The Correlation between Metal Mixed Exposure and Lung Function in Different Ages of the Population
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Participants
2.2. Measurement of Vital Capacity
2.3. Environmental Chemicals
2.4. Covariates
2.5. Statistical Analyses
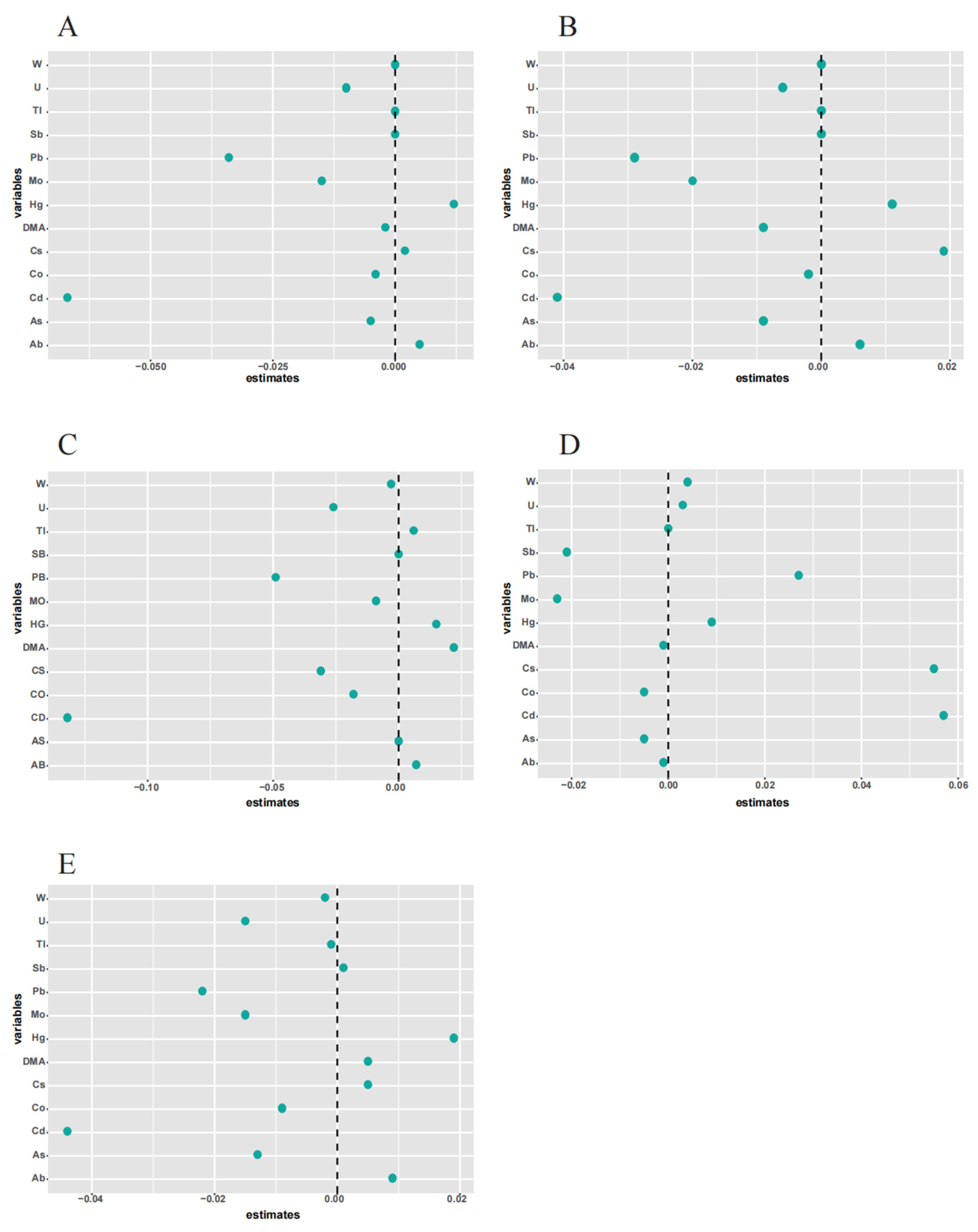
2.5.1. ENET Model and GLM
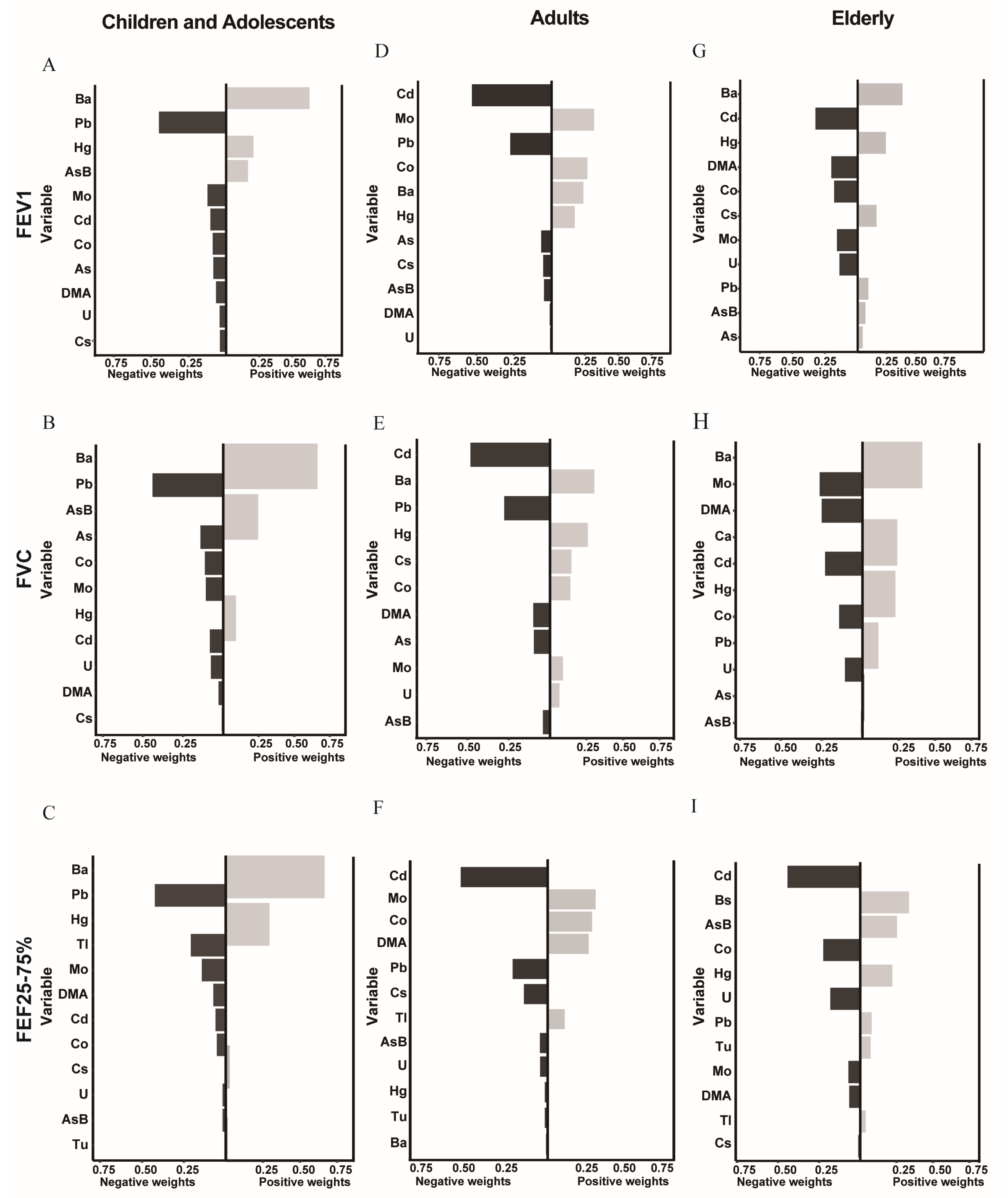
2.5.2. Qgcomp Model
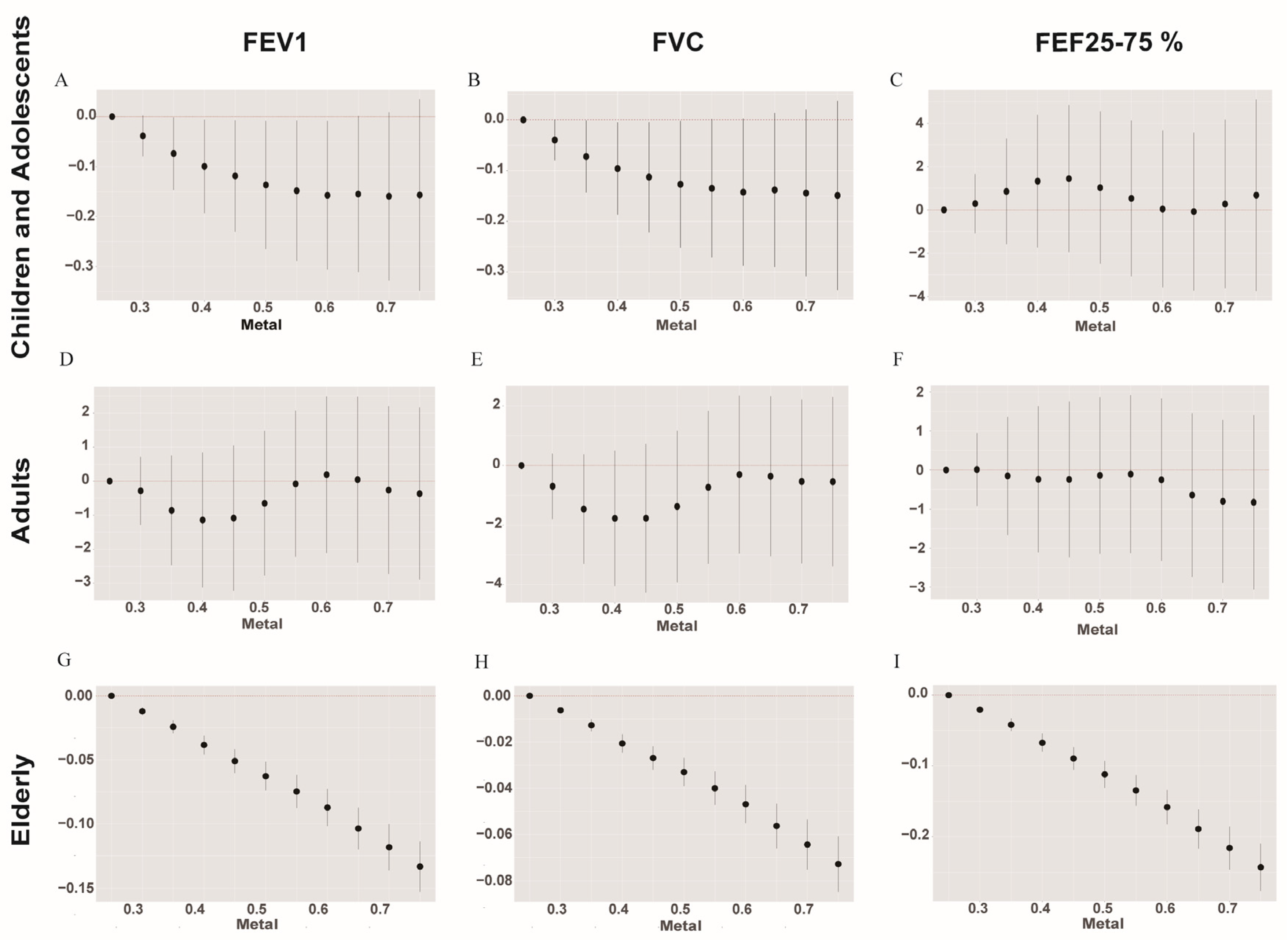
2.5.3. BKMR Model
3. Results
3.1. Population Characteristics
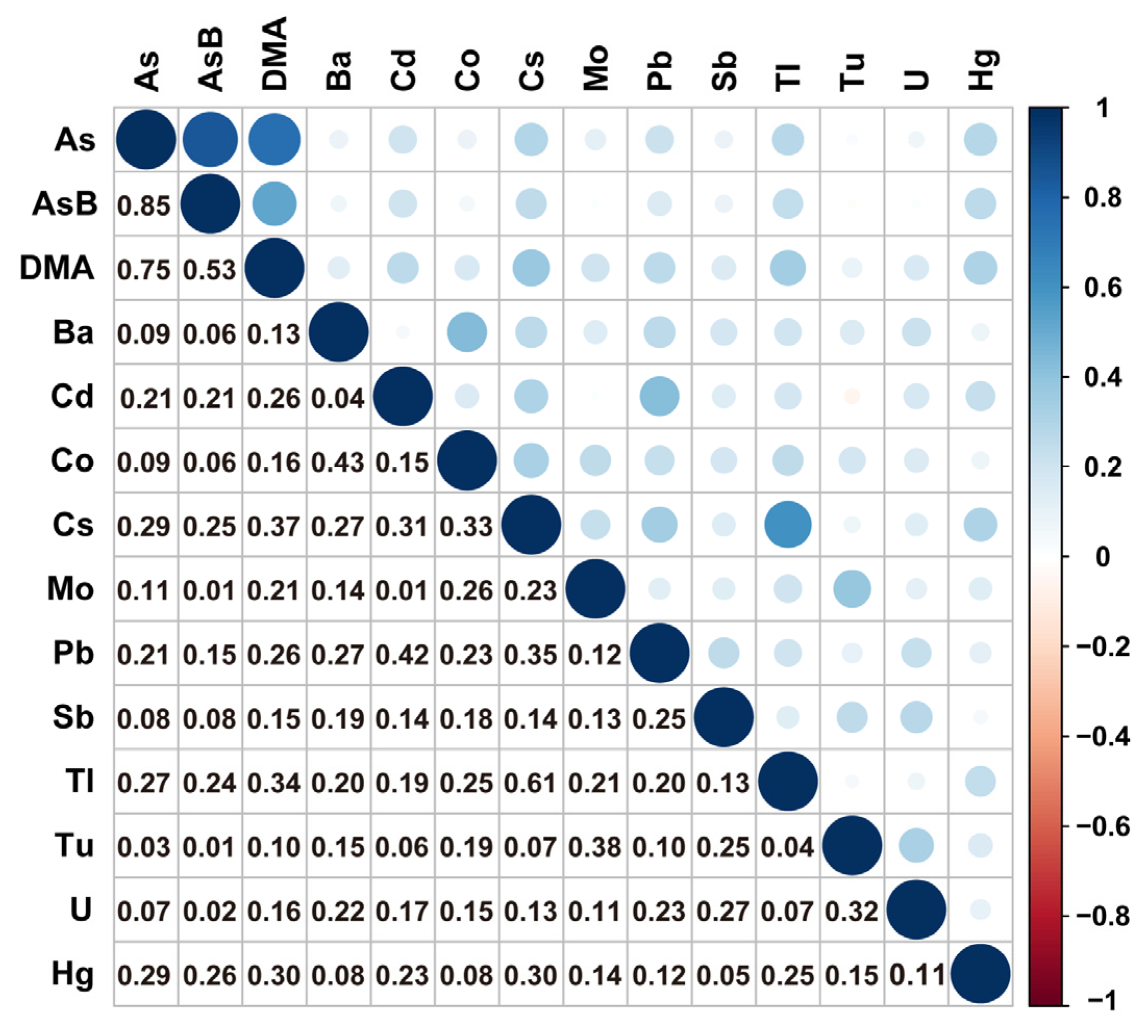
3.2. Urinary Metal Distribution, Correlation, and Selection
3.3. Association between Metal Exposure and Lung Functions
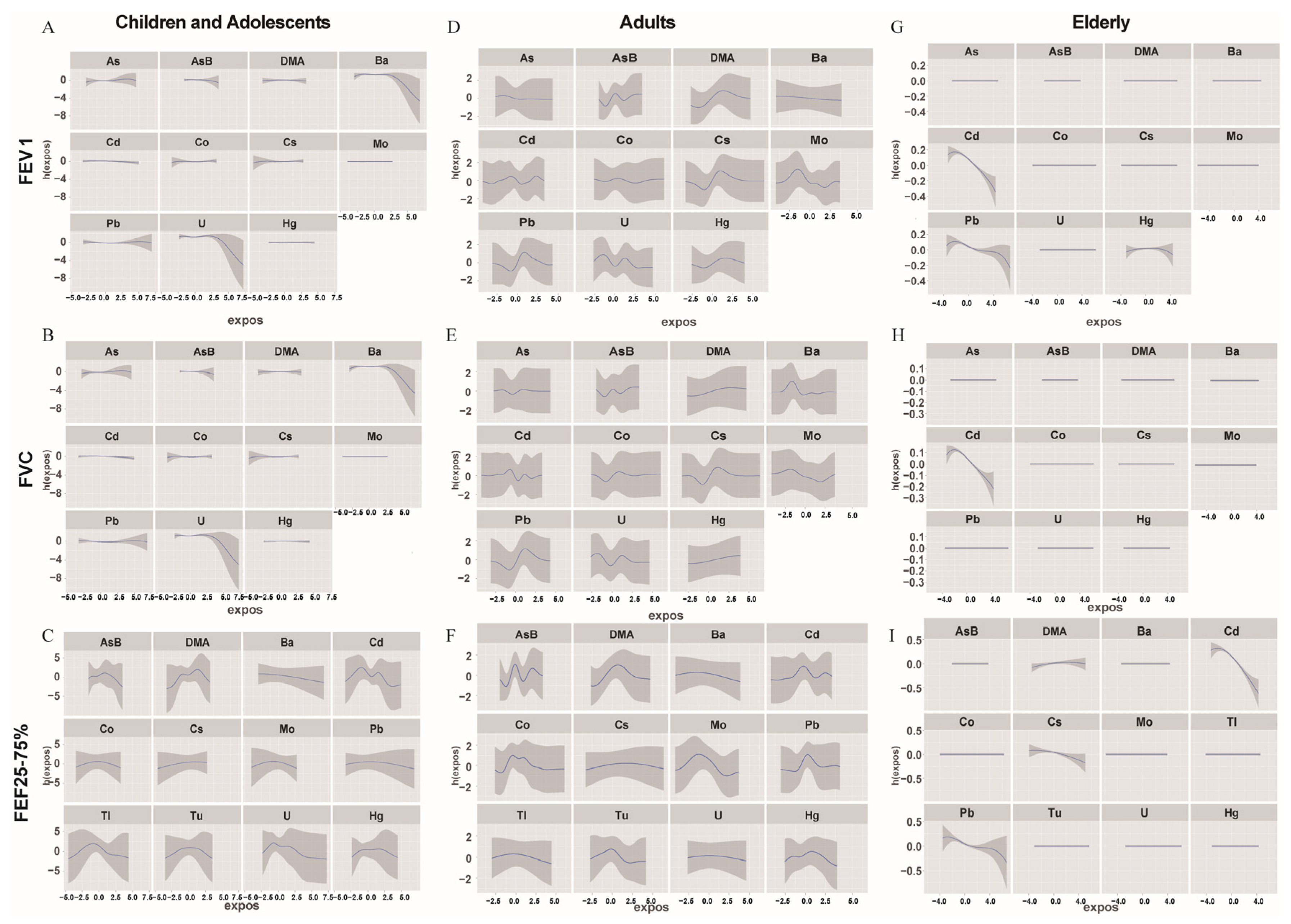
3.4. Association among Urinary Metals and Lung Functions Using the BMKR Model
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Vareda, J.P.; Valente, A.J.M.; Durães, L. Assessment of heavy metal pollution from anthropogenic activities and remediation strategies: A review. J. Environ. Manag. 2019, 246, 101–118. [Google Scholar] [CrossRef]
- Chen, L.; Sun, Q.; Peng, S.; Tan, T.; Mei, G.; Chen, H.; Zhao, Y.; Yao, P.; Tang, Y. Associations of blood and urinary heavy metals with rheumatoid arthritis risk among adults in NHANES 1999–2018. Chemosphere 2022, 289, 133147. [Google Scholar] [CrossRef] [PubMed]
- Witkowska, D.; Słowik, J.; Chilicka, K. Heavy Metals and Human Health: Possible Exposure Pathways and the Competition for Protein Binding Sites. Molecules 2021, 26, 6060. [Google Scholar] [CrossRef] [PubMed]
- Danziger, J.; Dodge, L.E.; Hu, H.; Mukamal, K.J. Susceptibility to Environmental Heavy Metal Toxicity among Americans with Kidney Disease. Kidney360 2022, 3, 1191–1196. [Google Scholar] [CrossRef] [PubMed]
- Kiani, B.; Amin, F.H.; Bagheri, N.; Bergquist, R.; Mohammadi, A.A.; Yousefi, M.; Faraji, H.; Roshandel, G.; Beirami, S.; Rahimzadeh, H.; et al. Association between heavy metals and colon cancer: An ecological study based on geographical information systems in North-Eastern Iran. BMC Cancer 2021, 21, 414. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, H.D.; Oh, H.; Hoang, N.H.M.; Jo, W.H.; Kim, M.-S. Environmental science and pollution research role of heavy metal concentrations and vitamin intake from food in depression: A national cross-sectional study (2009–2017). Environ. Sci. Pollut. Res. 2021, 29, 4574–4586. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Li, N.; Wang, H.; Su, W.; Song, Q.; Liang, Q.; Liang, M.; Sun, C.; Li, Y.; Lowe, S.; et al. Combined exposure to multiple metals on cardiovascular disease in NHANES under five statistical models. Environ. Res. 2022, 215 Pt 3, 114435. [Google Scholar] [CrossRef] [PubMed]
- Haddi, A.A.A.; Jaafar, M.H.; Ismail, H. Association between lung function impairment with urinary heavy metals in a community in Klang Valley, Malaysia. PeerJ 2022, 10, e13845. [Google Scholar] [CrossRef] [PubMed]
- Dwyer-Lindgren, L.; Bertozzi-Villa, A.; Stubbs, R.W.; Morozoff, C.; Shirude, S.; Naghavi, M.; Mokdad, A.H.; Murray, C.J.L. Trends and Patterns of Differences in Chronic Respiratory Disease Mortality Among US Counties, 1980–2014. JAMA 2017, 318, 1136–1149. [Google Scholar] [CrossRef]
- Sobel, M.; Navas-Acien, A.; Powers, M.; Grau-Perez, M.; Goessler, W.; Best, L.G.; Umans, J.; Oelsner, E.C.; Podolanczuk, A.; Sanchez, T.R. Environmental-level exposure to metals and metal-mixtures associated with spirometry-defined lung disease in American Indian adults: Evidence from the Strong Heart Study. Environ. Res. 2021, 207, 112194. [Google Scholar] [CrossRef]
- Assad, N.; Sood, A.; Campen, M.J.; Zychowski, K.E. Metal-Induced Pulmonary Fibrosis. Curr. Environ. Health Rep. 2018, 5, 486–498. [Google Scholar] [CrossRef]
- Wu, L.; Cui, F.; Ma, J.; Huang, Z.; Zhang, S.; Xiao, Z.; Li, J.; Ding, X.; Niu, P. Associations of multiple metals with lung function in welders by four statistical models. Chemosphere 2022, 298, 134202. [Google Scholar] [CrossRef] [PubMed]
- Oh, J.; Shin, S.H.; Choi, R.; Kim, S.; Park, H.-D.; Kim, S.-Y.; Han, S.A.; Koh, W.-J.; Lee, S.-Y. Assessment of 7 trace elements in serum of patients with nontuberculous mycobacterial lung disease. J. Trace Elements Med. Biol. 2019, 53, 84–90. [Google Scholar] [CrossRef]
- Wu, W.; Bromberg, P.A.; Samet, J.M. Zinc ions as effectors of environmental oxidative lung injury. Free. Radic. Biol. Med. 2013, 65, 57–69. [Google Scholar] [CrossRef]
- Chang, Z.; Qiu, J.; Wang, K.; Liu, X.; Fan, L.; Liu, X.; Zhao, Y.; Zhang, Y. The relationship between co-exposure to multiple heavy metals and liver damage. J. Trace Elements Med. Biol. 2023, 77, 127128. [Google Scholar] [CrossRef]
- Andjelkovic, M.; Djordjevic, A.B.; Antonijevic, E.; Antonijevic, B.; Stanic, M.; Kotur-Stevuljevic, J.; Spasojevic-Kalimanovska, V.; Jovanovic, M.; Boricic, N.; Wallace, D.; et al. Toxic Effect of Acute Cadmium and Lead Exposure in Rat Blood, Liver, and Kidney. Int. J. Environ. Res. Public Health 2019, 16, 274. [Google Scholar] [CrossRef]
- Wang, X.; Mukherjee, B.; Park, S.K. Associations of cumulative exposure to heavy metal mixtures with obesity and its comorbidities among U.S. adults in NHANES 2003–2014. Environ. Int. 2018, 121 Pt 1, 683–694. [Google Scholar] [CrossRef]
- Di Cicco, M.; Kantar, A.; Masini, B.; Nuzzi, G.; Ragazzo, V.; Peroni, D. Structural and functional development in airways throughout childhood: Children are not small adults. Pediatr. Pulmonol. 2020, 56, 240–251. [Google Scholar] [CrossRef] [PubMed]
- Smith, L.S.; Zimmerman, J.J.; Martin, T.R. Mechanisms of acute respiratory distress syndrome in children and adults: A review and suggestions for future research. Pediatr. Crit. Care Med. 2013, 14, 631–643. [Google Scholar] [CrossRef] [PubMed]
- Rissler, J.; Gudmundsson, A.; Nicklasson, H.; Swietlicki, E.; Wollmer, P.; Löndah, J. Deposition efficiency of inhaled particles (15–5000 nm) related to breathing pattern and lung function: An experimental study in healthy children and adults. Part. Fibre. Toxicol. 2017, 14, 10. [Google Scholar] [CrossRef] [PubMed]
- Liang, T.I.; Lee, E.Y. Interstitial Lung Diseases in Children, Adolescents, and Young Adults: Different from Infants and Older Adults. Radiol. Clin. N. Am. 2020, 58, 487–502. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Guo, L.; Huang, L.; Zhang, C.; Luo, R.; Zeng, L.; Liang, H.; Li, Q.; Lu, X.; Wang, X.; et al. Distinct Disease Severity Between Children and Older Adults with Coronavirus Disease 2019 (COVID-19): Impacts of ACE2 Expression, Distribution, and Lung Progenitor Cells. Clin. Infect. Dis. 2021, 73, e4154–e4165. [Google Scholar] [CrossRef] [PubMed]
- Sharma, G.; Goodwin, J. Effect of aging on respiratory system physiology and immunology. Clin. Interv. Aging 2006, 1, 253–260. [Google Scholar] [CrossRef] [PubMed]
- Tian, X.; Xue, B.; Wang, B.; Lei, R.; Shan, X.; Niu, J.; Luo, B. Physical activity reduces the role of blood cadmium on depression: A cross-sectional analysis with NHANES data. Environ. Pollut. 2022, 304, 119211. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Xue, Q.; Wen, Y.; Huang, Y.; Wang, Y.; Mahai, G.; Yan, T.; Liu, Y.; Rong, T.; Wang, Y.; et al. Environmental polycyclic aromatic hydrocarbon exposure in relation to metabolic syndrome in US adults. Sci. Total Environ. 2022, 840, 156673. [Google Scholar] [CrossRef] [PubMed]
- Hu, P.; Su, W.; Vinturache, A.; Gu, H.; Cai, C.; Lu, M.; Ding, G. Urinary 3-phenoxybenzoic acid (3-PBA) concentration and pulmonary function in children: A National Health and Nutrition Examination Survey (NHANES) 2007–2012 analysis. Environ. Pollut. 2020, 270, 116178. [Google Scholar] [CrossRef]
- Shi, Y.; Wang, H.; Zhu, Z.; Ye, Q.; Lin, F.; Cai, G. Association between exposure to phenols and parabens and cognitive function in older adults in the United States: A cross-sectional study. Sci. Total Environ. 2023, 858 Pt 3, 160129. [Google Scholar] [CrossRef]
- Che, Z.; Jia, H.; Chen, R.; Pan, K.; Fan, Z.; Su, C.; Wu, Z.; Zhang, T. Associations between exposure to brominated flame retardants and metabolic syndrome and its components in U.S. adults. Sci. Total Environ. 2023, 858 Pt 2, 159935. [Google Scholar] [CrossRef]
- Li, K.; Yin, R.; Wang, Y.; Zhao, D. Associations between exposure to polycyclic aromatic hydrocarbons and metabolic syndrome in U.S. adolescents: Cross-sectional results from the National Health and Nutrition Examination Survey (2003–2016) data. Environ. Res. 2021, 202, 111747. [Google Scholar] [CrossRef]
- Peng, K.; Li, Z.; Gao, T.-R.; Lv, J.; Wang, W.-J.; Zhan, P.; Yao, W.-C.; Zhao, H.; Wang, H.; Xu, D.-X.; et al. Polycyclic aromatic hydrocarbon exposure burden: Individual and mixture analyses of associations with chronic obstructive pulmonary disease risk. Environ. Res. 2023, 222, 115334. [Google Scholar] [CrossRef]
- Guo, X.; Wu, B.; Hu, W.; Wang, X.; Su, W.; Meng, J.; Lowe, S.; Zhao, D.; Huang, C.; Liang, M.; et al. Associations of perchlorate, nitrate, and thiocyanate with metabolic syndrome and its components among US adults: A cross-sectional study from NHANES. Sci. Total Environ. 2023, 879, 163083. [Google Scholar] [CrossRef] [PubMed]
- Kang, H.; Lee, J.P.; Choi, K. Exposure to phthalates and environmental phenols in association with chronic kidney disease (CKD) among the general US population participating in multi-cycle NHANES (2005–2016). Sci. Total Environ. 2021, 791, 148343. [Google Scholar] [CrossRef]
- Kim, S.S.; Meeker, J.D.; Carroll, R.; Zhao, S.; Mourgas, M.J.; Richards, M.J.; Aung, M.; Cantonwine, D.E.; McElrath, T.F.; Ferguson, K.K. Urinary trace metals individually and in mixtures in association with preterm birth. Environ. Int. 2018, 121 Pt 1, 582–590. [Google Scholar] [CrossRef] [PubMed]
- Teppola, P.; Taavitsainen, V.-M. Parsimonious and robust multivariate calibration with rational function Least Absolute Shrinkage and Selection Operator and rational function Elastic Net. Anal. Chim. Acta 2013, 768, 57–68. [Google Scholar] [CrossRef]
- Vuong, A.M.; Xie, C.; Jandarov, R.; Dietrich, K.N.; Zhang, H.; Sjödin, A.; Calafat, A.M.; Lanphear, B.P.; McCandless, L.; Braun, J.M.; et al. Prenatal exposure to a mixture of persistent organic pollutants (POPs) and child reading skills at school age. Int. J. Hyg. Environ. Health 2020, 228, 113527. [Google Scholar] [CrossRef] [PubMed]
- Keil, A.P.; Buckley, J.P.; O’brien, K.M.; Ferguson, K.K.; Zhao, S.; White, A.J. A Quantile-Based g-Computation Approach to Addressing the Effects of Exposure Mixtures. Environ. Health Perspect. 2020, 128, 47004. [Google Scholar] [CrossRef]
- Lee, K.-S.; Kim, K.-N.; Ahn, Y.D.; Choi, Y.-J.; Cho, J.; Jang, Y.; Lim, Y.-H.; Kim, J.I.; Shin, C.H.; Lee, Y.A.; et al. Prenatal and postnatal exposures to four metals mixture and IQ in 6-year-old children: A prospective cohort study in South Korea. Environ. Int. 2021, 157, 106798. [Google Scholar] [CrossRef]
- Bobb, J.F.; Henn, B.C.; Valeri, L.; Coull, B.A. Statistical software for analyzing the health effects of multiple concurrent exposures via Bayesian kernel machine regression. Environ. Health 2018, 17, 67. [Google Scholar] [CrossRef]
- Kim, S.S.; Meeker, J.D.; Keil, A.P.; Aung, M.T.; Bommarito, P.A.; Cantonwine, D.E.; McElrath, T.F.; Ferguson, K.K. Exposure to 17 trace metals in pregnancy and associations with urinary oxidative stress biomarkers. Environ. Res. 2019, 179, 108854. [Google Scholar] [CrossRef]
- Nemery, B. Metal toxicity and the respiratory tract. Eur. Respir. J. 1990, 3, 202–219. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Gao, E.; Xiao, F.; Wu, Q.; Liu, W.; Luo, Y.; Ren, X.; Chen, X.; He, K.; Huang, H.; et al. The relative and interactive effects of urinary multiple metals exposure on hyperuricemia among urban elderly in China. Front. Public Health 2023, 11, 1015202. [Google Scholar] [CrossRef] [PubMed]
- Braun, J.M.; Gennings, C.; Hauser, R.; Webster, T.F. What Can Epidemiological Studies Tell Us about the Impact of Chemical Mixtures on Human Health? Environ. Health Perspect. 2016, 124, A6–A9. [Google Scholar] [CrossRef] [PubMed]
- Ren, J.; Jin, H.; Zhang, C.; Liu, S.; Han, Y.; Xi, J.; Cao, J. Mixed exposure effect of seminal metals on semen quality, mediation of total antioxidant capacity, and moderation of GSTM1/GSTT1 gene deletion in Chinese reproductive-aged men. Environ. Res. 2023, 229, 115888. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.W. Risk factor analysis for the development and progression of retinopathy of prematurity. PLoS ONE 2019, 14, e0219934. [Google Scholar] [CrossRef] [PubMed]
- Su, L.; Ho, H.; Stock, C.T.; Quadri, S.M.; Williamson, C.; Servais, E.L. Surgeon Experience Is Associated with Prolonged Air Leak After Robotic-assisted Pulmonary Lobectomy. Ann. Thorac. Surg. 2021, 114, 434–441. [Google Scholar] [CrossRef]
- Li, Z.; Xu, Y.; Huang, Z.; Wei, Y.; Hou, J.; Long, T.; Wang, F.; Hu, H.; Duan, Y.; Guo, H.; et al. Association between exposure to arsenic, nickel, cadmium, selenium, and zinc and fasting blood glucose levels. Environ. Pollut. 2019, 255 Pt 2, 113325. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Hendryx, M. Metal mixtures and kidney function: An application of machine learning to NHANES data. Environ. Res. 2020, 191, 110126. [Google Scholar] [CrossRef]
- Xu, C.; Liang, J.; Xu, S.; Liu, Q.; Xu, J.; Gu, A. Increased serum levels of aldehydes are associated with cardiovascular disease and cardiovascular risk factors in adults. J. Hazard. Mater. 2020, 400, 123134. [Google Scholar] [CrossRef]
- Saintilnord, W.N.; Fondufe-Mittendorf, Y. Arsenic-induced epigenetic changes in cancer development. Semin. Cancer Biol. 2021, 76, 195–205. [Google Scholar] [CrossRef]
- Garbinski, L.D.; Rosen, B.P.; Chen, J. Pathways of arsenic uptake and efflux. Environ. Int. 2019, 126, 585–597. [Google Scholar] [CrossRef]
- Selman, M.; Pardo, A. Alveolar epithelial cell disintegrity and subsequent activation: A key process in pulmonary fibrosis. Am. J. Respir. Crit. Care Med. 2012, 186, 119–121. [Google Scholar] [CrossRef]
- Atkinson, J.J.; Senior, R.M. Matrix Metalloproteinase-9 in Lung Remodeling. Am. J. Respir. Cell Mol. Biol. 2003, 28, 12–24. [Google Scholar] [CrossRef]
- Moe, B.; Peng, H.; Lu, X.; Chen, B.; Chen, L.W.; Gabos, S.; Li, X.-F.; Le, X.C. Comparative cytotoxicity of fourteen trivalent and pentavalent arsenic species determined using real-time cell sensing. J. Environ. Sci. 2016, 49, 113–124. [Google Scholar] [CrossRef]
- R Roy, N.K.; Murphy, A.; Costa, M. Arsenic Methyltransferase and Methylation of Inorganic Arsenic. Biomolecules 2020, 10, 1351. [Google Scholar] [CrossRef]
- El-Ghiaty, M.A.; El-Kadi, A.O. The Duality of Arsenic Metabolism: Impact on Human Health. Annu. Rev. Pharmacol. Toxicol. 2023, 63, 341–358. [Google Scholar] [CrossRef]
- Cohen, S.M.; Arnold, L.L.; Beck, B.D.; Lewis, A.S.; Eldan, M. Evaluation of the carcinogenicity of inorganic arsenic. Crit. Rev. Toxicol. 2013, 43, 711–752. [Google Scholar] [CrossRef]
- Metryka, E.; Chibowska, K.; Gutowska, I.; Falkowska, A.; Kupnicka, P.; Barczak, K.; Chlubek, D.; Baranowska-Bosiacka, I. Lead (Pb) Exposure Enhances Expression of Factors Associated with Inflammation. Int. J. Mol. Sci. 2018, 19, 1813. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zhang, H.; Zhang, Y.; Wang, J.; Tan, H.; Piao, J.; Yang, L.; Yang, X. The Time Trend of Blood Lead and Cadmium Levels in Rural Chinese Children: China Nutrition and Health Survey 2002 and 2012. Biol. Trace Element Res. 2022, 201, 2162–2169. [Google Scholar] [CrossRef] [PubMed]
- Madaniyazi, L.; Guo, Y.; Ye, X.; Kim, D.; Zhang, Y.; Pan, X. Effects of Airborne Metals on Lung Function in Inner Mongolian Schoolchildren. J. Occup. Environ. Med. 2013, 55, 80–86. [Google Scholar] [CrossRef] [PubMed]
- Rosa, M.J.; Tamayo-Ortiz, M.; Garcia, A.M.; Rivera, N.Y.R.; Bush, D.; Lee, A.G.; Solano-González, M.; Amarasiriwardena, C.; Téllez-Rojo, M.M.; Wright, R.O.; et al. Prenatal lead exposure and childhood lung function: Influence of maternal cortisol and child sex. Environ. Res. 2021, 205, 112447. [Google Scholar] [CrossRef]
- Xiao, L.L.; Zhou, Y.; Cui, X.Q.; Huang, X.J.; Yuan, J.; Chen, W.H. Association of urinary metals and lung function in general Chinese population of Wuhan. Zhonghua Yu Fang Yi Xue Za Zhi 2016, 50, 680–688. [Google Scholar]
- Ahamed, M.; Akhtar, M.J.; Khan, M.M.; Alhadlaq, H.A.; Alshamsan, A. Barium Titanate (BaTiO3) Nanoparticles Exert Cytotoxicity through Oxidative Stress in Human Lung Carcinoma (A549) Cells. Nanomaterials 2020, 10, 2309. [Google Scholar] [CrossRef]
- Wang, W.-J.; Peng, K.; Lu, X.; Zhu, Y.-Y.; Li, Z.; Qian, Q.-H.; Yao, Y.-X.; Fu, L.; Wang, Y.; Huang, Y.-C.; et al. Long-term cadmium exposure induces chronic obstructive pulmonary disease-like lung lesions in a mouse model. Sci. Total Environ. 2023, 879, 163073. [Google Scholar] [CrossRef]
- Knoell, D.L.; Wyatt, T.A. The adverse impact of cadmium on immune function and lung host defense. Semin. Cell Dev. Biol. 2021, 115, 70–76. [Google Scholar] [CrossRef]
- Chasapis, C.T. Shared gene-network signatures between the human heavy metal proteome and neurological disorders and cancer types. Metallomics 2018, 10, 1678–1686. [Google Scholar] [CrossRef]
- Peana, M.; Pelucelli, A.; Chasapis, C.T.; Perlepes, S.P.; Bekiari, V.; Medici, S.; Zoroddu, M.A. Biological Effects of Human Exposure to Environmental Cadmium. Biomolecules 2022, 13, 36. [Google Scholar] [CrossRef]
- Larson-Casey, J.L.; Liu, S.; Pyles, J.M.; Lapi, S.E.; Saleem, K.; Antony, V.B.; Gonzalez, M.L.; Crossman, D.K.; Carter, A.B. Impaired PPARγ activation by cadmium exacerbates infection-induced lung injury. J. Clin. Investig. 2023, 8, 166608. [Google Scholar] [CrossRef]
- Oh, C.-M.; Oh, I.-H.; Lee, J.-K.; Park, Y.H.; Choe, B.-K.; Yoon, T.-Y.; Choi, J.-M. Blood cadmium levels are associated with a decline in lung function in males. Environ. Res. 2014, 132, 119–125. [Google Scholar] [CrossRef]
- Hu, X.; Fernandes, J.; Jones, D.P.; Go, Y.-M. Cadmium stimulates myofibroblast differentiation and mouse lung fibrosis. Toxicology 2017, 383, 50–56. [Google Scholar] [CrossRef]
- Niewoehner, D.E.; Hoidal, J.R. Lung fibrosis and emphysema: Divergent responses to a common injury? Science 1982, 217, 359–360. [Google Scholar] [CrossRef]
- Zeng, X.; Huo, X.; Xu, X.; Liu, D.; Wu, W. E-waste lead exposure and children’s health in China. Sci. Total Environ. 2020, 734, 139286. [Google Scholar] [CrossRef] [PubMed]
- Svendsen, E.R.; Kolpakov, I.E.; Karmaus, W.J.J.; Mohr, L.C.; Vdovenko, V.Y.; McMahon, D.M.; Jelin, B.A.; Stepanova, Y.I. Reduced Lung Function in Children Associated with Cesium 137 Body Burden. Ann. Am. Thorac. Soc. 2015, 12, 1050–1057. [Google Scholar] [CrossRef]
- Sierra-Vargas, M.P.; Montero-Vargas, J.M.; Debray-García, Y.; Vizuet-De-Rueda, J.C.; Loaeza-Román, A.; Terán, L.M. Oxidative Stress and Air Pollution: Its Impact on Chronic Respiratory Diseases. Int. J. Mol. Sci. 2023, 24, 853. [Google Scholar] [CrossRef]
- Nie, L.; Chu, H.; Liu, C.; Cole, S.R.; Vexler, A.; Schisterman, E.F. Linear Regression with an Independent Variable Subject to a Detection Limit. Epidemiology 2010, 21, S17–S24. [Google Scholar] [CrossRef] [PubMed]

| All Participants | Children and Adolescents (6–18) | Adults (19–59) | Elderly (≥60) | |
|---|---|---|---|---|
| Gender n (%) | ||||
| Male | 2217 (49.1) | 245 (48.5) | 1454 (49.9) | 518 (45.9) |
| Female | 2165 (50.9) | 235 (51.5) | 1431 (50.1) | 499 (54.1) |
| Race n (%) | ||||
| Mexican American | 750 (8.4) | 132 (14.0) | 497 (9.0) | 121 (3.6) |
| Other Hispanic | 461 (5.5) | 47 (4.8) | 311 (6.1) | 103 (3.1) |
| Non-Hispanic White | 1888 (68.9) | 164 (61.4) | 1225 (66.9) | 499 (80.3) |
| Non-Hispanic Black | 909 (10.7) | 110 (14.0) | 566 (10.9) | 233 (8.4) |
| Other race—including multi-racial | 374 (6.5) | 27 (5.8) | 286 (7.1) | 61 (4.6) |
| Family income poverty ratio n (%) | ||||
| <1.3 | 1382 (20.3) | 187 (26.8) | 919 (20.8) | 276 (15.8) |
| 1.3–3.5 | 1652 (35.9) | 182 (36.6) | 1048 (34.7) | 422 (40.2) |
| >3.5 | 1348 (43.8) | 111 (36.6) | 918 (44.5) | 319 (44.0) |
| Recreational activities n (%) | ||||
| Yes | 2397 (61.0) | 406 (88.1) | 1567 (61.2) | 424 (48.6) |
| No | 1985 (39.0) | 74 (11.9) | 1318 (38.8) | 593 (51.4) |
| Body mass index (BMI) n (%) | ||||
| <18.5 kg/m2 | 137 (2.6) | 74 (15.9) | 55 (1.7) | 8 (0.6) |
| 18.5 < 25 kg/m2 | 1373 (33.0) | 256 (57.6) | 896 (33.1) | 221 (22.7) |
| 25 < 30 kg/m2 | 1411 (32.7) | 84 (14.8) | 939 (33.1) | 388 (38.5) |
| ≥30 kg/m2 | 1461 (31.7) | 66 (11.7) | 995 (32.1) | 400 (38.2) |
| Pulmonary function Mean (SD) | ||||
| FEV1 (mL) | 8.29 (0.27) | 8.26 (0.22) | 8.34 (0.25) | 8.09 (0.29) |
| FVC (mL) | 8.05 (0.30) | 8.11 (0.23) | 8.11 (0.26) | 7.77 (0.31) |
| FEF25–75% (mL/s) | 8.98 (0.29) | 8.90 (0.22) | 9.03 (0.26) | 8.81 (0.33) |
| PEF (mL/s) | 7.93 (0.52) | 8.19 (0.32) | 8.03 (0.43) | 7.41 (0.58) |
| FET (s) | 2.30 (0.37) | 1.92 (0.39) | 2.29 (0.33) | 2.50 (0.35) |
| NHANES cycles n (%) | ||||
| 2007–2008 | 1446 (32.9) | 206 (47.9) | 885 (31.7) | 355 (31.5) |
| 2009–2010 | 1751 (35.6) | 274 (52.1) | 1104 (34.3) | 373 (34.2) |
| 2011–2012 | 1185 (31.5) | 0 (0.0) | 896 (34.0) | 289 (34.3) |
| Metal Metabolites | Detection Rate N (%) | Mean | LOD | Percentiles | ||||
|---|---|---|---|---|---|---|---|---|
| P5 | P25 | P50 | P75 | P95 | ||||
| Urinary total arsenic | 4326 (98.72) | 16.90 | 0.26 | 2.38 | 4.28 | 6.97 | 14.84 | 53.30 |
| Urinary arsenobetaine | 2537 (57.89) | 8.72 | 1.19 | 0.16 | 0.48 | 1.49 | 5.49 | 35.87 |
| Urinary dimethylarsonic acid | 3577 (81.63) | 4.95 | 1.91 | 1.40 | 2.34 | 3.51 | 5.73 | 12.39 |
| Urinary barium | 4361 (99.52) | 2.29 | 0.06 | 0.36 | 0.85 | 1.49 | 2.62 | 6.14 |
| Urinary cadmium | 4020 (91.74) | 0.30 | 0.04 | 0.05 | 0.11 | 0.19 | 0.36 | 0.86 |
| Urinary cobalt | 4358 (99.45) | 0.48 | 0.02 | 0.14 | 0.23 | 0.34 | 0.51 | 1.13 |
| Urinary cesium | 4382 (100.0) | 4.96 | 0.09 | 1.98 | 3.14 | 4.27 | 5.98 | 9.90 |
| Urinary molybdenum | 4380 (99.95) | 50.04 | 0.08 | 14.94 | 27.75 | 40.56 | 60.42 | 114.25 |
| Urinary lead | 4242 (96.81) | 0.59 | 0.03 | 0.15 | 0.28 | 0.43 | 0.68 | 1.45 |
| Urinary antimony | 3095 (70.63) | 0.08 | 0.02 | 0.02 | 0.04 | 0.06 | 0.08 | 0.19 |
| Urinary thallium | 4356 (99.41) | 0.18 | 0.02 | 0.07 | 0.11 | 0.15 | 0.21 | 0.37 |
| Urinary tungsten | 3846 (87.77) | 0.12 | 0.02 | 0.02 | 0.05 | 0.08 | 0.14 | 0.37 |
| Urinary uranium | 3787 (86.42) | 0.01 | 0.01 | 0.00 | 0.00 | 0.01 | 0.01 | 0.03 |
| Urinary mercury | 4382 (100.0) | 0.69 | 0.13 | 0.10 | 0.21 | 0.43 | 0.82 | 2.12 |
| Serum cotinine | 4382 (100.0) | 51.74 | 0.01 | 0.01 | 0.02 | 0.05 | 4.07 | 328.70 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Z.; Gu, H.; Zhou, R.; Cheng, S. The Correlation between Metal Mixed Exposure and Lung Function in Different Ages of the Population. Metabolites 2024, 14, 139. https://doi.org/10.3390/metabo14030139
Chen Z, Gu H, Zhou R, Cheng S. The Correlation between Metal Mixed Exposure and Lung Function in Different Ages of the Population. Metabolites. 2024; 14(3):139. https://doi.org/10.3390/metabo14030139
Chicago/Turabian StyleChen, Zhongwen, Huiwen Gu, Ruiqi Zhou, and Shuqun Cheng. 2024. "The Correlation between Metal Mixed Exposure and Lung Function in Different Ages of the Population" Metabolites 14, no. 3: 139. https://doi.org/10.3390/metabo14030139
APA StyleChen, Z., Gu, H., Zhou, R., & Cheng, S. (2024). The Correlation between Metal Mixed Exposure and Lung Function in Different Ages of the Population. Metabolites, 14(3), 139. https://doi.org/10.3390/metabo14030139







