2,3-diol | 0.00 (0.16) | −0.33 (0.18) | 5.21 (1.28) | 5.21 (1.28) | 1.30 × 10−4 | 5.53 (1.29) | 5.97 × 10−5 | −0.32 (0.24) | 1.80 × 10−1 |
| Methionine | 0.01 (0.20) | −0.05 (0.22) | 1.76 (0.45) | 1.75 (0.49) | 7.16 × 10−4 | 1.81 (0.50) | 6.16 × 10−4 | −0.06 (0.30) | 8.51 × 10−1 |
| Creatinine | 0.09 (0.17) | −0.01 (0.18) | 1.15 (0.31) | 1.06 (0.34) | 2.92 × 10−3 | 1.16 (0.35) | 1.37 × 10−3 | −0.10 (0.23) | 6.73 × 10−1 |
| Glucose | −0.09 (0.16) | −0.13 (0.28) | −0.86 (0.14) | −0.78 (0.21) | 4.83 × 10−4 | −0.73 (0.32) | 2.38 × 10−2 | −0.05 (0.33) | 8.83 × 10−1 |
| Alanine | −0.10 (0.19) | 0.15 (0.20) | 0.75 (0.17) | 0.85 (0.26) | 1.55 × 10−3 | 0.60 (0.27) | 2.67 × 10−2 | 0.25 (0.27) | 3.65 × 10−1 |
| Allantoin | 1.39 (2.19) | 0.11 (0.17) | 0.95 (0.21) | −0.44 (2.20) | 8.43 × 10−1 | 0.84 (0.27) | 2.66 × 10−3 | −1.27 (2.20) | 5.64 × 10−1 |
| Galactonic acid | −0.01 (0.26) | 0.13 (0.20) | −0.79 (0.15) | −0.78 (0.30) | 1.03 × 10−2 | −0.92 (0.25) | 4.01 × 10−4 | −0.14 (0.32) | 6.68 × 10−1 |
| Glutamine | 0.05 (0.17) | −0.08 (0.20) | 0.93 (0.24) | 0.88 (0.30) | 4.54 × 10−3 | 1.01 (0.32) | 2.38 × 10−3 | 0.13 (0.25) | 6.08 × 10−1 |
| Mannose | −0.09 (0.21) | 0.12 (0.22) | −0.76 (0.16) | −0.67 (0.26) | 1.23 × 10−2 | −0.88 (0.27) | 1.94 × 10−3 | −0.21 (0.29) | 4.81 × 10−1 |
| N-methylalanine | 0.04 (0.17) | −0.23 (0.20) | 0.75 (0.24) | 0.71 (0.29) | 1.71 × 10−2 | 0.98 (0.31) | 2.83 × 10−3 | −0.27 (0.26) | 3.12 × 10−1 |
| Metabolites | IMI | BAND | RYGB | RYGB vs. IMI | RYGB vs. BAND | BAND vs. IMI | |||
|---|---|---|---|---|---|---|---|---|---|
| β* (SE) | β* (SE) | β* (SE) | β† (SE) | p Value | β† (SE) | p Value | β† (SE) | p Value | |
| 1,5-anhydroglucitol | −0.14 (0.15) | 0.17 (0.13) | 0.83 (0.14) | 0.97 (0.21) | 1.44 × 10−5 | 0.66 (0.19) | 9.77 × 10−4 | 0.31 (0.20) | 1.19 × 10−1 |
| Hydroxylamine | 0.02 (0.14) | 0.10 (0.13) | 0.83 (0.19) | 0.81 (0.23) | 6.37 × 10−4 | 0.73 (0.22) | 1.70 × 10−3 | 0.08 (0.18) | 6.58 × 10−1 |
| Nicotinic acid | 0.02 (0.12) | 0.13 (0.14) | 0.93 (0.23) | 0.90 (0.26) | 7.76 × 10−4 | 0.80 (0.27) | 4.07 × 10−3 | 0.11 (0.18) | 5.50 × 10−1 |
| Phosphoethanolamine | −0.02 (0.13) | 0.09 (0.15) | 0.67 (0.17) | 0.69 (0.20) | 1.16 × 10−3 | 0.58 (0.22) | 1.04 × 10−2 | 0.11 (0.19) | 5.51 × 10−1 |
| Allantoin | −0.63 (1.63) | −0.05 (0.13) | 0.77 (0.15) | 1.41 (1.64) | 3.94 × 10−1 | 0.83 (0.20) | 9.99 × 10−5 | 0.58 (1.64) | 7.24 × 10−1 |
| Pyrophosphate | −0.02 (2.76) | −0.01 (0.08) | 9.58 (2.31) | 9.59 (3.65) | 1.08 × 10−2 | 9.59 (2.31) | 1.00 × 10−4 | 0.00 (2.77) | 9.99 × 10−1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, M.; Miao, G.; Huo, Z.; Peng, H.; Wen, X.; Anton, S.; Zhang, D.; Hu, G.; Brock, R.; Brantley, P.J.; et al. Longitudinal Profiling of Fasting Plasma Metabolome in Response to Weight-Loss Interventions in Patients with Morbid Obesity. Metabolites 2024, 14, 116. https://doi.org/10.3390/metabo14020116
Chen M, Miao G, Huo Z, Peng H, Wen X, Anton S, Zhang D, Hu G, Brock R, Brantley PJ, et al. Longitudinal Profiling of Fasting Plasma Metabolome in Response to Weight-Loss Interventions in Patients with Morbid Obesity. Metabolites. 2024; 14(2):116. https://doi.org/10.3390/metabo14020116
Chicago/Turabian StyleChen, Mingjing, Guanhong Miao, Zhiguang Huo, Hao Peng, Xiaoxiao Wen, Stephen Anton, Dachuan Zhang, Gang Hu, Ricky Brock, Phillip J. Brantley, and et al. 2024. "Longitudinal Profiling of Fasting Plasma Metabolome in Response to Weight-Loss Interventions in Patients with Morbid Obesity" Metabolites 14, no. 2: 116. https://doi.org/10.3390/metabo14020116
APA StyleChen, M., Miao, G., Huo, Z., Peng, H., Wen, X., Anton, S., Zhang, D., Hu, G., Brock, R., Brantley, P. J., & Zhao, J. (2024). Longitudinal Profiling of Fasting Plasma Metabolome in Response to Weight-Loss Interventions in Patients with Morbid Obesity. Metabolites, 14(2), 116. https://doi.org/10.3390/metabo14020116









