Metabolomic Profiles and Pathways in Osteoarthritic Human Cartilage: A Comparative Analysis with Healthy Cartilage
Abstract
1. Introduction
2. Materials and Methods
2.1. Articular Cartilage Sample Obtainment
2.2. Metabolite Extraction and Mass Spectrometry Analysis
2.3. Statistical and Metabolomic Profiling
2.4. Metabolite Identification
3. Results
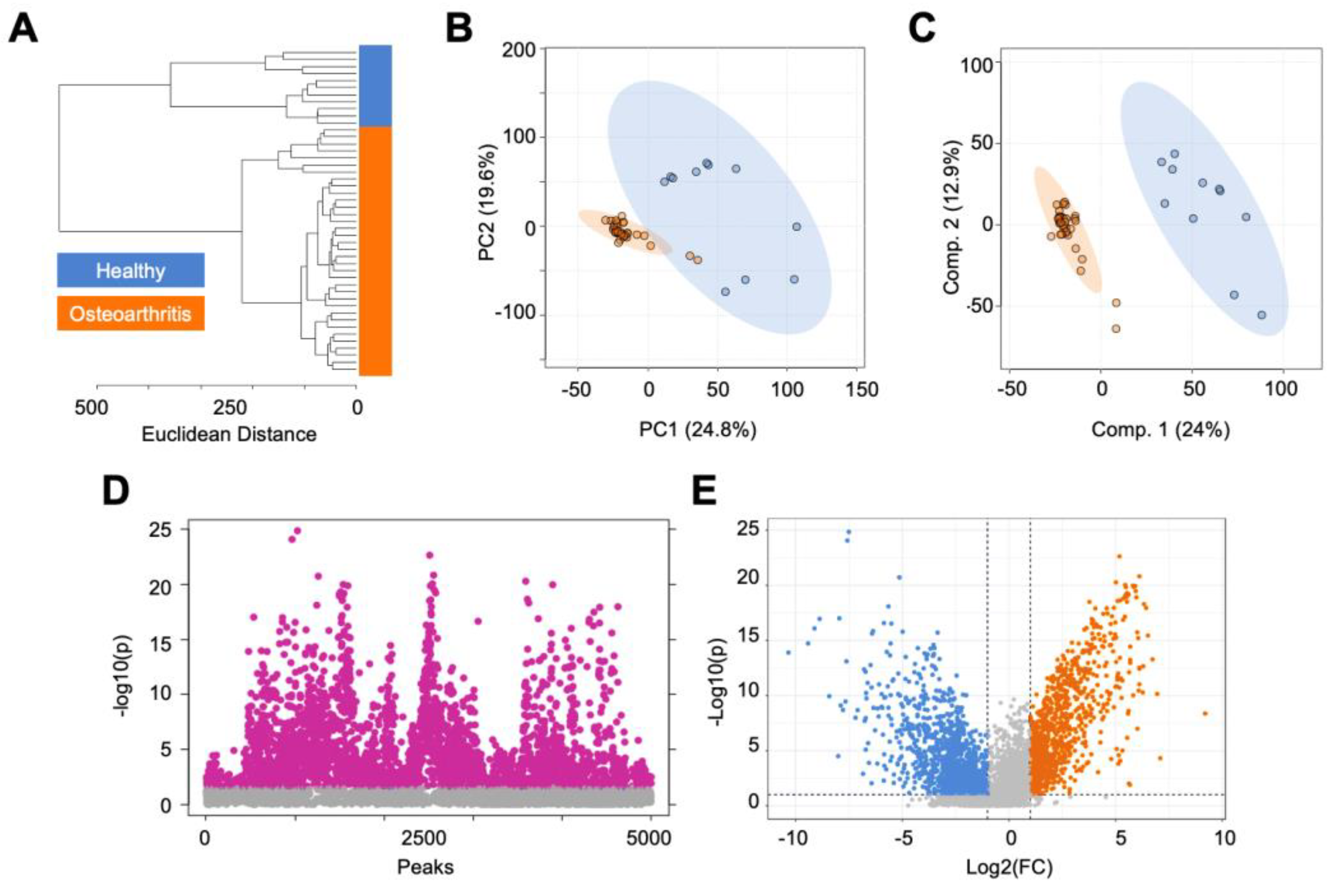
3.1. Global Metabolomic Profiles of Osteoarthritis and Healthy Cartilage Unveil Altered Cellular Mechanisms Associated with Disease
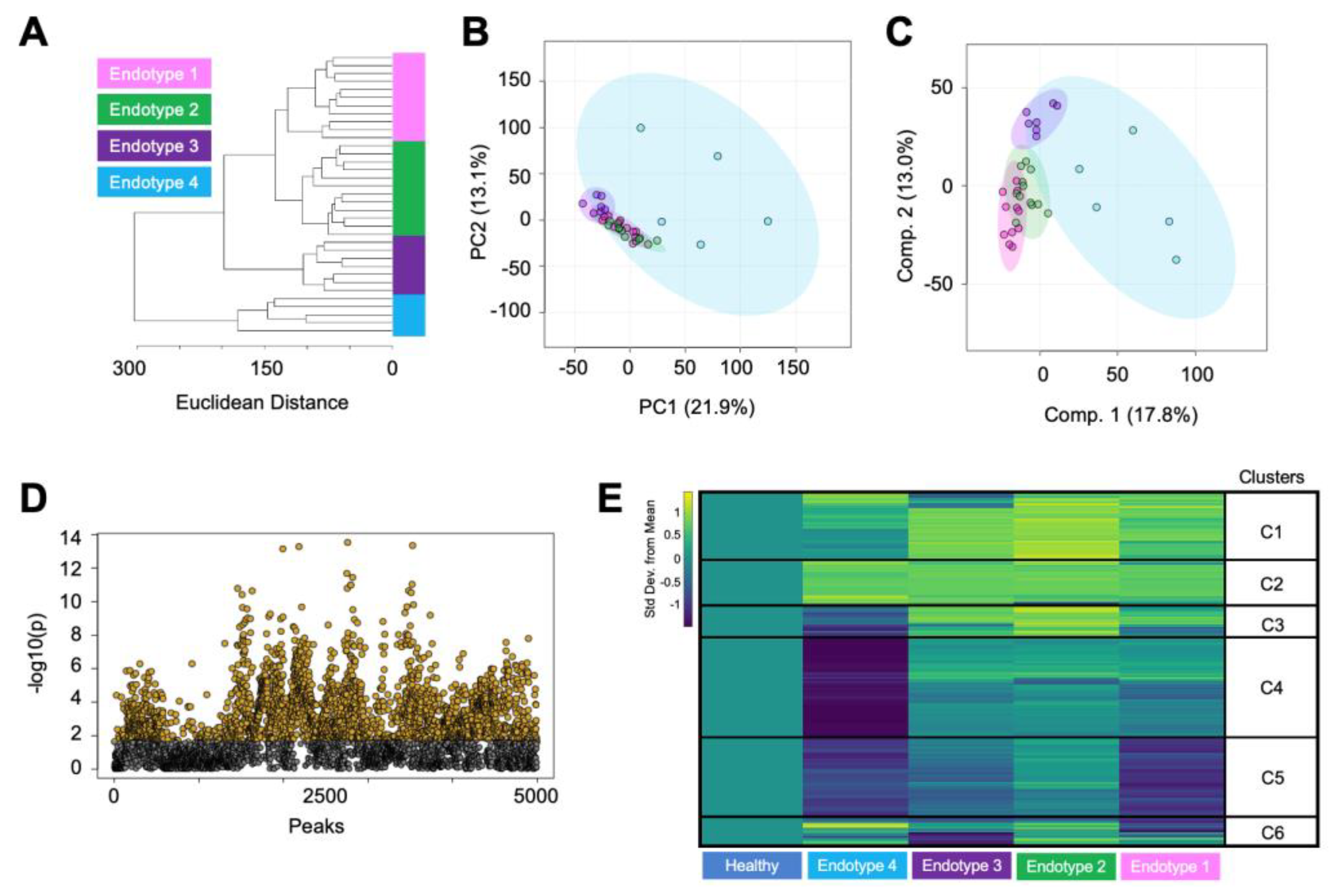
3.2. Endotype Characterization Supports the Heterogenous Nature of Osteoarthritis
4. Discussion
4.1. Matrix Metabolism
4.2. Lipid and Mitochondria-Related Metabolism
4.3. Vitamin Metabolism
4.4. Amino Acid Metabolism
4.5. Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Long, H.; Liu, Q.; Yin, H.; Wang, K.; Diao, N.; Zhang, Y.; Lin, J.; Guo, A. Prevalence trends of site-specific osteoarthritis from 1990 to 2019: Findings from the Global Burden of Disease Study 2019. Arthritis Rheumatol. 2022, 74, 1172–1183. [Google Scholar] [CrossRef]
- Vina, E.R.; Kwoh, C.K. Epidemiology of osteoarthritis: Literature update. Curr. Opin. Rheumatol. 2018, 30, 160–167. [Google Scholar] [CrossRef] [PubMed]
- Barbour, K.E.; Helmick, C.G.; Boring, M.; Brady, T.J. Vital Signs: Prevalence of Doctor-Diagnosed Arthritis and Arthritis-Attributable Activity Limitation-United States, 2013–2015. MMWR Morb. Mortal. Wkly. Rep. 2017, 66, 246–253. [Google Scholar] [CrossRef] [PubMed]
- Bitton, R. The economic burden of osteoarthritis. Am. J. Manag. Care 2009, 15, S230–S235. [Google Scholar]
- Hootman, J.M.; Helmick, C.G.; Barbour, K.E.; Theis, K.A.; Boring, M.A. Updated Projected Prevalence of Self-Reported Doctor-Diagnosed Arthritis and Arthritis-Attributable Activity Limitation Among US Adults, 2015–2040. Arthritis Rheumatol. 2016, 68, 1582–1587. [Google Scholar] [CrossRef]
- Neogi, T. The epidemiology and impact of pain in osteoarthritis. Osteoarthr. Cartil. 2013, 21, 1145–1153. [Google Scholar] [CrossRef]
- Shet, K.; Siddiqui, S.M.; Yoshihara, H.; Kurhanewicz, J.; Ries, M.; Li, X. High-resolution magic angle spinning NMR spectroscopy of human osteoarthritic cartilage. NMR Biomed. 2012, 25, 538–544. [Google Scholar] [CrossRef] [PubMed]
- Xue, M.; Huang, N.; Luo, Y.; Yang, X.; Wang, Y.; Fang, M. Combined Transcriptomics and Metabolomics Identify Regulatory Mechanisms of Porcine Vertebral Chondrocyte Development In Vitro. Int. J. Mol. Sci. 2024, 25, 1189. [Google Scholar] [CrossRef] [PubMed]
- Zignego, D.L.; Hilmer, J.K.; June, R.K. Mechanotransduction in primary human osteoarthritic chondrocytes is mediated by metabolism of energy, lipids, and amino acids. J. Biomech. 2015, 48, 4253–4261. [Google Scholar] [CrossRef]
- Bartlett, S.J.; Ling, S.M.; Mayo, N.E.; Scott, S.C.; Bingham, C.O., 3rd. Identifying common trajectories of joint space narrowing over two years in knee osteoarthritis. Arthritis. Care Res. 2011, 63, 1722–1728. [Google Scholar] [CrossRef]
- Collins, J.E.; Katz, J.N.; Dervan, E.E.; Losina, E. Trajectories and risk profiles of pain in persons with radiographic, symptomatic knee osteoarthritis: Data from the osteoarthritis initiative. Osteoarthr. Cartil. 2014, 22, 622–630. [Google Scholar] [CrossRef] [PubMed]
- Karsdal, M.A.; Bihlet, A.; Byrjalsen, I.; Alexandersen, P.; Ladel, C.; Michaels, M.; Andersen, J.R.; Riis, B.J.; Kraus, V.; Bay-Jensen, A.C.; et al. OA phenotypes, rather than disease stage, drive structural progression--identification of structural progressors from 2 phase III randomized clinical studies with symptomatic knee OA. Osteoarthr. Cartil. 2015, 23, 550–558. [Google Scholar] [CrossRef]
- Bruyere, O.; Cooper, C.; Arden, N.; Branco, J.; Brandi, M.L.; Herrero-Beaumont, G.; Berenbaum, F.; Dennison, E.; Devogelaer, J.P.; Hochberg, M.; et al. Can we identify patients with high risk of osteoarthritis progression who will respond to treatment? A focus on epidemiology and phenotype of osteoarthritis. Drugs Aging 2015, 32, 179–187. [Google Scholar] [CrossRef]
- Deveza, L.A.; Nelson, A.E.; Loeser, R.F. Phenotypes of osteoarthritis: Current state and future implications. Clin. Exp. Rheumatol. 2019, 37 (Suppl. S120), 64–72. [Google Scholar]
- Patti, G.J.; Yanes, O.; Siuzdak, G. Innovation: Metabolomics: The apogee of the omics trilogy. Nat. Rev. Mol. Cell. Biol. 2012, 13, 263–269. [Google Scholar] [CrossRef]
- Carlson, A.K.; Rawle, R.A.; Wallace, C.W.; Brooks, E.G.; Adams, E.; Greenwood, M.C.; Olmer, M.; Lotz, M.K.; Bothner, B.; June, R.K. Characterization of synovial fluid metabolomic phenotypes of cartilage morphological changes associated with osteoarthritis. Osteoarthr. Cartil. 2019, 27, 1174–1184. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Likhodii, S.; Zhang, Y.; Aref-Eshghi, E.; Harper, P.E.; Randell, E.; Green, R.; Martin, G.; Furey, A.; Sun, G.; et al. Classification of osteoarthritis phenotypes by metabolomics analysis. BMJ Open 2014, 4, e006286. [Google Scholar] [CrossRef] [PubMed]
- Welhaven, H.D.; Viles, E.; Starke, J.; Wallace, C.; Bothner, B.; June, R.K.; Hahn, A.K. Metabolomic profiles of cartilage and bone reflect tissue type, radiography-confirmed osteoarthritis, and spatial location within the joint. Biochem. Biophys. Res. Commun. 2024, 703, 149683. [Google Scholar] [CrossRef]
- Welhaven, H.D.; Welfley, A.H.; Pershad, P.; Satalich, J.; O’Connell, R.; Bothner, B.; Vap, A.R.; June, R.K. Metabolic phenotypes reflect patient sex and injury status: A cross-sectional analysis of human synovial fluid. Osteoarthr. Cartil. 2023, Preprint. [Google Scholar] [CrossRef]
- Kessner, D.; Chambers, M.; Burke, R.; Agus, D.; Mallick, P. ProteoWizard: Open source software for rapid proteomics tools development. Bioinformatics 2008, 24, 2534–2536. [Google Scholar] [CrossRef]
- Smith, C.A.; Want, E.J.; O’Maille, G.; Abagyan, R.; Siuzdak, G. XCMS: Processing mass spectrometry data for metabolite profiling using nonlinear peak alignment, matching, and identification. Anal. Chem. 2006, 78, 779–787. [Google Scholar] [CrossRef] [PubMed]
- Welhaven, H.D.; Vahidi, G.; Walk, S.T.; Bothner, B.; Martin, S.A.; Heveran, C.M.; June, R.K. The Cortical Bone Metabolome of C57BL/6J Mice Is Sexually Dimorphic. JBMR Plus 2022, 6, e10654. [Google Scholar] [CrossRef] [PubMed]
- Pang, Z.; Zhou, G.; Ewald, J.; Chang, L.; Hacariz, O.; Basu, N.; Xia, J. Using MetaboAnalyst 5.0 for LC-HRMS spectra processing, multi-omics integration and covariate adjustment of global metabolomics data. Nat. Protoc. 2022, 17, 1735–1761. [Google Scholar] [CrossRef] [PubMed]
- Xiao, J.F.; Zhou, B.; Ressom, H.W. Metabolite identification and quantitation in LC-MS/MS-based metabolomics. Trends Analyt. Chem. 2012, 32, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Wishart, D.S.; Guo, A.; Oler, E.; Wang, F.; Anjum, A.; Peters, H.; Dizon, R.; Sayeeda, Z.; Tian, S.; Lee, B.L.; et al. HMDB 5.0: The Human Metabolome Database for 2022. Nucleic Acids Res. 2022, 50, D622–D631. [Google Scholar] [CrossRef] [PubMed]
- Elliott, R.J.; Gardner, D.L. Changes with age in the glycosaminoglycans of human articular cartilage. Ann. Rheum. Dis. 1979, 38, 371–377. [Google Scholar] [CrossRef] [PubMed]
- Hjertquist, S.O.; Lemperg, R. Identification and concentration of the glycosaminoglycans of human articular cartilage in relation to age and osteoarthritis. Calcif. Tissue Res. 1972, 10, 223–237. [Google Scholar] [CrossRef] [PubMed]
- Thonar, E.J.; Masuda, K.; Hauselmann, H.J.; Uebelhart, D.; Lenz, M.E.; Manicourt, D.H. Keratan sulfate in body fluids in joint disease. Acta Orthop. Scand. Suppl. 1995, 266, 103–106. [Google Scholar] [CrossRef]
- Jay, G.D.; Torres, J.R.; Warman, M.L.; Laderer, M.C.; Breuer, K.S. The role of lubricin in the mechanical behavior of synovial fluid. Proc. Natl. Acad. Sci. USA 2007, 104, 6194–6199. [Google Scholar] [CrossRef]
- Ioan-Facsinay, A.; Kloppenburg, M. Bioactive lipids in osteoarthritis: Risk or benefit? Curr. Opin. Rheumatol. 2018, 30, 108–113. [Google Scholar] [CrossRef]
- Van de Vyver, A.; Clockaerts, S.; van de Lest, C.H.A.; Wei, W.; Verhaar, J.; Van Osch, G.; Bastiaansen-Jenniskens, Y.M. Synovial Fluid Fatty Acid Profiles Differ between Osteoarthritis and Healthy Patients. Cartilage 2020, 11, 473–478. [Google Scholar] [CrossRef] [PubMed]
- Cillero-Pastor, B.; Eijkel, G.; Kiss, A.; Blanco, F.J.; Heeren, R.M. Time-of-flight secondary ion mass spectrometry-based molecular distribution distinguishing healthy and osteoarthritic human cartilage. Anal. Chem. 2012, 84, 8909–8916. [Google Scholar] [CrossRef] [PubMed]
- Baker, K.R.; Matthan, N.R.; Lichtenstein, A.H.; Niu, J.; Guermazi, A.; Roemer, F.; Grainger, A.; Nevitt, M.C.; Clancy, M.; Lewis, C.E.; et al. Association of plasma n-6 and n-3 polyunsaturated fatty acids with synovitis in the knee: The MOST study. Osteoarthr. Cartil. 2012, 20, 382–387. [Google Scholar] [CrossRef] [PubMed]
- Lippiello, L.; Walsh, T.; Fienhold, M. The association of lipid abnormalities with tissue pathology in human osteoarthritic articular cartilage. Metabolism 1991, 40, 571–576. [Google Scholar] [CrossRef] [PubMed]
- Dalmao-Fernandez, A.; Lund, J.; Hermida-Gomez, T.; Vazquez-Mosquera, M.E.; Rego-Perez, I.; Blanco, F.J.; Fernandez-Moreno, M. Impaired Metabolic Flexibility in the Osteoarthritis Process: A Study on Transmitochondrial Cybrids. Cells 2020, 9, 809. [Google Scholar] [CrossRef]
- Smith, R.L.; Soeters, M.R.; Wust, R.C.I.; Houtkooper, R.H. Metabolic Flexibility as an Adaptation to Energy Resources and Requirements in Health and Disease. Endocr. Rev. 2018, 39, 489–517. [Google Scholar] [CrossRef]
- Blanco, F.J.; Lopez-Armada, M.J.; Maneiro, E. Mitochondrial dysfunction in osteoarthritis. Mitochondrion 2004, 4, 715–728. [Google Scholar] [CrossRef] [PubMed]
- Blanco, F.J.; Rego, I.; Ruiz-Romero, C. The role of mitochondria in osteoarthritis. Nat. Rev. Rheumatol. 2011, 7, 161–169. [Google Scholar] [CrossRef]
- Lane, R.S.; Fu, Y.; Matsuzaki, S.; Kinter, M.; Humphries, K.M.; Griffin, T.M. Mitochondrial respiration and redox coupling in articular chondrocytes. Arthritis. Res. Ther. 2015, 17, 54. [Google Scholar] [CrossRef]
- Wu, L.; Liu, H.; Li, L.; Liu, H.; Cheng, Q.; Li, H.; Huang, H. Mitochondrial pathology in osteoarthritic chondrocytes. Curr. Drug Targets 2014, 15, 710–719. [Google Scholar] [CrossRef]
- Dambrova, M.; Makrecka-Kuka, M.; Kuka, J.; Vilskersts, R.; Nordberg, D.; Attwood, M.M.; Smesny, S.; Sen, Z.D.; Guo, A.C.; Oler, E.; et al. Acylcarnitines: Nomenclature, Biomarkers, Therapeutic Potential, Drug Targets, and Clinical Trials. Pharmacol. Rev. 2022, 74, 506–551. [Google Scholar] [CrossRef] [PubMed]
- Collins, J.A.; Wood, S.T.; Nelson, K.J.; Rowe, M.A.; Carlson, C.S.; Chubinskaya, S.; Poole, L.B.; Furdui, C.M.; Loeser, R.F. Oxidative Stress Promotes Peroxiredoxin Hyperoxidation and Attenuates Pro-survival Signaling in Aging Chondrocytes. J. Biol. Chem. 2016, 291, 6641–6654. [Google Scholar] [CrossRef]
- Davies, M.R.; Ribeiro, L.R.; Downey-Jones, M.; Needham, M.R.; Oakley, C.; Wardale, J. Ligands for retinoic acid receptors are elevated in osteoarthritis and may contribute to pathologic processes in the osteoarthritic joint. Arthritis. Rheum. 2009, 60, 1722–1732. [Google Scholar] [CrossRef] [PubMed]
- Underhill, T.M.; Weston, A.D. Retinoids and their receptors in skeletal development. Microsc. Res. Tech. 1998, 43, 137–155. [Google Scholar] [CrossRef]
- Flannery, C.R.; Little, C.B.; Caterson, B.; Hughes, C.E. Effects of culture conditions and exposure to catabolic stimulators (IL-1 and retinoic acid) on the expression of matrix metalloproteinases (MMPs) and disintegrin metalloproteinases (ADAMs) by articular cartilage chondrocytes. Matrix Biol. 1999, 18, 225–237. [Google Scholar] [CrossRef]
- Misra, D.; Booth, S.L.; Tolstykh, I.; Felson, D.T.; Nevitt, M.C.; Lewis, C.E.; Torner, J.; Neogi, T. Vitamin K deficiency is associated with incident knee osteoarthritis. Am. J. Med. 2013, 126, 243–248. [Google Scholar] [CrossRef]
- Shea, M.K.; Kritchevsky, S.B.; Hsu, F.C.; Nevitt, M.; Booth, S.L.; Kwoh, C.K.; McAlindon, T.E.; Vermeer, C.; Drummen, N.; Harris, T.B.; et al. The association between vitamin K status and knee osteoarthritis features in older adults: The Health, Aging and Body Composition Study. Osteoarthr. Cartil. 2015, 23, 370–378. [Google Scholar] [CrossRef]
- Wallin, R.; Schurgers, L.J.; Loeser, R.F. Biosynthesis of the vitamin K-dependent matrix Gla protein (MGP) in chondrocytes: A fetuin-MGP protein complex is assembled in vesicles shed from normal but not from osteoarthritic chondrocytes. Osteoarthr. Cartil. 2010, 18, 1096–1103. [Google Scholar] [CrossRef]
- Luo, G.; Ducy, P.; McKee, M.D.; Pinero, G.J.; Loyer, E.; Behringer, R.R.; Karsenty, G. Spontaneous calcification of arteries and cartilage in mice lacking matrix GLA protein. Nature 1997, 386, 78–81. [Google Scholar] [CrossRef]
- Price, P.A.; Williamson, M.K.; Haba, T.; Dell, R.B.; Jee, W.S. Excessive mineralization with growth plate closure in rats on chronic warfarin treatment. Proc. Natl. Acad. Sci. USA 1982, 79, 7734–7738. [Google Scholar] [CrossRef]
- Zhang, Q.; Li, H.; Zhang, Z.; Yang, F.; Chen, J. Serum metabolites as potential biomarkers for diagnosis of knee osteoarthritis. Dis. Markers 2015, 2015, 684794. [Google Scholar] [CrossRef] [PubMed]
- Zhai, G.; Wang-Sattler, R.; Hart, D.J.; Arden, N.K.; Hakim, A.J.; Illig, T.; Spector, T.D. Serum branched-chain amino acid to histidine ratio: A novel metabolomic biomarker of knee osteoarthritis. Ann. Rheum. Dis. 2010, 69, 1227–1231. [Google Scholar] [CrossRef] [PubMed]
- Abdelrazig, S.; Ortori, C.A.; Doherty, M.; Valdes, A.M.; Chapman, V.; Barrett, D.A. Metabolic signatures of osteoarthritis in urine using liquid chromatography-high resolution tandem mass spectrometry. Metabolomics 2021, 17, 29. [Google Scholar] [CrossRef]
- Igari, T.; Tsuchizawa, M.; Shimamura, T. Alteration of tryptophan metabolism in the synovial fluid of patients with rheumatoid arthritis and osteoarthritis. Tohoku J. Exp. Med. 1987, 153, 79–86. [Google Scholar] [CrossRef] [PubMed]
| Group | Regulation | Pathway |
|---|---|---|
| Osteoarthritis | FC > 2, p < 0.05 | Carnitine shuttle |
| Osteoarthritis | FC > 2, p < 0.05 | De novo fatty acid biosynthesis |
| Osteoarthritis | FC > 2, p < 0.05 | Fatty acid activation |
| Osteoarthritis | FC > 2, p < 0.05 | Fatty acid metabolism |
| Osteoarthritis | FC > 2, p < 0.05 | Fatty acid oxidation |
| Osteoarthritis | FC > 2, p < 0.05 | Fatty acid oxidation, peroxisome |
| Osteoarthritis | FC > 2, p < 0.05 | Glycosphingolipid biosynthesis—ganglioseries |
| Osteoarthritis | FC > 2, p < 0.05 | Glycosphingolipid biosynthesis—globoseries |
| Osteoarthritis | FC > 2, p < 0.05 | Leukotriene metabolism |
| Osteoarthritis | FC > 2, p < 0.05 | N-glycan degradation |
| Osteoarthritis | FC > 2, p < 0.05 | Omega-3 fatty acid metabolism |
| Osteoarthritis | FC > 2, p < 0.05 | Omega-6 fatty acid metabolism |
| Osteoarthritis | FC > 2, p < 0.05 | Phosphatidylinositol phosphate metabolism |
| Osteoarthritis | FC > 2, p < 0.05 | Phytanic acid peroxisomal oxidation |
| Osteoarthritis | FC > 2, p < 0.05 | Polyunsaturated fatty acid biosynthesis |
| Osteoarthritis | FC > 2, p < 0.05 | R group synthesis |
| Osteoarthritis | FC > 2, p < 0.05 | Saturated fatty acid beta-oxidation |
| Healthy | FC < −2, p < 0.05 | Aspartate and asparagine metabolism |
| Healthy | FC < −2, p < 0.05 | Glycerophospholipid metabolism |
| Healthy | FC < −2, p < 0.05 | Glycine, serine, alanine and threonine metabolism |
| Healthy | FC < −2, p < 0.05 | Histidine metabolism |
| Healthy | FC < −2, p < 0.05 | Methionine and cysteine metabolism |
| Healthy | FC < −2, p < 0.05 | Purine metabolism |
| Healthy | FC < −2, p < 0.05 | Squalene and cholesterol biosynthesis |
| Healthy | FC < −2, p < 0.05 | Tryptophan metabolism |
| Healthy | FC < −2, p < 0.05 | Urea cycle/amino group metabolism |
| Healthy | FC < −2, p < 0.05 | Vitamin E metabolism |
| Healthy | FC < −2, p < 0.05 | Vitamin K metabolism |
| Cluster | Pathway |
|---|---|
| 1 | Fatty acid activation |
| 1 | Saturated fatty acids beta-oxidation |
| 1 | De novo fatty acid biosynthesis |
| 1 | Fatty acid metabolism |
| 1 | Omega-6 fatty acid metabolism |
| 1 | Carnitine shuttle |
| 1 | R group synthesis |
| 1 | Fatty acid oxidation |
| 1 | Fatty acid oxidation, peroxisome |
| 1 | Leukotriene metabolism |
| 2 | Fatty acid oxidation |
| 2 | Polyunsaturated fatty acid biosynthesis |
| 2 | De novo fatty acid biosynthesis |
| 2 | Phytanic acid peroxisomal oxidation |
| 2 | R group synthesis |
| 2 | Selenoamino acid metabolism |
| 3 | Phytanic acid peroxisomal oxidation |
| 3 | Omega-6 fatty acid metabolism |
| 4 | Glycosphingolipid biosynthesis—globoseries |
| 4 | Lysine metabolism |
| 4 | Tyrosine metabolism |
| 4 | Polyunsaturated fatty acid biosynthesis |
| 4 | Glycosphingolipid biosynthesis—ganglioseries |
| 4 | Keratan sulfate degradation |
| 4 | N-glycan degradation |
| 4 | Linoleate metabolism |
| 4 | Vitamin A (retinol) metabolism |
| 4 | Butanoate metabolism |
| 4 | Trihydroxycoprostanoyl-CoA beta-oxidation |
| 4 | Glycerophospholipid metabolism |
| 4 | Omega-3 fatty acid metabolism |
| 4 | Starch and sucrose metabolism |
| 5 | Purine metabolism |
| 5 | Leukotriene metabolism |
| 5 | Urea cycle/amino group metabolism |
| 5 | Methionine and cysteine metabolism |
| 5 | Tryptophan metabolism |
| 5 | Aminosugar metabolism |
| 5 | Aspartate and asparagine metabolism |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Welhaven, H.D.; Welfley, A.H.; Brahmachary, P.; Bergstrom, A.R.; Houske, E.; Glimm, M.; Bothner, B.; Hahn, A.K.; June, R.K. Metabolomic Profiles and Pathways in Osteoarthritic Human Cartilage: A Comparative Analysis with Healthy Cartilage. Metabolites 2024, 14, 183. https://doi.org/10.3390/metabo14040183
Welhaven HD, Welfley AH, Brahmachary P, Bergstrom AR, Houske E, Glimm M, Bothner B, Hahn AK, June RK. Metabolomic Profiles and Pathways in Osteoarthritic Human Cartilage: A Comparative Analysis with Healthy Cartilage. Metabolites. 2024; 14(4):183. https://doi.org/10.3390/metabo14040183
Chicago/Turabian StyleWelhaven, Hope D., Avery H. Welfley, Priyanka Brahmachary, Annika R. Bergstrom, Eden Houske, Matthew Glimm, Brian Bothner, Alyssa K. Hahn, and Ronald K. June. 2024. "Metabolomic Profiles and Pathways in Osteoarthritic Human Cartilage: A Comparative Analysis with Healthy Cartilage" Metabolites 14, no. 4: 183. https://doi.org/10.3390/metabo14040183
APA StyleWelhaven, H. D., Welfley, A. H., Brahmachary, P., Bergstrom, A. R., Houske, E., Glimm, M., Bothner, B., Hahn, A. K., & June, R. K. (2024). Metabolomic Profiles and Pathways in Osteoarthritic Human Cartilage: A Comparative Analysis with Healthy Cartilage. Metabolites, 14(4), 183. https://doi.org/10.3390/metabo14040183






