Determination of Bile Acids in Canine Biological Samples: Diagnostic Significance
Abstract
1. Introduction
2. Bile Acids in the Body: Physiological and Biochemical Aspects
2.1. Enterohepatic Circulation

2.2. Cytotoxicity and Properties
2.3. Roles
- On the one hand, these molecules exert influence within the enterohepatic circulation. While certain bile salts increase the effectiveness of antibiotics produced by bacterial species, others are capable of stabilizing the composition of the intestinal microbiome because they can be used as substrates for microbes [56].
- On the other hand, BAs were found to impact systems beyond the traditional scope of enterohepatic circulation. From some points of view, both the ab ovo synthesized and bacterially transformed BAs can be considered active metabolites with hormonal effects, the pathological patterns of which play a role in the manifestation of neuroinflammatory or neurodegenerative diseases mediated by the gut–brain axis [16,38,43,73,74,75]. These molecules can also lead to the improvement of barrier function and the mediation of anti-inflammatory mechanisms and can play a regulatory role at specific points of intermediate metabolism, e.g., in the metabolic regulatory system of carbohydrates or synthesis and oxidation of fatty acids [38,40,71,76,77,78].
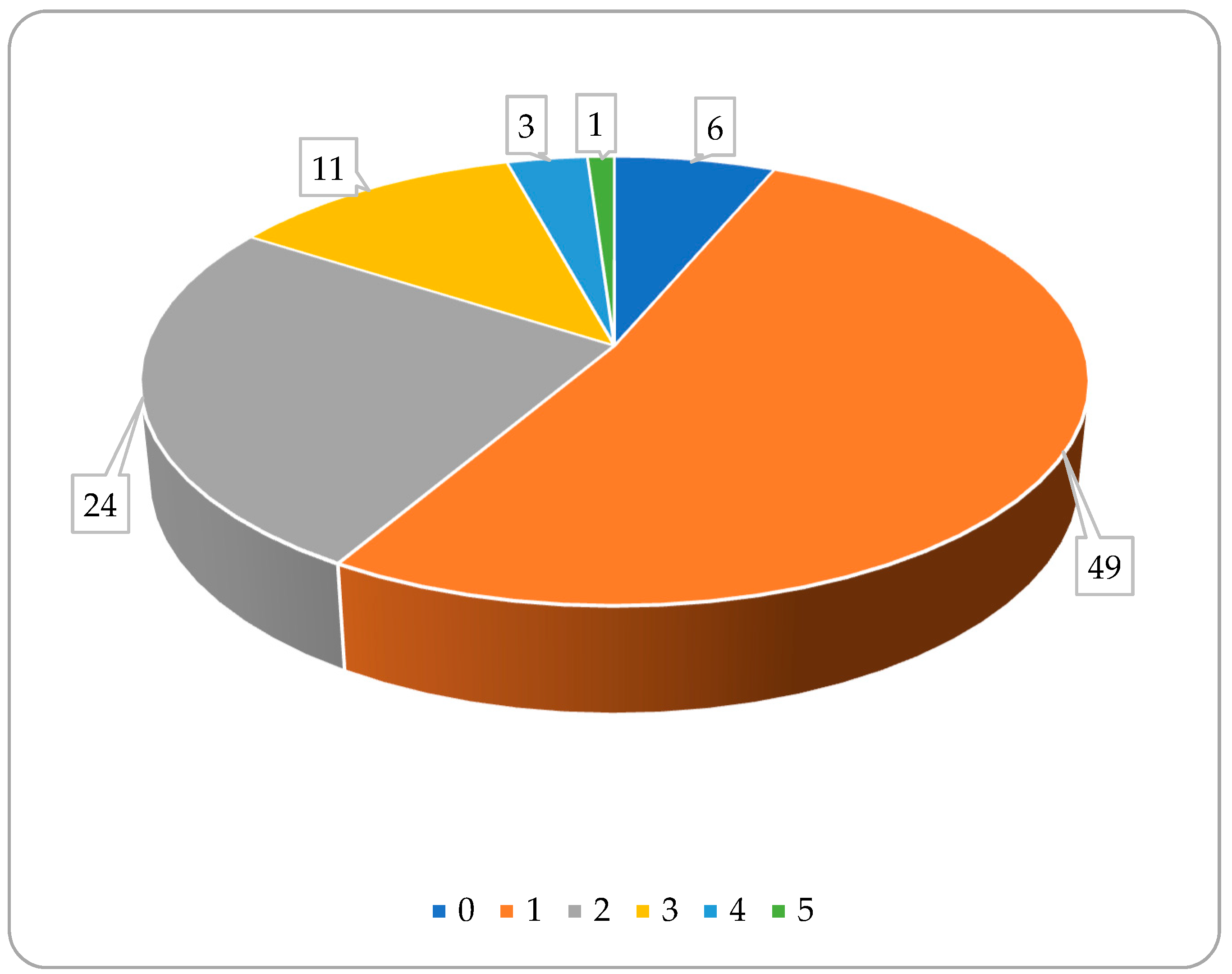
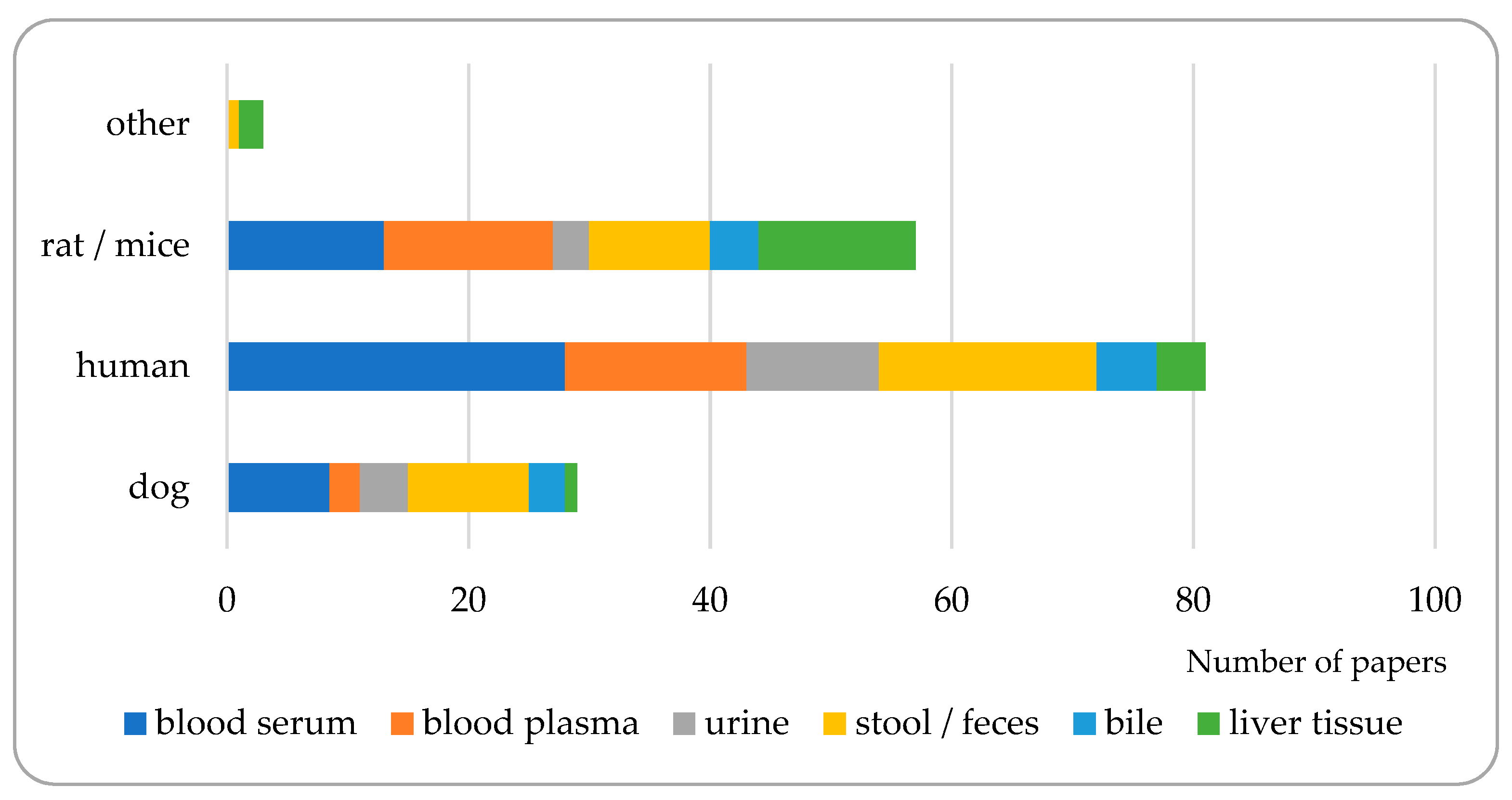
3. Matrices to Be Analyzed for Bile Acid Composition
| Matrix | Bibliographic Sources | |
|---|---|---|
| blood serum and plasma | [105,106,107,108] | |
| + urine, feces, and bile | [109] | |
| + urine | [86,110] | |
| + feces | [111] | |
| + liver tissue | [89] | |
| blood serum and urine | [112,113,114] | |
| + feces | [115] | |
| + feces and bile | [116] | |
| + feces and liver tissue | [90] | |
| blood serum and feces | [54,64,67,117,118] | |
| + bile | [75] | |
| + liver tissue | [28,91] | |
| blood serum | [119,120,121,122,123,124,125,126,127,128,129,130,131,132] | |
| + bile | [133,134,135] | |
| + liver tissue | [92,93] | |
| blood plasma | [3,102,136,137,138,139,140,141] | |
| + urine, feces and bile | [142] | |
| + urine | [143] | |
| + feces | [10] | |
| + feces and liver tissue | [63,94] | |
| + liver tissue | [95,96,97,98] | |
| blood, without detailing whether it was serum or plasma | [144] | |
| urine | [145,146,147,148] | |
| feces | [19,52,65,83,149,150,151,152,153,154,155,156,157,158,159,160,161,162] | |
| + bile | [103] | |
| bile | [163,164] | |
| liver tissue | [99,100,101] | |
| no specific matrix (or pure solutions) | [18,57,104,165,166,167] | |
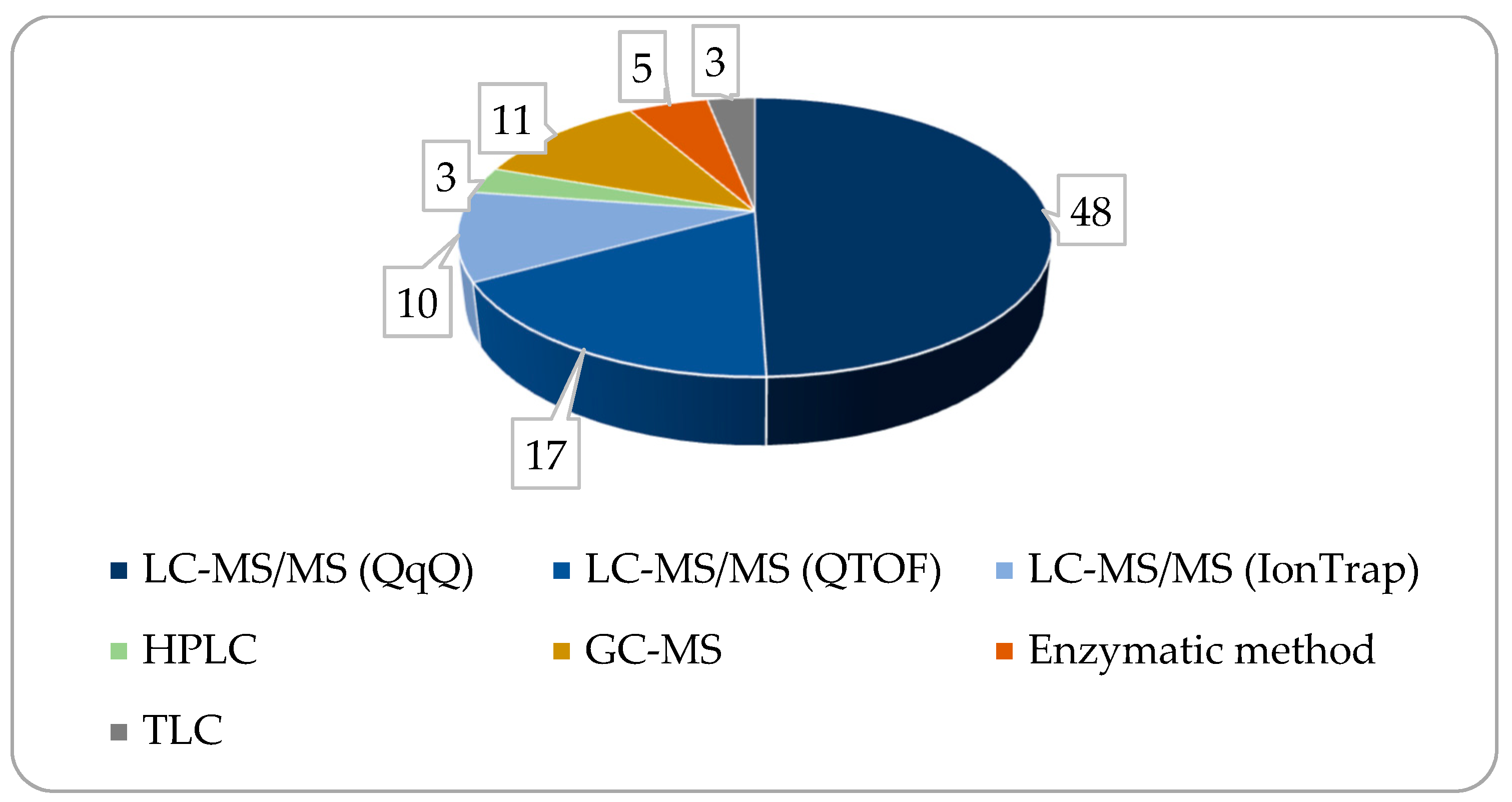
4. Measurement Methods and Instruments Used
5. Factors Influencing the Enterohepatic Circulation of BA
5.1. Effects of Feeding State on the Bile Acid Levels
5.2. Impact of Motility and Transit Times
5.3. Impact of Breeds and Body Sizes
5.4. Impact of Age and Aging
5.5. Impact of Nutrient Content and Microbial Aspects
6. Major Causes of Elevated TBA or Paired TBA
6.1. Intrahepatic Diseases
6.2. Portosystemic Vascular Anomalies (PSVAs)
6.3. Cholestasis
6.4. Other Causes of Elevated TBA Level
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Deschamps, C.; Humbert, D.; Zentek, J.; Denis, S.; Priymenko, N.; Apper, E.; Blanquet-Diot, S. From Chihuahua to Saint-Bernard: How did digestion and microbiota evolve with dog sizes. Int. J. Biol. Sci. 2022, 18, 5086–5102. [Google Scholar] [CrossRef]
- Niu, X.; Xu, Y.; Yang, Q.; Tang, X.; Li, Y.; Wang, Z. Analytical methods for characterization of bile acids and its application in quality control of cow-bezoar and bear bile powder. Am. J. Appl. Chem. 2014. [Google Scholar] [CrossRef]
- Sangaraju, D.; Katavolos, P.; Liang, X.; Chou, C.; Zabka, T.S.; Dean, B.; Maher, J. Establishment of baseline profiles of 50 bile acids in preclinical toxicity species: A comprehensive assessment of translational differences and study design considerations for biomarker development. Toxicol. Appl. Pharmacol. 2022, 443, 116008. [Google Scholar] [CrossRef]
- Molinero, N.; Ruiz, L.; Sánchez, B.; Margolles, A.; Delgado, S. Intestinal bacteria interplay with bile and cholesterol metabolism: Implications on host physiology. Front. Physiol. 2019, 10, 185. [Google Scholar] [CrossRef]
- Russell, D.W. Fifty years of advances in bile acid synthesis and metabolism. J. Lipid Res. 2009, 50, S120–S125. [Google Scholar] [CrossRef]
- Patel, S.K.; Billingsley, M.M.; Mukalel, A.J.; Thatte, A.S.; Hamilton, A.G.; Gong, N.; El-Mayta, R.; Safford, H.C.; Merolle, M.; Mitchell, M.J. Bile acid-containing lipid nanoparticles enhance extrahepatic mRNA delivery. Theranostics 2024, 14, 1–16. [Google Scholar] [CrossRef]
- Ridlon, J.M.; Daniel, S.L.; Gaskins, H.R. The Hylemon-Björkhem pathway of bile acid 7-dehydroxylation: History, biochemistry, and microbiology. J. Lipid Res. 2023, 64, 100392. [Google Scholar] [CrossRef]
- Ridlon, J.M.; Kang, D.J.; Hylemon, P.B. Bile salt biotransformations by human intestinal bacteria. J. Lipid Res. 2006, 47, 241–259. [Google Scholar] [CrossRef]
- Larabi, A.B.; Masson, H.L.P.; Bäumler, A.J. Bile acids as modulators of gut microbiota composition and function. Gut Microbes 2023, 15, 2172671. [Google Scholar] [CrossRef]
- Behr, C.; Slopianka, M.; Haake, V.; Strauss, V.; Sperber, S.; Kamp, H.; Walk, T.; Beekmann, K.; Rietjens, I.; van Ravenzwaay, B. Analysis of metabolome changes in the bile acid pool in feces and plasma of antibiotic-treated rats. Toxicol. Appl. Pharmacol. 2019, 363, 79–87. [Google Scholar] [CrossRef]
- Di Ciaula, A.; Garruti, G.; Baccetto, R.L.; Molina-Molina, E.; Bonfrate, L.; Wang, D.Q.-H.; Portincasa, P. Bile Acid Physiology. Ann. Hepatol. 2017, 16, S4–S14. [Google Scholar] [CrossRef]
- Kakimoto, T.; Kanemoto, H.; Fukushima, K.; Ohno, K.; Tsujimoto, H. Effect of a high-fat–high-cholesterol diet on gallbladder bile acid composition and gallbladder motility in dogs. Am. J. Vet. Res. 2017, 78, 1406–1413. [Google Scholar] [CrossRef]
- Bridger, N.; Glanemann, B.; Neiger, R. Comparison of postprandial and ceruletide serum bile acid stimulation in dogs. J. Vet. Intern. Med. 2008, 22, 873–878. [Google Scholar] [CrossRef]
- Center, S.A.; Thompson, M.; Guida, L. 3α-Hydroxylated bile acid profiles in clinically normal cats, cats with severe hepatic lipidosis, and cats with complete extrahepatic bile duct occlusion. Am. J. Vet. Res. 1993, 54, 681–688. [Google Scholar] [CrossRef]
- Bhowmik, S.; Chiu, H.; Jones, D.H.; Chiu, H.; Miller, M.D.; Xu, Q.; Farr, C.L.; Ridlon, J.M.; Wells, J.E.; Elsliger, M.; et al. Structure and functional characterization of a bile acid 7α dehydratase BaiE in secondary bile acid synthesis. Proteins Struct. Funct. Bioinform. 2016, 84, 316–331. [Google Scholar] [CrossRef]
- Jia, H.-M.; Yu, M.; Ma, L.-Y.; Zhang, H.-W.; Zou, Z.-M. Chaihu-Shu-Gan-San regulates phospholipids and bile acid metabolism against hepatic injury induced by chronic unpredictable stress in rat. J. Chromatogr. B 2017, 1064, 14–21. [Google Scholar] [CrossRef]
- Staley, C.; Weingarden, A.R.; Khoruts, A.; Sadowsky, M.J. Interaction of gut microbiota with bile acid metabolism and its influence on disease states. Appl. Microbiol. Biotechnol. 2017, 101, 47–64. [Google Scholar] [CrossRef]
- Doden, H.; Sallam, L.A.; Devendran, S.; Ly, L.; Doden, G.; Daniel, S.L.; Alves, J.M.P.; Ridlon, J.M. Metabolism of Oxo-Bile Acids and Characterization of Recombinant 12α-Hydroxysteroid Dehydrogenases from Bile Acid 7α-Dehydroxylating Human Gut Bacteria. Appl. Environ. Microbiol. 2018, 84, e00235-18. [Google Scholar] [CrossRef]
- Blake, A.B.; Cigarroa, A.; Klein, H.L.; Khattab, M.R.; Keating, T.; Van De Coevering, P.; Lidbury, J.A.; Steiner, J.M.; Suchodolski, J.S. Developmental stages in microbiota, bile acids, and clostridial species in healthy puppies. J. Vet. Intern. Med. 2020, 34, 2345–2356. [Google Scholar] [CrossRef]
- Zwicker, B.L.; Agellon, L.B. Transport and biological activities of bile acids. Int. J. Biochem. Cell Biol. 2013, 45, 1389–1398. [Google Scholar] [CrossRef]
- Dawson, P.A.; Karpen, S.J. Intestinal transport and metabolism of bile acids. J. Lipid Res. 2015, 56, 1085–1099. [Google Scholar] [CrossRef]
- Ridlon, J.M.; Kang, D.J.; Hylemon, P.B.; Bajaj, J.S. Bile acids and the gut microbiome. Curr. Opin. Gastroenterol. 2014, 30, 332–338. [Google Scholar] [CrossRef]
- Sayin, S.I.; Wahlström, A.; Felin, J.; Jäntti, S.; Marschall, H.-U.; Bamberg, K.; Angelin, B.; Hyötyläinen, T.; Orešič, M.; Bäckhed, F. Gut microbiota regulates bile acid metabolism by reducing the levels of tauro-beta-muricholic acid, a naturally occurring FXR antagonist. Cell Metab. 2013, 17, 225–235. [Google Scholar] [CrossRef]
- Wells, J.E.; Hylemon, P.B. Identification and characterization of a bile Acid 7α-dehydroxylation operon in Clostridium sp. strain TO-931, a highly active 7α-dehydroxylating strain isolated from human feces. Appl. Environ. Microbiol. 2000, 66, 1107–1113. [Google Scholar] [CrossRef]
- Campbell, C.; McKenney, P.T.; Konstantinovsky, D.; Isaeva, O.I.; Schizas, M.; Verter, J.; Mai, C.; Jin, W.-B.; Guo, C.-J.; Violante, S.; et al. Bacterial metabolism of bile acids promotes generation of peripheral regulatory T cells. Nature 2020, 581, 475–479. [Google Scholar] [CrossRef]
- Winston, J.A.; Theriot, C.M. Diversification of host bile acids by members of the gut microbiota. Gut Microbes 2020, 11, 158–171. [Google Scholar] [CrossRef]
- Poland, J.C.; Flynn, C.R. Bile acids, their receptors, and the gut microbiota. Physiology 2021, 36, 235–245. [Google Scholar] [CrossRef]
- Tang, Y.; Zhang, J.; Li, J.; Lei, X.; Xu, D.; Wang, Y.; Li, C.; Li, X.; Mao, Y. Turnover of bile acids in liver, serum and caecal content by high-fat diet feeding affects hepatic steatosis in rats. Biochim. et Biophys. Acta (BBA) Mol. Cell Biol. Lipids 2019, 1864, 1293–1304. [Google Scholar] [CrossRef]
- Ridlon, J.M.; Harris, S.C.; Bhowmik, S.; Kang, D.-J.; Hylemon, P.B. Consequences of bile salt biotransformations by intestinal bacteria. Gut Microbes 2016, 7, 22–39. [Google Scholar] [CrossRef]
- Zheng, L. New insights into the interplay between intestinal flora and bile acids in inflammatory bowel disease. World J. Clin. Cases 2022, 10, 10823–10839. [Google Scholar] [CrossRef]
- Kim, K.H.; Park, D.; Jia, B.; Baek, J.H.; Hahn, Y.; Jeon, C.O. Identification and Characterization of Major Bile Acid 7α-Dehydroxylating Bacteria in the Human Gut. mSystems 2022, 7, e0045522. [Google Scholar] [CrossRef]
- Griffiths, W.J.; Sjovall, J. Bile acids: Analysis in biological fluids and tissues. J. Lipid Res. 2010, 51, 23–41. [Google Scholar] [CrossRef]
- James, S.B.; Raphael, B.L.; Clippinger, T. Diagnosis and Treatment of Hepatic Lipidosis in a Barred Owl (Strix varia). J. Avian Med. Surg. 2000, 14, 268–272. [Google Scholar] [CrossRef]
- Harris, S.C.; Devendran, S.; García, C.M.; Mythen, S.M.; Wright, C.L.; Fields, C.J.; Hernandez, A.G.; Cann, I.; Hylemon, P.B.; Ridlon, J.M. Bile acid oxidation by Eggerthella lenta strains C592 and DSM 2243T. Gut Microbes 2018, 9, 523–539. [Google Scholar] [CrossRef]
- Anwer, M.S.; Stieger, B. Sodium-dependent bile salt transporters of the SLC10A transporter family: More than solute transporters. Pflügers Arch. Eur. J. Physiol. 2014, 466, 77–89. [Google Scholar] [CrossRef]
- Dawson, P.A. Role of the intestinal bile acid transporters in bile acid and drug disposition. Handb. Exp. Pharmacol. 2011, 201, 169–203. [Google Scholar] [CrossRef]
- Zhang, Y.; Jiang, R.; Zheng, X.; Lei, S.; Huang, F.; Xie, G.; Kwee, S.; Yu, H.; Farrar, C.; Sun, B.; et al. Ursodeoxycholic acid accelerates bile acid enterohepatic circulation. Br. J. Pharmacol. 2019, 176, 2848–2863. [Google Scholar] [CrossRef]
- Kliewer, S.A.; Mangelsdorf, D.J. Bile Acids as Hormones: The FXR-FGF15/19 Pathway. Dig. Dis. 2015, 33, 327–331. [Google Scholar] [CrossRef]
- Dawson, P.A.; Lan, T.; Rao, A. Bile acid transporters. J. Lipid Res. 2009, 50, 2340–2357. [Google Scholar] [CrossRef]
- Copple, B.L.; Li, T. Pharmacology of bile acid receptors: Evolution of bile acids from simple detergents to complex signaling molecules. Pharmacol. Res. 2016, 104, 9–21. [Google Scholar] [CrossRef]
- Fang, C.; Filipp, F.V.; Smith, J.W. Unusual binding of ursodeoxycholic acid to ileal bile acid binding protein: Role in activation of FXRα. J. Lipid Res. 2012, 53, 664–673. [Google Scholar] [CrossRef]
- Chiang, J.Y.L. Bile Acid Metabolism and Signaling. Compr. Physiol. 2013, 3, 1191–1212. [Google Scholar]
- Yeo, X.Y.; Tan, L.Y.; Chae, W.R.; Lee, D.-Y.; Lee, Y.-A.; Wuestefeld, T.; Jung, S. Liver’s influence on the brain through the action of bile acids. Front. Neurosci. 2023, 17, 1123967. [Google Scholar] [CrossRef]
- Ticho, A.L.; Malhotra, P.; Dudeja, P.K.; Gill, R.K.; Alrefai, W.A. Intestinal absorption of bile acids in health and disease. Compr. Physiol. 2019, 10, 21–56. [Google Scholar] [CrossRef]
- Ridlon, J.M.; Devendran, S.; Alves, J.M.; Doden, H.; Wolf, P.G.; Pereira, G.V.; Ly, L.; Volland, A.; Takei, H.; Nittono, H.; et al. The ‘in vivo lifestyle’ of bile acid 7α-dehydroxylating bacteria: Comparative genomics, metatranscriptomic, and bile acid metabolomics analysis of a defined microbial community in gnotobiotic mice. Gut Microbes 2020, 11, 381–404. [Google Scholar] [CrossRef]
- Center, S.A. Serum Bile Acids in Companion Animal—Medicine. Vet. Clin. N. Am. Small Anim. Pr. 1993, 23, 625–657. [Google Scholar] [CrossRef]
- Dawson, P.A.; Haywood, J.; Craddock, A.L.; Wilson, M.; Tietjen, M.; Kluckman, K.; Maeda, N.; Parks, J.S. Targeted deletion of the ileal bile acid transporter eliminates enterohepatic cycling of bile acids in mice. J. Biol. Chem. 2003, 278, 33920–33927. [Google Scholar] [CrossRef]
- Deschamps, C.; Denis, S.; Humbert, D.; Priymenko, N.; Chalancon, S.; De Bodt, J.; Van de Wiele, T.; Ipharraguerre, I.; Alvarez-Acero, I.; Achard, C.; et al. Canine Mucosal Artificial Colon: Development of a new colonic in vitro model adapted to dog sizes. Appl. Microbiol. Biotechnol. 2024, 108, 166. [Google Scholar] [CrossRef]
- Praslickova, D.; Torchia, E.C.; Sugiyama, M.G.; Magrane, E.J.; Zwicker, B.L.; Kolodzieyski, L.; Agellon, L.B. The ileal lipid binding protein is required for efficient absorption and transport of bile acids in the distal portion of the murine small intestine. PLoS ONE 2012, 7, e50810. [Google Scholar] [CrossRef]
- Kook, P.H.; Schellenberg, S.; Rentsch, K.M.; Reusch, C.E.; Glaus, T.M. Effect of twice-daily oral administration of hydrocortisone on the bile acids composition of gallbladder bile in dogs. Am. J. Vet. Res. 2011, 72, 1607–1612. [Google Scholar] [CrossRef]
- Jones, H.; Alpini, G.; Francis, H. Bile acid signaling and biliary functions. Acta Pharm. Sin. B 2015, 5, 123–128. [Google Scholar] [CrossRef]
- Herstad, K.M.V.; Rønning, H.T.; Bakke, A.M.; Moe, L.; Skancke, E. Changes in the faecal bile acid profile in dogs fed dry food vs high content of beef: A pilot study. Acta Vet. Scand. 2018, 60, 29. [Google Scholar] [CrossRef]
- Sitkin, S.; Pokrotnieks, J. Bad “good” bile acids and gut microbiota dysbiosis in inflammatory bowel disease: Mice and humans are not the same. Dig. Dis. Sci. 2021, 66, 925–927. [Google Scholar] [CrossRef]
- Ju, J.; Zhang, C.; Yang, J.; Yang, Q.; Yin, P.; Sun, X. Deoxycholic acid exacerbates intestinal inflammation by modulating interleukin-1β expression and tuft cell proportion in dextran sulfate sodium-induced murine colitis. PeerJ 2023, 11, e14842. [Google Scholar] [CrossRef]
- Lenci, I.; Milana, M.; Signorello, A.; Grassi, G.; Baiocchi, L. Secondary bile acids and the biliary epithelia: The good and the bad. World J. Gastroenterol. 2023, 29, 357–366. [Google Scholar] [CrossRef]
- Kang, J.D.; Myers, C.J.; Harris, S.C.; Kakiyama, G.; Lee, I.-K.; Yun, B.-S.; Matsuzaki, K.; Furukawa, M.; Min, H.-K.; Bajaj, J.S.; et al. Bile Acid 7α-Dehydroxylating Gut Bacteria Secrete Antibiotics that Inhibit Clostridium difficile: Role of Secondary Bile Acids. Cell Chem. Biol. 2019, 26, 27–34.e4. [Google Scholar] [CrossRef]
- Watanabe, M.; Fukiya, S.; Yokota, A. Comprehensive evaluation of the bactericidal activities of free bile acids in the large intestine of humans and rodents. J. Lipid Res. 2017, 58, 1143–1152. [Google Scholar] [CrossRef]
- Kurdi, P.; Kawanishi, K.; Mizutani, K.; Yokota, A. Mechanism of growth inhibition by free bile acids in lactobacilli and bifidobacteria. J. Bacteriol. 2006, 188, 1979–1986. [Google Scholar] [CrossRef]
- Schadt, H.S.; Wolf, A.; Pognan, F.; Chibout, S.-D.; Merz, M.; Kullak-Ublick, G.A. Bile acids in drug induced liver injury: Key players and surrogate markers. Clin. Res. Hepatol. Gastroenterol. 2016, 40, 257–266. [Google Scholar] [CrossRef]
- Yu, Q.; Jiang, Z.; Zhang, L. Bile acid regulation: A novel therapeutic strategy in non-alcoholic fatty liver disease. Pharmacol. Ther. 2018, 190, 81–90. [Google Scholar] [CrossRef]
- Jiang, L.; Schnabl, B. Gut microbiota in liver disease: What do we know and what do we not know? Physiology 2020, 35, 261–274. [Google Scholar] [CrossRef]
- Slattery, S.A.; Niaz, O.; Aziz, Q.; Ford, A.C.; Farmer, A.D. Systematic review with meta-analysis: The prevalence of bile acid malabsorption in the irritable bowel syndrome with diarrhoea. Aliment. Pharmacol. Ther. 2015, 42, 3–11. [Google Scholar] [CrossRef]
- Li, L.; Liu, T.; Gu, Y.; Wang, X.; Xie, R.; Sun, Y.; Wang, B.; Cao, H. Regulation of gut microbiota-bile acids axis by probiotics in inflammatory bowel disease. Front. Immunol. 2022, 13, 974305. [Google Scholar] [CrossRef]
- Wang, X.; Chen, L.; Wang, H.; Cai, W.; Xie, Q. Modulation of bile acid profile by gut microbiota in chronic hepatitis B. J. Cell Mol. Med. 2020, 24, 2573–2581. [Google Scholar] [CrossRef]
- Blake, A.B.; Guard, B.C.; Honneffer, J.B.; Lidbury, J.A.; Steiner, J.M.; Suchodolski, J.S. Altered microbiota, fecal lactate, and fecal bile acids in dogs with gastrointestinal disease. PLoS ONE 2019, 14, e0224454. [Google Scholar] [CrossRef]
- Luo, L.; Aubrecht, J.; Li, D.; Warner, R.L.; Johnson, K.J.; Kenny, J.; Colangelo, J.L. Assessment of serum bile acid profiles as biomarkers of liver injury and liver disease in humans. PLoS ONE 2018, 13, e0193824. [Google Scholar] [CrossRef]
- Kakiyama, G.; Pandak, W.M.; Gillevet, P.M.; Hylemon, P.B.; Heuman, D.M.; Daita, K.; Takei, H.; Muto, A.; Nittono, H.; Ridlon, J.M.; et al. Modulation of the fecal bile acid profile by gut microbiota in cirrhosis. J. Hepatol. 2013, 58, 949–955. [Google Scholar] [CrossRef]
- Swann, J.R.; Want, E.J.; Geier, F.M.; Spagou, K.; Wilson, I.D.; Sidaway, J.E.; Nicholson, J.K.; Holmes, E. Systemic gut microbial modulation of bile acid metabolism in host tissue compartments. Proc. Natl. Acad. Sci. USA 2011, 108 (Suppl. S1), 4523–4530. [Google Scholar] [CrossRef]
- van Best, N.; Rolle-Kampczyk, U.; Schaap, F.G.; Basic, M.; Damink, S.W.M.O.; Bleich, A.; Savelkoul, P.H.M.; von Bergen, M.; Penders, J.; Hornef, M.W. Bile acids drive the newborn’s gut microbiota maturation. Nat. Commun. 2020, 11, 3692. [Google Scholar] [CrossRef]
- Kelly, O.B.; Mroz, M.S.; Ward, J.B.J.; Colliva, C.; Scharl, M.; Pellicciari, R.; Gilmer, J.F.; Fallon, P.G.; Hofmann, A.F.; Roda, A.; et al. Ursodeoxycholic acid attenuates colonic epithelial secretory function. J. Physiol. 2013, 591, 2307–2318. [Google Scholar] [CrossRef]
- da Silva, T.C.; Polli, J.E.; Swaan, P.W. The solute carrier family 10 (SLC10): Beyond bile acid transport. Mol. Asp. Med. 2013, 34, 252–269. [Google Scholar] [CrossRef]
- Ward, J.B.J.; Lajczak, N.K.; Kelly, O.B.; O’dwyer, A.M.; Giddam, A.K.; Gabhann, J.N.; Franco, P.; Tambuwala, M.M.; Jefferies, C.A.; Keely, S.; et al. Ursodeoxycholic acid and lithocholic acid exert anti-inflammatory actions in the colon. Am. J. Physiol. Liver Physiol. 2017, 312, G550–G558. [Google Scholar] [CrossRef]
- MahmoudianDehkordi, S.; Arnold, M.; Nho, K.; Ahmad, S.; Jia, W.; Xie, G.; Louie, G.; Kueider-Paisley, A.; Moseley, M.A.; Thompson, J.W.; et al. Altered bile acid profile associates with cognitive impairment in Alzheimer’s disease—An emerging role for gut microbiome. Alzheimer’s Dement. 2019, 15, 76–92. [Google Scholar] [CrossRef]
- Chiang, J.Y.L. Bile acids: Regulation of synthesis. J. Lipid Res. 2009, 50, 1955–1966. [Google Scholar] [CrossRef]
- Winston, J.A.; Rivera, A.; Cai, J.; Patterson, A.D.; Theriot, C.M. Secondary bile acid ursodeoxycholic acid alters weight, the gut microbiota, and the bile acid pool in conventional mice. PLoS ONE 2021, 16, e0246161. [Google Scholar] [CrossRef]
- Yang, Z.; Danzeng, A.; Liu, Q.; Zeng, C.; Xu, L.; Mo, J.; Pingcuo, C.; Wang, X.; Wang, C.; Zhang, B.; et al. The Role of Nuclear Receptors in the Pathogenesis and Treatment of Non-alcoholic Fatty Liver Disease. Int. J. Biol. Sci. 2024, 20, 113–126. [Google Scholar] [CrossRef]
- Calzadilla, N.; Comiskey, S.M.; Dudeja, P.K.; Saksena, S.; Gill, R.K.; Alrefai, W.A. Bile acids as inflammatory mediators and modulators of intestinal permeability. Front. Immunol. 2022, 13, 1021924. [Google Scholar] [CrossRef]
- O’kell, A.L.; Garrett, T.J.; Wasserfall, C.; Atkinson, M.A. Untargeted metabolomic analysis in naturally occurring canine diabetes mellitus identifies similarities to human Type 1 Diabetes. Sci. Rep. 2017, 7, 9467. [Google Scholar] [CrossRef]
- Li, M.; Cai, S.-Y.; Boyer, J.L. Mechanisms of bile acid mediated inflammation in the liver. Mol. Asp. Med. 2017, 56, 45–53. [Google Scholar] [CrossRef]
- Mackei, M.; Talabér, R.; Müller, L.; Sterczer, Á.; Fébel, H.; Neogrády, Z.; Mátis, G. Altered intestinal production of volatile fatty acids in dogs triggered by lactulose and psyllium treatment. Vet. Sci. 2022, 9, 206. [Google Scholar] [CrossRef]
- Li, B.; Zhang, J.; Chen, Y.; Wang, Q.; Yan, L.; Wang, R.; Wei, Y.; You, Z.; Li, Y.; Miao, Q.; et al. Alterations in microbiota and their metabolites are associated with beneficial effects of bile acid sequestrant on icteric primary biliary Cholangitis. Gut Microbes 2021, 13, 1946366. [Google Scholar] [CrossRef]
- Alshawaqfeh, M.K.; Wajid, B.; Minamoto, Y.; Markel, M.; Lidbury, J.A.; Steiner, J.M.; Serpedin, E.; Suchodolski, J.S. A dysbiosis index to assess microbial changes in fecal samples of dogs with chronic inflammatory enteropathy. FEMS Microbiol. Ecol. 2017, 93, fix136. [Google Scholar] [CrossRef] [PubMed]
- Guard, B.C.; Honneffer, J.B.; Jergens, A.E.; Jonika, M.M.; Toresson, L.; Lawrence, Y.A.; Webb, C.B.; Hill, S.; Lidbury, J.A.; Steiner, J.M.; et al. Longitudinal assessment of microbial dysbiosis, fecal unconjugated bile acid concentrations, and disease activity in dogs with steroid-responsive chronic inflammatory enteropathy. J. Vet. Intern. Med. 2019, 33, 1295–1305. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Rong, Z.; Xiang, D.; Zhang, C.; Liu, D. Detection technologies and metabolic profiling of bile acids: A comprehensive review. Lipids Health Dis. 2018, 17, 121. [Google Scholar] [CrossRef] [PubMed]
- Vaz, F.M.; Ferdinandusse, S. Bile acid analysis in human disorders of bile acid biosynthesis. Mol. Asp. Med. 2017, 56, 10–24. [Google Scholar] [CrossRef] [PubMed]
- Sarafian, M.H.; Lewis, M.R.; Pechlivanis, A.; Ralphs, S.; McPhail, M.J.W.; Patel, V.C.; Dumas, M.-E.; Holmes, E.; Nicholson, J.K. Bile acid profiling and quantification in biofluids using ultra-performance liquid chromatography tandem mass spectrometry. Anal. Chem. 2015, 87, 9662–9670. [Google Scholar] [CrossRef] [PubMed]
- Dosedělová, V.; Itterheimová, P.; Kubáň, P. Analysis of bile acids in human biological samples by microcolumn separation techniques: A review. Electrophoresis 2021, 42, 68–85. [Google Scholar] [CrossRef] [PubMed]
- Łuczykowski, K.; Warmuzińska, N.; Bojko, B. Current approaches to the analysis of bile and the determination of bile acids in various biological matrices as supportive tools to traditional diagnostic testing for liver dysfunction and biliary diseases. TrAC Trends Anal. Chem. 2021, 142, 116307. [Google Scholar] [CrossRef]
- Gómez, C.; Stücheli, S.; Kratschmar, D.V.; Bouitbir, J.; Odermatt, A. Development and Validation of a Highly Sensitive LC-MS/MS Method for the Analysis of Bile Acids in Serum, Plasma, and Liver Tissue Samples. Metabolites 2020, 10, 282. [Google Scholar] [CrossRef]
- Qin, S.; Tian, J.; Wang, L.; Zhao, Y.; Wang, D.; Wang, F.; Meng, J.; Liu, M.; Liang, A. Ultra-performance chromatography-electrospray tandem mass spectrometry analysis of bile acid profiles in the enterohepatic circulation following geniposide and acetaminophen-induced liver injury. J. Chromatogr. A 2022, 1680, 463417. [Google Scholar] [CrossRef]
- Tian, M.; Yan, J.; Zhang, H.; Wei, Y.; Zhang, M.; Rao, Z.; Zhang, M.; Wang, H.; Wang, Y.; Li, X. Screening and validation of biomarkers for cadmium-induced liver injury based on targeted bile acid metabolomics. Environ. Pollut. 2022, 300, 118837. [Google Scholar] [CrossRef]
- García-Cañaveras, J.C.; Donato, M.T.; Castell, J.V.; Lahoz, A. Targeted profiling of circulating and hepatic bile acids in human, mouse, and rat using a UPLC-MRM-MS-validated method. J. Lipid Res. 2012, 53, 2231–2241. [Google Scholar] [CrossRef]
- Suzuki, Y.; Kaneko, R.; Nomura, M.; Naito, H.; Kitamori, K.; Nakajima, T.; Ogawa, T.; Hattori, H.; Seno, H.; Ishii, A. Simple and rapid quantitation of 21 bile acids in rat serum and liver by UPLC-MS-MS: Effect of high fat diet on glycine conjugates of rat bile acids. Nagoya J. Med. Sci. 2013, 75, 57–71. [Google Scholar] [PubMed]
- Hagio, M.; Matsumoto, M.; Fukushima, M.; Hara, H.; Ishizuka, S. Improved analysis of bile acids in tissues and intestinal contents of rats using LC/ESI-MS. J. Lipid Res. 2009, 50, 173–180. [Google Scholar] [CrossRef]
- La Frano, M.R.; Hernandez-Carretero, A.; Weber, N.; Borkowski, K.; Pedersen, T.L.; Osborn, O.; Newman, J.W. Diet-induced obesity and weight loss alter bile acid concentrations and bile acid–sensitive gene expression in insulin target tissues of C57BL/6J mice. Nutr. Res. 2017, 46, 11–21. [Google Scholar] [CrossRef]
- Liu, Z.; Zhang, Y.; Zhang, R.; Gu, L.; Chen, X. Promotion of classic neutral bile acids synthesis pathway is responsible for cholesterol-lowing effect of Si-miao-yong-an decoction: Application of LC–MS/MS method to determine 6 major bile acids in rat liver and plasma. J. Pharm. Biomed. Anal. 2017, 135, 167–175. [Google Scholar] [CrossRef] [PubMed]
- Slopianka, M.; Herrmann, A.; Pavkovic, M.; Ellinger-Ziegelbauer, H.; Ernst, R.; Mally, A.; Keck, M.; Riefke, B. Quantitative targeted bile acid profiling as new markers for DILI in a model of methapyrilene-induced liver injury in rats. Toxicology 2017, 386, 1–10. [Google Scholar] [CrossRef]
- Wu, W.; Liu, X.; Peng, X.; Xue, R.; Ji, L.; Shen, X.; Chen, S.; Gu, J.; Zhang, S. Bile acids override steatosis in farnesoid X receptor deficient mice in a model of non-alcoholic steatohepatitis. Biochem. Biophys. Res. Commun. 2014, 448, 50–55. [Google Scholar] [CrossRef] [PubMed]
- Thakare, R.; Alamoudi, J.A.; Gautam, N.; Rodrigues, A.D.; Alnouti, Y. Species differences in bile acids II. Bile acid metabolism. J. Appl. Toxicol. 2018, 38, 1336–1352. [Google Scholar] [CrossRef]
- Wang, W.; Shi, Z.; Zhang, R.; Yu, J.; Wang, C.; Hou, J.; Sun, J.; Liu, Y.; Qin, K.; Liu, Y.; et al. Liver proteomics analysis reveals abnormal metabolism of bile acid and arachidonic acid in Chinese hamsters with type 2 diabetes mellitus. J. Proteom. 2021, 239, 104186. [Google Scholar] [CrossRef]
- Wei, Y.; Cheng, J.; Luo, M.; Yang, S.; Xing, Q.; Cheng, J.; Lv, J.; Yu, C.; Sun, L.; Shi, D.; et al. Targeted metabolomics analysis of bile acids and cell biology studies reveal the critical role of glycodeoxycholic acid in buffalo follicular atresia. J. Steroid Biochem. Mol. Biol. 2022, 221, 106115. [Google Scholar] [CrossRef]
- Humbert, L.; Rainteau, D.; Tuvignon, N.; Wolf, C.; Seksik, P.; Laugier, R.; Carrière, F. Postprandial bile acid levels in intestine and plasma reveal altered biliary circulation in chronic pancreatitis patients. J. Lipid Res. 2018, 59, 2202–2213. [Google Scholar] [CrossRef] [PubMed]
- Naritaka, N.; Suzuki, M.; Sato, H.; Takei, H.; Murai, T.; Kurosawa, T.; Iida, T.; Nittono, H.; Shimizu, T. Profile of bile acids in fetal gallbladder and meconium using liquid chromatography-tandem mass spectrometry. Clin. Chim. Acta 2015, 446, 76–81. [Google Scholar] [CrossRef] [PubMed]
- Ďurč, P.; Dosedělová, V.; Foret, F.; Dolina, J.; Konečný, Š.; Himmelsbach, M.; Buchberger, W.; Kubáň, P. Analysis of major bile acids in saliva samples of patients with Barrett’s esophagus using high-performance liquid chromatography-electrospray ionization-mass spectrometry. J. Chromatogr. A 2020, 1625, 461278. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Liu, Y.; Wang, R.; Yang, J.; Ling, V.; Borchers, C.H. Metabolic profiling of bile acids in human and mouse blood by lc–ms/ms in combination with phospholipid-depletion solid-phase extraction. Anal. Chem. 2015, 87, 1127–1136. [Google Scholar] [CrossRef] [PubMed]
- Scherer, M.; Gnewuch, C.; Schmitz, G.; Liebisch, G. Rapid quantification of bile acids and their conjugates in serum by liquid chromatography–tandem mass spectrometry. J. Chromatogr. B 2009, 877, 3920–3925. [Google Scholar] [CrossRef]
- Ulaszewska, M.M.; Mancini, A.; Garcia-Aloy, M.; Del Bubba, M.; Tuohy, K.M.; Vrhovsek, U. Isotopic dilution method for bile acid profiling reveals new sulfate glycine-conjugated dihydroxy bile acids and glucuronide bile acids in serum. J. Pharm. Biomed. Anal. 2019, 173, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.J.; Shields, E.E.; Snow, K.J.; Nelson, D.M.; Olah, T.V.; Reily, M.D.; Robertson, D.G.; Shipkova, P.A.; Stryker, S.A.; Xin, B.; et al. The utility of stable isotope labeled (SIL) analogues in the bioanalysis of endogenous compounds by LC-MS applied to the study of bile acids in a metabolomics assay. Anal. Biochem. 2016, 503, 71–78. [Google Scholar] [CrossRef] [PubMed]
- Amplatz, B.; Zöhrer, E.; Haas, C.; Schäffer, M.; Stojakovic, T.; Jahnel, J.; Fauler, G. Bile acid preparation and comprehensive analysis by high performance liquid chromatography–high-resolution mass spectrometry. Clin. Chim. Acta 2017, 464, 85–92. [Google Scholar] [CrossRef]
- Peng, Z.; Zhang, Q.; Mao, Z.; Wang, J.; Liu, C.; Lin, X.; Li, X.; Ji, W.; Fan, J.; Wang, M.; et al. A rapid quantitative analysis of bile acids, lysophosphatidylcholines and polyunsaturated fatty acids in biofluids based on ultraperformance liquid chromatography coupled with triple quadrupole tandem massspectrometry. J. Chromatogr. B 2017, 1068–1069, 343–351. [Google Scholar] [CrossRef]
- Mouzaki, M.; Wang, A.Y.; Bandsma, R.; Comelli, E.M.; Arendt, B.M.; Zhang, L.; Fung, S.; Fischer, S.E.; McGilvray, I.G.; Allard, J.P. Bile Acids and Dysbiosis in Non-Alcoholic Fatty Liver Disease. PLoS ONE 2016, 11, e0151829. [Google Scholar] [CrossRef]
- Balkman, C.E.; Center, S.A.; Randolph, J.F.; Trainor, D.; Warner, K.L.; Crawford, M.A.; Adachi, K.; Erb, H.N. Evaluation of urine sulfated and nonsulfated bile acids as a diagnostic test for liver disease in dogs. J. Am. Vet. Med Assoc. 2003, 222, 1368–1375. [Google Scholar] [CrossRef]
- Ferslew, B.C.; Xie, G.; Johnston, C.K.; Su, M.; Stewart, P.W.; Jia, W.; Brouwer, K.L.; Barritt, A.S. Altered Bile Acid Metabolome in Patients with Nonalcoholic Steatohepatitis. Dig. Dis. Sci. 2015, 60, 3318–3328. [Google Scholar] [CrossRef]
- Zhu, P.; Zhang, J.; Chen, Y.; Yin, S.; Su, M.; Xie, G.; Brouwer, K.L.R.; Liu, C.; Lan, K.; Jia, W. Analysis of human C24 bile acids metabolome in serum and urine based on enzyme digestion of conjugated bile acids and LC-MS determination of unconjugated bile acids. Anal. Bioanal. Chem. 2018, 410, 5287–5300. [Google Scholar] [CrossRef] [PubMed]
- Gui, L.; Wu, Q.; Hu, Y.; Zeng, W.; Tan, X.; Zhu, P.; Li, X.; Yang, L.; Jia, W.; Liu, C.; et al. Compensatory Transition of Bile Acid Metabolism from Fecal Disposition of Secondary Bile Acids to Urinary Excretion of Primary Bile Acids Underlies Rifampicin-Induced Cholestasis in Beagle Dogs. ACS Pharmacol. Transl. Sci. 2021, 4, 1001–1013. [Google Scholar] [CrossRef] [PubMed]
- Humbert, L.; Maubert, M.A.; Wolf, C.; Duboc, H.; Mahé, M.; Farabos, D.; Seksik, P.; Mallet, J.M.; Trugnan, G.; Masliah, J.; et al. Bile acid profiling in human biological samples: Comparison of extraction procedures and application to normal and cholestatic patients. J. Chromatogr. B 2012, 899, 135–145. [Google Scholar] [CrossRef]
- Leibovitzh, H.; Nayeri, S.; Borowski, K.; Hernandez-Rocha, C.; Lee, S.H.; Turpin, W.; Stempak, J.; Sandhu, I.; Milgrom, R.; I Smith, M.; et al. P066 Lower serum concentration of sulfated bile acids is associated with Primary Sclerosing Cholangitis Inflammatory Bowel Disease comorbidity and advanced liver fibrosis. J. Crohn’s Colitis 2022, 16, i173–i174. [Google Scholar] [CrossRef]
- Vijayvargiya, P.; Busciglio, I.; Burton, D.; Donato, L.; Lueke, A.; Camilleri, M. Bile acid deficiency in a subgroup of patients with irritable bowel syndrome with constipation based on biomarkers in serum and fecal samples. Clin. Gastroenterol. Hepatol. 2018, 16, 522–527. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Xu, B.; Zhang, X.; He, Y.; Shao, Y.; Ding, M. Diagnostic and therapeutic profiles of serum bile acids in women with intrahepatic cholestasis of pregnancy-a pseudo-targeted metabolomics study. Clin. Chim. Acta 2018, 483, 135–141. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Xu, B.; Zhang, X.; Cui, Y.; Deng, L.; Shi, Z.; Shao, Y.; Ding, M. Association between serum bile acid profiles and gestational diabetes mellitus: A targeted metabolomics study. Clin. Chim. Acta 2016, 459, 63–72. [Google Scholar] [CrossRef] [PubMed]
- Lawrance, D.; Williamson, C.; Boutelle, M.G.; Cass, A.E.G. Development of a disposable bile acid biosensor for use in the management of cholestasis. Anal. Methods 2015, 7, 3714–3719. [Google Scholar] [CrossRef]
- Luo, C.; Zhang, X.; He, Y.; Chen, H.; Liu, M.; Wang, H.; Tang, L.; Tu, G.; Ding, M. A pseudo-targeted metabolomics study based on serum bile acids profiling for the differential diagnosis of benign and malignant breast lesions. Steroids 2021, 175, 108914. [Google Scholar] [CrossRef]
- Melgarejo, T.; Williams, D.A.; O’connell, N.C.; Setchell, K.D. Serum unconjugated bile acids as a test for intestinal bacterial overgrowth in dogs. Dig. Dis. Sci. 2000, 45, 407–414. [Google Scholar] [CrossRef] [PubMed]
- Qi, Y.; Jiang, C.; Cheng, J.; Krausz, K.W.; Li, T.; Ferrell, J.M.; Gonzalez, F.J.; Chiang, J.Y. Bile acid signaling in lipid metabolism: Metabolomic and lipidomic analysis of lipid and bile acid markers linked to anti-obesity and anti-diabetes in mice. Biochim. et Biophys. Acta (BBA) Mol. Cell Biol. Lipids 2015, 1851, 19–29. [Google Scholar] [CrossRef]
- Steiner, C.; von Eckardstein, A.; Rentsch, K.M. Quantification of the 15 major human bile acids and their precursor 7α-hydroxy-4-cholesten-3-one in serum by liquid chromatography–tandem mass spectrometry. J. Chromatogr. B 2010, 878, 2870–2880. [Google Scholar] [CrossRef]
- Sugita, T.; Amano, K.; Nakano, M.; Masubuchi, N.; Sugihara, M.; Matsuura, T. Analysis of the serum bile acid composition for differential diagnosis in patients with liver disease. Gastroenterol. Res. Pract. 2015, 2015, 717431. [Google Scholar] [CrossRef]
- Taguchi, K.; Fukusaki, E.; Bamba, T. Simultaneous and rapid analysis of bile acids including conjugates by supercritical fluid chromatography coupled to tandem mass spectrometry. J. Chromatogr. A 2013, 1299, 103–109. [Google Scholar] [CrossRef]
- Thompson, M.B.; Chappell, J.D.; Kunze, D.J.; Blair, P.C. Bile acid profile in a dog with cholangiocarcinoma. Vet. Pathol. 1989, 26, 75–78. [Google Scholar] [CrossRef] [PubMed]
- Washizu, T.; Tomoda, I.; Kaneko, J.J. Serum bile acid composition of the dog, cow, horse and human. J. Vet. Med Sci. 1991, 53, 81–86. [Google Scholar] [CrossRef]
- Weiler, K.; Kleber, K.; Zielinsky, S.; Moritz, A.; Bauer, N. Analytical performance and method comparison of a quantitative point-of-care immunoassay for measurement of bile acids in cats and dogs. J. Vet. Diagn. Investig. 2020, 33, 35–46. [Google Scholar] [CrossRef]
- Xie, G.; Wang, Y.; Wang, X.; Zhao, A.; Chen, T.; Ni, Y.; Wong, L.; Zhang, H.; Zhang, J.; Liu, C.; et al. Profiling of serum bile acids in a healthy chinese population using UPLC–MS/MS. J. Proteome Res. 2015, 14, 850–859. [Google Scholar] [CrossRef]
- Xiong, Y.; Shi, C.; Zhong, F.; Liu, X.; Yang, P. LC-MS/MS and SWATH based serum metabolomics enables biomarker discovery in pancreatic cancer. Clin. Chim. Acta 2020, 506, 214–221. [Google Scholar] [CrossRef]
- Gookin, J.L.; Mathews, K.G.; Cullen, J.; Seiler, G. Qualitative metabolomics profiling of serum and bile from dogs with gallbladder mucocele formation. PLoS ONE 2018, 13, e0191076. [Google Scholar] [CrossRef] [PubMed]
- Washizu, T.; Ishida, T.; Washizu, M.; Tomoda, I.; Kaneko, J.J. Changes in bile acid composition of serum and gallbladder bile in bile duct ligated dogs. J. Vet. Med Sci. 1994, 56, 299–303. [Google Scholar] [CrossRef] [PubMed]
- Woolbright, B.L.; Dorko, K.; Antoine, D.J.; Clarke, J.I.; Gholami, P.; Li, F.; Kumer, S.C.; Schmitt, T.M.; Forster, J.; Fan, F.; et al. Bile acid-induced necrosis in primary human hepatocytes and in patients with obstructive cholestasis. Toxicol. Appl. Pharmacol. 2015, 283, 168–177. [Google Scholar] [CrossRef] [PubMed]
- Ashritha, A.; Lal, B.B.; Sood, V.; Khanna, R.; Tripathi, G.; Maras, J.; Alam, S. Analysis of plasma bile acid composition in Indian children with progressive familial intrahepatic cholestasis. J. Clin. Exp. Hepatol. 2023, 13, S2–S3. [Google Scholar] [CrossRef]
- De Giorgi, S.; Campos, V.; Egli, L.; Toepel, U.; Carrel, G.; Cariou, B.; Rainteau, D.; Schneiter, P.; Tappy, L.; Giusti, V. Long-term effects of Roux-en-Y gastric bypass on postprandial plasma lipid and bile acids kinetics in female non diabetic subjects: A cross-sectional pilot study. Clin. Nutr. 2015, 34, 911–917. [Google Scholar] [CrossRef] [PubMed]
- Gu, P.; Liu, R.-J.; Cheng, M.-L.; Wu, Y.; Zheng, L.; Liu, Y.-J.; Ma, P.-C.; Ding, L. Simultaneous quantification of chlorogenic acid and taurocholic acid in human plasma by LC-MS/MS and its application to a pharmacokinetic study after oral administration of Shuanghua Baihe tablets. Chin. J. Nat. Med. 2016, 14, 313–320. [Google Scholar] [CrossRef] [PubMed]
- de Paiva, M.J.N.; Menezes, H.C.; da Silva, J.C.C.; Resende, R.R.; Cardeal, Z.d.L. New method for the determination of bile acids in human plasma by liquid-phase microextraction using liquid chromatography-ion-trap-time-of-flight mass spectrometry. J. Chromatogr. A 2015, 1388, 102–109. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Li, H.; Yun, Y.; Wang, H.; Meng, B.; Mu, Y.; Gao, S.; Tao, X.; Chen, W. A dynamic multiple reaction monitoring strategy to develop and optimize targeted metabolomics methods: Analyzing bile acids in capecitabine-induced diarrhea. J. Pharm. Biomed. Anal. 2022, 219, 114938. [Google Scholar] [CrossRef]
- Zhang, X.; Yang, Z.; Shi, Z.; Zhu, Z.; Li, C.; Du, Z.; Zhang, Y.; Wang, Z.; Jiao, Z.; Tian, X.; et al. Analysis of bile acid profile in plasma to differentiate cholangiocarcinoma from benign biliary diseases and healthy controls. J. Steroid Biochem. Mol. Biol. 2021, 205, 105775. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Li, W.; Gong, X.; Niu, X.; Zheng, J.; Yu, J.; Li, J.; Tu, P.; Song, Y. Widely quasi-quantitative analysis enables temporal bile acids-targeted metabolomics in rat after oral administration of ursodeoxycholic acid. Anal. Chim. Acta 2022, 1212, 339885. [Google Scholar] [CrossRef] [PubMed]
- Thakare, R.; Alamoudi, J.A.; Gautam, N.; Rodrigues, A.D.; Alnouti, Y. Species differences in bile acids I. Plasma and urine bile acid composition. J. Appl. Toxicol. 2018, 38, 1323–1335. [Google Scholar] [CrossRef] [PubMed]
- Nakagaki, M.; Danzinger, R.G.; Hofmann, A.F.; DiPietro, R.A. Biliary secretion and hepatic metabolism of taurine-conjugated 7 alpha-hydroxy and 7 beta-hydroxy bile acids in the dog. Defective hepatic transport and bile hyposecretion. Gastroenterology 1984, 87, 647–659. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Zhang, X.; Xu, B.; Cui, Y.; He, Y.; Yang, T.; Shao, Y.; Ding, M. The urinary bile acid profiling analysis of asymptomatic hypercholanemia of pregnancy: A pseudo-targeted metabolomics study. Clin. Chim. Acta 2019, 497, 67–75. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhang, X.; Chen, J.; Feng, C.; He, Y.; Shao, Y.; Ding, M. Targeted metabolomics of sulfated bile acids in urine for the diagnosis and grading of intrahepatic cholestasis of pregnancy. Genes Dis. 2018, 5, 358–366. [Google Scholar] [CrossRef] [PubMed]
- Lyu, J.; Li, H.; Yin, D.; Zhao, M.; Sun, Q.; Guo, M. Analysis of eight bile acids in urine of gastric cancer patients based on covalent organic framework enrichment coupled with liquid chromatography-tandem mass spectrometry. J. Chromatogr. A 2021, 1653, 462422. [Google Scholar] [CrossRef]
- Muto, A.; Takei, H.; Unno, A.; Murai, T.; Kurosawa, T.; Ogawa, S.; Iida, T.; Ikegawa, S.; Mori, J.; Ohtake, A.; et al. Detection of Δ4-3-oxo-steroid 5β-reductase deficiency by LC–ESI-MS/MS measurement of urinary bile acids. J. Chromatogr. B 2012, 900, 24–31. [Google Scholar] [CrossRef]
- Chaitman, J.; Ziese, A.-L.; Pilla, R.; Minamoto, Y.; Blake, A.B.; Guard, B.C.; Isaiah, A.; Lidbury, J.A.; Steiner, J.M.; Unterer, S.; et al. Fecal microbial and metabolic profiles in dogs with acute diarrhea receiving either fecal microbiota transplantation or oral metronidazole. Front. Vet. Sci. 2020, 7, 192. [Google Scholar] [CrossRef]
- Grundy, S.M.; Ahrens, E.; Miettinen, T.A. Quantitative isolation and gas–liquid chromatographic analysis of total fecal bile acids. J. Lipid Res. 1965, 6, 397–410. [Google Scholar] [CrossRef]
- He, Z.; Ma, Y.; Yang, S.; Zhang, S.; Liu, S.; Xiao, J.; Wang, Y.; Wang, W.; Yang, H.; Li, S.; et al. Gut microbiota-derived ursodeoxycholic acid from neonatal dairy calves improves intestinal homeostasis and colitis to attenuate extended-spectrum β-lactamase-producing enteroaggregative Escherichia coli infection. Microbiome 2022, 10, 79. [Google Scholar] [CrossRef]
- Horvath, T.D.; Haidacher, S.J.; Hoch, K.M.; Auchtung, J.M.; Haag, A.M. A high-throughput LC-MS/MS method for the measurement of the bile acid/salt content in microbiome-derived sample sets. MethodsX 2020, 7, 100951. [Google Scholar] [CrossRef]
- Hu, C.; Wang, W.; Garey, K.W. Heterogeneity and lyophilization comparison of stool processing for gastrointestinal bile acid measurement by LC-MS/MS. J. Chromatogr. B 2023, 1214, 123569. [Google Scholar] [CrossRef]
- Jergens, A.E.; Guard, B.C.; Redfern, A.; Rossi, G.; Mochel, J.P.; Pilla, R.; Chandra, L.; Seo, Y.-J.; Steiner, J.M.; Lidbury, J.; et al. Microbiota-Related Changes in Unconjugated Fecal Bile Acids Are Associated with Naturally Occurring, Insulin-Dependent Diabetes Mellitus in Dogs. Front. Vet. Sci. 2019, 6, 199. [Google Scholar] [CrossRef]
- Kakiyama, G.; Muto, A.; Takei, H.; Nittono, H.; Murai, T.; Kurosawa, T.; Hofmann, A.F.; Pandak, W.M.; Bajaj, J.S. A simple and accurate HPLC method for fecal bile acid profile in healthy and cirrhotic subjects: Validation by GC-MS and LC-MS. J. Lipid Res. 2014, 55, 978–990. [Google Scholar] [CrossRef]
- Manchester, A.C.; Webb, C.B.; Blake, A.B.; Sarwar, F.; Lidbury, J.A.; Steiner, J.M.; Suchodolski, J.S. Long-term impact of tylosin on fecal microbiota and fecal bile acids of healthy dogs. J. Vet. Intern. Med. 2019, 33, 2605–2617. [Google Scholar] [CrossRef]
- Marclay, M.; Dwyer, E.; Suchodolski, J.S.; Lidbury, J.A.; Steiner, J.M.; Gaschen, F.P. Recovery of Fecal Microbiome and Bile Acids in Healthy Dogs after Tylosin Administration with and without Fecal Microbiota Transplantation. Vet. Sci. 2022, 9, 324. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, M.; Unterer, S.; Suchodolski, J.S.; Honneffer, J.B.; Guard, B.C.; Lidbury, J.A.; Steiner, J.M.; Fritz, J.; Kölle, P. The fecal microbiome and metabolome differs between dogs fed Bones and Raw Food (BARF) diets and dogs fed commercial diets. PLoS ONE 2018, 13, e0201279. [Google Scholar] [CrossRef] [PubMed]
- Shin, A.; Camilleri, M.; Vijayvargiya, P.; Busciglio, I.; Burton, D.; Ryks, M.; Rhoten, D.; Lueke, A.; Saenger, A.; Girtman, A.; et al. Bowel functions, fecal unconjugated primary and secondary bile acids, and colonic transit in patients with irritable bowel syndrome. Clin. Gastroenterol. Hepatol. 2013, 11, 1270–1275.e1. [Google Scholar] [CrossRef] [PubMed]
- Cardinelli, C.d.S.; Torrinhas, R.S.; Sala, P.; Pudenzi, M.A.; Angolini, C.F.F.; da Silva, M.M.; Machado, N.M.; Ravacci, G.; Eberlin, M.N.; Waitzberg, D.L. Fecal bile acid profile after Roux-en-Y gastric bypass and its association with the remission of type 2 diabetes in obese women: A preliminary study. Clin. Nutr. 2019, 38, 2906–2912. [Google Scholar] [CrossRef]
- Vijayvargiya, P.; Camilleri, M.; Chedid, V.; Carlson, P.; Busciglio, I.; Burton, D.; Donato, L.J. Analysis of fecal primary bile acids detects increased stool weight and colonic transit in patients with chronic functional diarrhea. Clin. Gastroenterol. Hepatol. 2019, 17, 922–929.e2. [Google Scholar] [CrossRef]
- Weingarden, A.R.; Chen, C.; Zhang, N.; Graiziger, C.T.B.; Dosa, P.I.; Steer, C.J.; Shaughnessy, M.K.; Johnson, J.R.; Sadowsky, M.J.; Khoruts, A. Ursodeoxycholic Acid Inhibits Clostridium difficile Spore Germination and Vegetative Growth, and Prevents the Recurrence of Ileal Pouchitis Associated With the Infection. J. Clin. Gastroenterol. 2016, 50, 624–630. [Google Scholar] [CrossRef]
- Lee, G.; Lee, H.; Hong, J.; Lee, S.H.; Jung, B.H. Quantitative profiling of bile acids in rat bile using ultrahigh-performance liquid chromatography–orbitrap mass spectrometry: Alteration of the bile acid composition with aging. J. Chromatogr. B 2016, 1031, 37–49. [Google Scholar] [CrossRef]
- Rees, D.O.; Crick, P.J.; Jenkins, G.J.; Wang, Y.; Griffiths, W.J.; Brown, T.H.; Al-Sarireh, B. Comparison of the composition of bile acids in bile of patients with adenocarcinoma of the pancreas and benign disease. J. Steroid Biochem. Mol. Biol. 2017, 174, 290–295. [Google Scholar] [CrossRef]
- Mooranian, A.; Ionescu, C.M.; Walker, D.; Jones, M.; Wagle, S.R.; Kovacevic, B.; Chester, J.; Foster, T.; Johnston, E.; Kuthubutheen, J.; et al. Single-Cellular Biological Effects of Cholesterol-Catabolic Bile Acid-Based Nano/Micro Capsules as Anti-Inflammatory Cell Protective Systems. Biomolecules 2022, 12, 73. [Google Scholar] [CrossRef] [PubMed]
- Qiao, X.; Ye, M.; Liu, C.-F.; Yang, W.-Z.; Miao, W.-J.; Dong, J.; Guo, D.-A. A tandem mass spectrometric study of bile acids: Interpretation of fragmentation pathways and differentiation of steroid isomers. Steroids 2012, 77, 204–211. [Google Scholar] [CrossRef] [PubMed]
- Trifunović, J.; Borčić, V.; Vukmirović, S.; Kon, S.G.; Mikov, M. Retention data of bile acids and their oxo derivatives in characterization of pharmacokinetic properties and in silico ADME modeling. Eur. J. Pharm. Sci. 2016, 92, 194–202. [Google Scholar] [CrossRef] [PubMed]
- Dutta, M.; Cai, J.; Gui, W.; Patterson, A.D. A review of analytical platforms for accurate bile acid measurement. Anal. Bioanal. Chem. 2019, 411, 4541–4549. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Liu, Z.; Sun, F.; Yao, L.; Yang, G.; Wang, K. Bile Acid Detection Techniques and Bile Acid-Related Diseases. Front. Physiol. 2022, 13, 826740. [Google Scholar] [CrossRef] [PubMed]
- Schermerhorn, T.; Center, S.A.; Dykes, N.L.; Rowland, P.H.; Yeager, A.; Erb, H.; Oberhansley, K.; Bonda, M. Characterization of hepatoportal microvascular dysplasia in a kindred of cairn terriers. J. Vet. Intern. Med. 1996, 10, 219–230. [Google Scholar] [CrossRef] [PubMed]
- Pena-Ramos, J.; Barker, L.; Saiz, R.; Walker, D.J.; Tappin, S.; Hare, C.H.Z.; Roberts, M.L.; Williams, T.L.; Bexfield, N. Resting and postprandial serum bile acid concentrations in dogs with liver disease. J. Vet. Intern. Med. 2021, 35, 1333–1341. [Google Scholar] [CrossRef]
- McLaughlin, J. Fatty Acid-Induced Cholecystokinin Secretion and Effects of Human Gastric Motility. Ph.D. Thesis, Hope Hospital, Department of Medicine, Manchester, UK, 1998. [Google Scholar]
- Pasman, W.J.; Heimerikx, J.; Rubingh, C.M.; Berg, R.v.D.; O’Shea, M.; Gambelli, L.; Hendriks, H.F.; Einerhand, A.W.; Scott, C.; Keizer, H.G.; et al. The effect of Korean pine nut oil on in vitro CCK release, on appetite sensations and on gut hormones in post-menopausal overweight women. Lipids Health Dis. 2008, 7, 10. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.J.; Cui, Z.J. How does cholecystokinin stimulate exocrine pancreatic secretion? From birds, rodents, to humans. Am. J. Physiol. Integr. Comp. Physiol. 2007, 292, R666–R678. [Google Scholar] [CrossRef] [PubMed]
- van Straten, G.; Spee, B.; Rothuizen, J.; Favier, R. Diagnostic value of the rectal ammonia tolerance test, fasting plasma ammonia and fasting plasma bile acids for canine portosystemic shunting. Vet. J. 2015, 204, 282–286. [Google Scholar] [CrossRef] [PubMed]
- Konstantinidis, A.O.; Patsikas, M.N.; Papazoglou, L.G.; Adamama-Moraitou, K.K. Congenital portosystemic shunts in dogs and cats: Classification, pathophysiology, clinical presentation and diagnosis. Vet. Sci. 2023, 10, 160. [Google Scholar] [CrossRef] [PubMed]
- Gerritzen-Bruning, M.J.; van den Ingh, T.S.G.A.M.; Rothuizen, J. Diagnostic value of fasting plasma ammonia and bile acid concentrations in the identification of portosystemic shunting in dogs. J. Vet. Intern. Med. 2006, 20, 13–19. [Google Scholar] [CrossRef] [PubMed]
- Weber, M.; Canin, A.R.; de France, P.A.V. Influence of size on the dog’s digestive function. Bull. L’académie Vétérinaire Fr. 2006, 159, 327–332. [Google Scholar] [CrossRef]
- Weber, M.P.; Biourge, V.C.; Nguyen, P.G. Digestive sensitivity varies according to size of dogs: A review. J. Anim. Physiol. Anim. Nutr. 2017, 101, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Koziolek, M.; Grimm, M.; Bollmann, T.; Schäfer, K.J.; Blattner, S.M.; Lotz, R.; Boeck, G.; Weitschies, W. Characterization of the GI transit conditions in Beagle dogs with a telemetric motility capsule. Eur. J. Pharm. Biopharm. 2019, 136, 221–230. [Google Scholar] [CrossRef]
- Tolbert, M.K.; Telles, N.J.; Simon, B.T.; Scallan, E.M.; Price, J.M.; Gould, E.N.; Papich, M.G.; Lidbury, J.A.; Steiner, J.M.; Kathrani, A. Gastrointestinal transit time is faster in Beagle dogs compared to cats. J. Am. Vet. Med Assoc. 2022, 260, S8–S14. [Google Scholar] [CrossRef]
- Sato, M.; Shibata, C.; Kikuchi, D.; Ikezawa, F.; Imoto, H.; Sasaki, I. Effects of biliary and pancreatic juice diversion into the ileum on gastrointestinal motility and gut hormone secretion in conscious dogs. Surgery 2010, 148, 1012–1019. [Google Scholar] [CrossRef] [PubMed]
- Tanprasertsuk, J.; Shmalberg, J.; Maughan, H.; Tate, D.E.; Perry, L.M.; Jha, A.R.; Honaker, R.W. Heterogeneity of gut microbial responses in healthy household dogs transitioning from an extruded to a mildly cooked diet. PeerJ 2021, 9, e11648. [Google Scholar] [CrossRef] [PubMed]
- Belchik, S.E.; Oba, P.M.; Wyss, R.; Asare, P.T.; Vidal, S.; Miao, Y.; Adesokan, Y.; Suchodolski, J.S.; Swanson, K.S. Effects of a milk oligosaccharide biosimilar on fecal characteristics, microbiota, and bile acid, calprotectin, and immunoglobulin concentrations of healthy adult dogs treated with metronidazole. J. Anim. Sci. 2023, 101, skad011. [Google Scholar] [CrossRef] [PubMed]
- Merlen, G.; Ursic-Bedoya, J.; Jourdainne, V.; Kahale, N.; Glenisson, M.; Doignon, I.; Rainteau, D.; Tordjmann, T. Bile acids and their receptors during liver regeneration: “Dangerous protectors”. Mol. Asp. Med. 2017, 56, 25–33. [Google Scholar] [CrossRef] [PubMed]
- Chow, M.D.; Lee, Y.-H.; Guo, G.L. The role of bile acids in nonalcoholic fatty liver disease and nonalcoholic steatohepatitis. Mol. Asp. Med. 2017, 56, 34–44. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Jang, K.; Jo, H.M.; Kim, S.E. Laparoscopic attenuation of a congenital extrahepatic portosystemic shunt in a dog—A thin-film banding for splenophrenic shunt: A case report. Front. Vet. Sci. 2022, 9, 918153. [Google Scholar] [CrossRef]
- Anglin, E.V.; Lux, C.N.; Sun, X.; Folk, C.A.; Fazio, C. Clinical characteristics of, prognostic factors for, and long-term outcome of dogs with multiple acquired portosystemic shunts: 72 cases (2000–2018). J. Am. Vet. Med Assoc. 2021, 260, S30–S39. [Google Scholar] [CrossRef]
- Ishigaki, K.; Asano, K.; Tamura, K.; Sakurai, N.; Terai, K.; Heishima, T.; Yoshida, O. Percutaneous transvenous coil embolization (PTCE) for treatment of single extrahepatic portosystemic shunt in dogs. BMC Vet. Res. 2023, 19, 215. [Google Scholar] [CrossRef]
- Kim, D.; Oh, H.; Ahn, H.; An, B.; Park, D.; NA, K.-J.; Kim, G. Comparison of serum bile acid concentrations between maltese and other breeds of dogs with portosystemic shunt. Vivo 2023, 37, 709–713. [Google Scholar] [CrossRef] [PubMed]
- Baptista, L.; Pollard, D.; Di Bella, A. Evaluation of Resting Serum Bile Acid Concentrations in Dogs with Sepsis. Vet. Sci. 2022, 9, 627. [Google Scholar] [CrossRef] [PubMed]
- Ullal, T.V.; Lakin, S.; Gallagher, B.; Sbardellati, N.; Abdo, Z.; Twedt, D.C. Demographic and histopathologic features of dogs with abnormally high concentrations of hepatic copper. J. Vet. Intern. Med. 2022, 36, 2016–2027. [Google Scholar] [CrossRef] [PubMed]
- Alvarenga, I.C.; Aldrich, C.G.; Jewell, D.E. Influence of Liver Condition and Copper on Selective Parameters of Post-Mortem Dog Tissue Samples. Animals 2018, 8, 237. [Google Scholar] [CrossRef] [PubMed]
- Spee, B.; Arends, B.; van den Ingh, T.S.G.A.M.; Penning, L.C.; Rothuizen, J. Copper metabolism and oxidative stress in chronic inflammatory and cholestatic liver diseases in dogs. J. Vet. Intern. Med. 2006, 20, 1085–1092. [Google Scholar] [CrossRef] [PubMed]
- Tai, N.; Wong, F.S.; Wen, L. The role of gut microbiota in the development of type 1, type 2 diabetes mellitus and obesity. Rev. Endocr. Metab. Disord. 2015, 16, 55–65. [Google Scholar] [CrossRef] [PubMed]
- Wewalka, M.; Patti, M.-E.; Barbato, C.; Houten, S.M.; Goldfine, A.B. Fasting serum taurine-conjugated bile acids are elevated in type 2 diabetes and do not change with intensification of insulin. J. Clin. Endocrinol. Metab. 2014, 99, 1442–1451. [Google Scholar] [CrossRef]
- German, A.; Day, M.; Ruaux, C.; Steiner, J.; Williams, D.; Hall, E. Comparison of Direct and Indirect Tests for Small Intestinal Bacterial Overgrowth and Antibiotic-Responsive Diarrhea in Dogs. J. Vet. Intern. Med. 2003, 17, 33–43. [Google Scholar] [CrossRef]
- Comito, R.; Porru, E.; Interino, N.; Conti, M.; Terragni, R.; Gotti, R.; Candela, M.; Simoni, P.; Roda, A.; Fiori, J. Metabolic bile acid profile impairments in dogs affected by chronic inflammatory enteropathy. Metabolites 2023, 13, 980. [Google Scholar] [CrossRef] [PubMed]

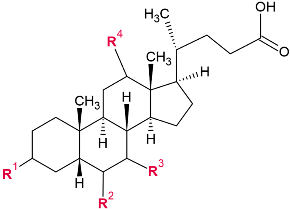 | |||
|---|---|---|---|
| Common Name | Chemical Name | Abbreviation | |
| cholic acid | 5b-cholanic acid-3a,7a,12a-triol | CA | PRIMARY BILE ACIDS |
| taurocholic acid | 5b-cholanic acid-3a,7a,12a-triol-N-(2-sulpho-ethyl)-amide | TCA | |
| glycocholic acid | 5b-cholanic acid-3a,7a,12a-triol-N-(carboxymethyl)-amide | GCA | |
| chenodeoxycholic acid | 5b-cholanicacid-3a,7a-diol | CDCA | |
| taurochenodeoxycholic acid | 5b-cholanic acid-3a,7a-diol-N-(2-sulpho-ethyl)-amide | TCDCA | |
| glycochenodeoxycholic acid | 5b-cholanic acid-3a,7a-diol-N-(carboxymethyl)-amide | GCDCA | |
| deoxycholic acid | 5b-cholanic acid-3a,12a-diol | DCA | SECONDARY BILE ACIDS |
| taurodeoxycholic acid | 5b-cholanic acid-3a,12a-diol-N-(2-sulphoethyl)-amide | TDCA | |
| glycodeoxycholic acid | 5b-cholanic acid-3a,12a-diol-N-(carboxymethyl)-amide | GDCA | |
| lithocholic acid | 5b-cholanic acid-3a-ol | LCA | |
| taurolithocholic acid | 5b-cholanicacid-3a-ol-N-(2-sulphoethyl)-amide | TLCA | |
| glycolithocholic acid | 5b-cholanic acid-3a-ol-N-(carboxymethyl)-amide | GLCA | |
| ursodeoxycholic acid | 5b-cholanic acid-3a,7b-diol | UDCA | |
| tauroursodeoxycholic acid | 5b-cholanic acid-3a,7b-diol-N-(2-sulphoethyl)-amide | TUDCA | |
| glycoursodeoxycholic acid | 5b-cholanic acid-3a,7b-diol-N-(carboxymethyl)-amide | GUDCA | |
| Bibliographic Source | Illness(es) |
|---|---|
| [115] | rifampicin-induced cholestasis (DILI) |
| [112] | healthy, hepatic disorders, clinical signs of hepatic disorder |
| [133] | gallbladder mucocele formation |
| [134] | biliary obstruction produced by surgical ligation of the common bile duct |
| [123] | culture-proven SIBO, indirectly diagnosed SIBO |
| [128] | metastatic cholangiocarcinoma |
| [129] | none (healthy dogs) |
| [130] | none (healthy dogs) |
| [144] | none (healthy dogs) |
| [143] | none (healthy dogs) |
| [3] | none (healthy dogs) |
| [65] | gastrointestinal diseases: chronic enteropathy (CE) and exocrine pancreatic insufficiency (EPI) |
| [19] | none (healthy dogs) |
| [149] | acute diarrhea (AD) |
| [83] | steroid-responsive chronic inflammatory enteropathy (CE) |
| [52] | none (healthy dogs) |
| [154] | insulin-dependent diabetes mellitus |
| [156] | none (healthy dogs) |
| [157] | none (healthy dogs) |
| [158] | none (healthy dogs) |
| [99] | none (healthy dogs) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Németh, K.; Sterczer, Á.; Kiss, D.S.; Lányi, R.K.; Hemző, V.; Vámos, K.; Bartha, T.; Buzás, A.; Lányi, K. Determination of Bile Acids in Canine Biological Samples: Diagnostic Significance. Metabolites 2024, 14, 178. https://doi.org/10.3390/metabo14040178
Németh K, Sterczer Á, Kiss DS, Lányi RK, Hemző V, Vámos K, Bartha T, Buzás A, Lányi K. Determination of Bile Acids in Canine Biological Samples: Diagnostic Significance. Metabolites. 2024; 14(4):178. https://doi.org/10.3390/metabo14040178
Chicago/Turabian StyleNémeth, Krisztián, Ágnes Sterczer, Dávid Sándor Kiss, Réka Katalin Lányi, Vivien Hemző, Kriszta Vámos, Tibor Bartha, Anna Buzás, and Katalin Lányi. 2024. "Determination of Bile Acids in Canine Biological Samples: Diagnostic Significance" Metabolites 14, no. 4: 178. https://doi.org/10.3390/metabo14040178
APA StyleNémeth, K., Sterczer, Á., Kiss, D. S., Lányi, R. K., Hemző, V., Vámos, K., Bartha, T., Buzás, A., & Lányi, K. (2024). Determination of Bile Acids in Canine Biological Samples: Diagnostic Significance. Metabolites, 14(4), 178. https://doi.org/10.3390/metabo14040178








