Characterization of Crocetin Isomers in Serum Samples via UHPLC-DAD-MS/MS and NMR after Saffron Extract (Safr’Inside™) Consumption
Abstract
:1. Introduction
2. Materials and Methods
2.1. Material and Reagents
2.2. Identification of Trans-Geometrical Isomers of Crocetins
2.2.1. Isomerization of Trans-Crocetin through Heat Treatment
2.2.2. UHPLC-DAD-MS/MS Characterization of Geometrical Trans-Crocetin Isomer
2.2.3. NMR Structural Identification of Geometrical Trans-Crocetin Isomer
2.3. Identification of Trans-Geometrical Isomers of Crocetin after Oral Administration of Saffron Extract (Safr’Inside™) in Human Samples
2.3.1. Clinical Study
2.3.2. Analysis of Samples
2.4. Method Validation
2.4.1. Standard Solutions and Quality Control Serum
2.4.2. UHPLC-MS/MS Method Development
- Instrumentation
- Chromatographic conditions
- MS Conditions
2.4.3. Method Validation
- Linearity
- Precision and accuracy
- Limits of detection and quantification
- Recovery and matrix effect
- Stability
3. Results
3.1. Identification of Trans-Geometrical Isomers of Crocetin Standard
3.1.1. Detection of Trans-Geometrical Isomers of Crocetin via UHPLC-DAD/MS after Heat Treatment
3.1.2. Characterization of Trans-Crocetin Isomer via NMR after UHPLC-DAD/MS Identification
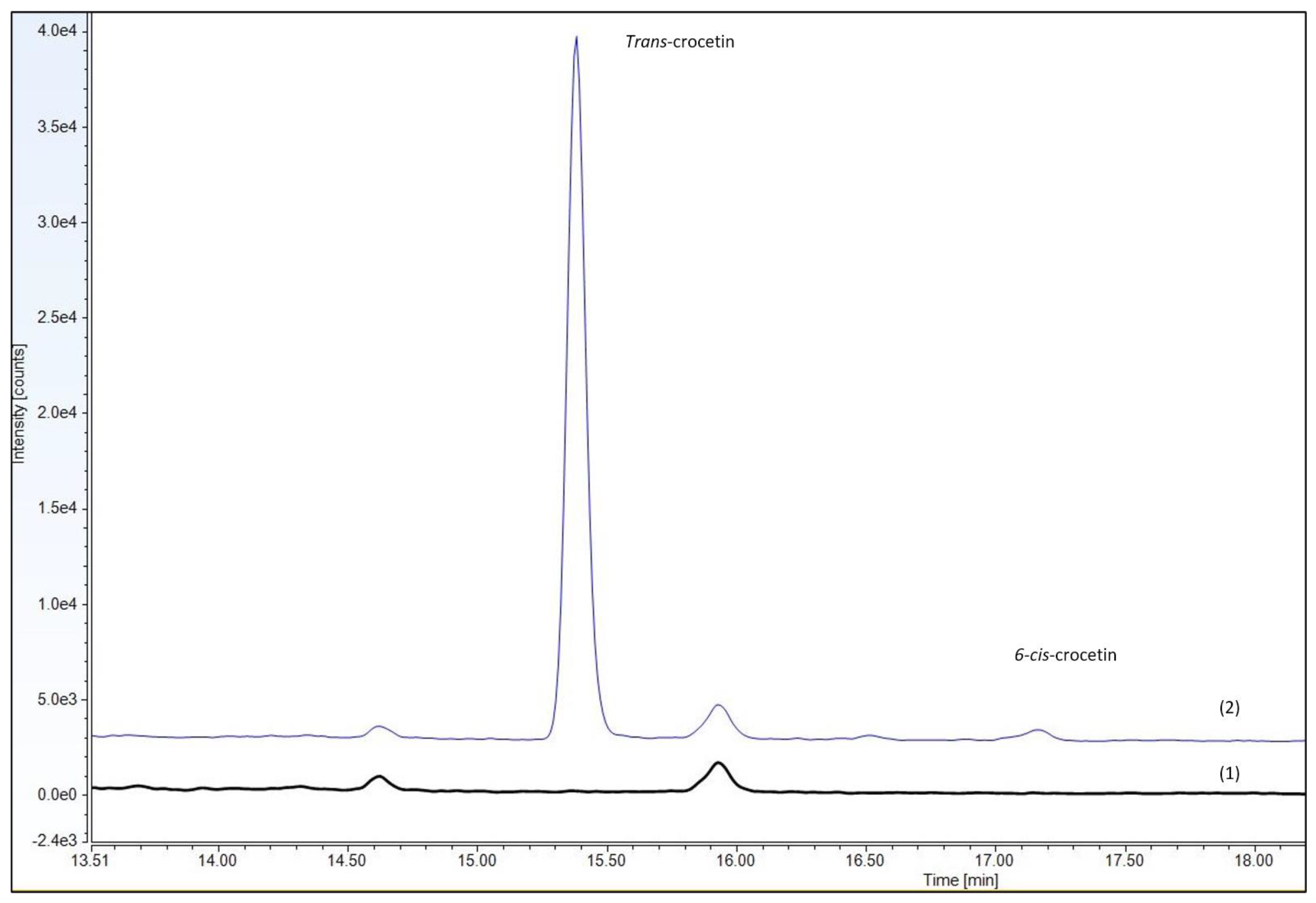
3.2. Identification of Trans-Geometrical Isomers of Crocetin after Oral Administration of Saffron Extract (Safr’Inside™) in Human Samples
3.3. Method Validation
4. Discussion
5. Patents
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Moratalla-López, N.; Bagur, M.J.; Lorenzo, C.; Martínez-Navarro, M.E.; Rosario Salinas, M.; Alonso, G.L. Bioactivity and Bioavailability of the Major Metabolites of Crocus sativus L. Flower. Molecules 2019, 24, 2827. [Google Scholar] [CrossRef] [PubMed]
- Butnariu, M.; Quispe, C.; Herrera-Bravo, J.; Sharifi-Rad, J.; Singh, L.; Aborehab, N.M.; Bouyahya, A.; Venditti, A.; Sen, S.; Acharya, K.; et al. The Pharmacological Activities of Crocus sativus L.: A Review Based on the Mechanisms and Therapeutic Opportunities of Its Phytoconstituents. Oxid. Med. Cell. Longev. 2022, 2022, 8214821. [Google Scholar] [CrossRef]
- Anaeigoudari, F.; Anaeigoudari, A.; Kheirkhah-Vakilabad, A. A Review of Therapeutic Impacts of Saffron (Crocus sativus L.) and Its Constituents. Physiol. Rep. 2023, 11, e15785. [Google Scholar] [CrossRef]
- Shahi, T.; Assadpour, E.; Jafari, S.M. Main Chemical Compounds and Pharmacological Activities of Stigmas and Tepals of ‘Red Gold’; Saffron. Trends Food Sci. Technol. 2016, 58, 69–78. [Google Scholar] [CrossRef]
- Hashemi, M.; Hosseinzadeh, H. A Comprehensive Review on Biological Activities and Toxicology of Crocetin. Food Chem. Toxicol. 2019, 130, 44–60. [Google Scholar] [CrossRef] [PubMed]
- Marjan Razavi Targeted, B.; Esmaealzadeh, D.; Moodi Ghalibaf, A.; Shariati Rad, M.; Rezaee, R.; Marjan Razavi, B.; Hosseinzadeh, H.; Ghalibaf, M.A.; Rad, S.M. Spectroscopic Investigation on the Interaction of DNA with Superparamagnetic Iron Oxide Nanoparticles Doped with Chromene via Dopamine as Cross Linker. Iran J. Basic Med. Sci 2023, 26, 1131–1143. [Google Scholar] [CrossRef]
- Lautenschläger, M.; Sendker, J.; Hüwel, S.; Galla, H.J.; Brandt, S.; Düfer, M.; Riehemann, K.; Hensel, A. Intestinal Formation of Trans-Crocetin from Saffron Extract (Crocus sativus L.) and in Vitro Permeation through Intestinal and Blood Brain Barrier. Phytomedicine 2015, 22, 36–44. [Google Scholar] [CrossRef]
- Mori, K.; Kurihara, T.; Miyauchi, M.; Ishida, A.; Jiang, X.; Ikeda, S.-I.; Torii, H.; Tsubota, K. Oral Crocetin Administration Suppressed Refractive Shift and Axial Elongation in a Murine Model of Lens-Induced Myopia. Nat. Sci. Rep. 2019, 9, 295. [Google Scholar] [CrossRef]
- Shakya, R.; Nepal, M.R.; Kang, M.J.; Jeong, T.C. Effects of Intestinal Microbiota on Pharmacokinetics of Crocin and Crocetin in Male Sprague-Dawley Rats. Metabolites 2020, 10, 424. [Google Scholar] [CrossRef]
- Hooshang Mohammadpour, A.; Ramezani, M.; Tavakoli Anaraki, N.; Malaekeh-Nikouei, B.; Amel Farzad, S.; Hosseinzadeh, H.; Anaraki, T.N.; Farzad, A.S.; Houshang Mohamadpour, A. Development and Validation of HPLC Method for Determination of Crocetin, a Constituent of Saffron. Iran. J. Basic Med. Sci. 2013, 16, 47. [Google Scholar]
- Asai, A.; Nakano, T.; Takahashi, M.; Nagao, A. Orally Administered Crocetin and Crocins Are Absorbed into Blood Plasma as Crocetin and Its Glucuronide Conjugates in Mice. J. Agric. Food Chem. 2005, 53, 7302–7306. [Google Scholar] [CrossRef]
- Christodoulou, E.; Grafakou, M.E.; Skaltsa, E.; Kadoglou, N.; Kostomitsopoulos, N.; Valsami, G. Preparation, Chemical Characterization and Determination of Crocetin’s Pharmacokinetics after Oral and Intravenous Administration of Saffron (Crocus sativus L.) Aqueous Extract to C57/BL6J Mice. J. Pharm. Pharmacol. 2019, 71, 753–764. [Google Scholar] [CrossRef] [PubMed]
- Karkoula, E.; Angelis, A.; Koulakiotis, N.S.; Gikas, E.; Halabalaki, M.; Tsarbopoulos, A.; Skaltsounis, A.L. Rapid Isolation and Characterization of Crocins, Picrocrocin, and Crocetin from Saffron Using Centrifugal Partition Chromatography and LC–MS. J. Sep. Sci. 2018, 41, 4105–4114. [Google Scholar] [CrossRef] [PubMed]
- Pang, Q.; Zhang, W.; Li, C.; Li, H.; Zhang, Y.; Li, L.; Zang, C.; Yao, X.; Zhang, D.; Yu, Y. Antidementia Effects, Metabolic Profiles and Pharmacokinetics of GJ-4, a Crocin-Rich Botanical Candidate from: Gardeniae Fructus. Food Funct. 2020, 11, 8825–8836. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Long, Z.; Dai, J.; Bi, K.; Chen, X. Identification of Multiple Constituents in the Traditional Chinese Medicine Formula Zhi-Zi-Chi Decoction and Rat Plasma after Oral Administration by Liquid Chromatography Coupled to Quadrupole Time-of-Flight Tandem Mass Spectrometry. Rapid Commun. Mass Spectrom. 2012, 26, 2443–2453. [Google Scholar] [CrossRef] [PubMed]
- Hosotani, K.; Kitagawa, M. Improved Simultaneous Determination Method of β-Carotene and Retinol with Saponification in Human Serum and Rat Liver. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2003, 791, 305–313. [Google Scholar] [CrossRef]
- Richelle, M.; Sanchez, B.; Tavazzi, I.; Lambelet, P.; Bortlik, K.; Williamson, G. Lycopene Isomerisation Takes Place within Enterocytes during Absorption in Human Subjects. Br. J. Nutr. 2010, 103, 1800–1807. [Google Scholar] [CrossRef] [PubMed]
- Bohm, V.; Puspitasari-Nienaber, N.L.; Ferruzzi, M.G.; Schwartz, S.J. Trolox Equivalent Antioxidant Capacity of Different Geometrical Isomers of α-Carotene, β-Carotene, Lycopene, and Zeaxanthin. J. Agric. Food Chem. 2001, 50, 221–226. [Google Scholar] [CrossRef] [PubMed]
- Wauquier, F.; Boutin-Wittrant, L.; Pourtau, L.; Gaudout, D.; Moras, B.; Vignault, A.; Monchaux De Oliveira, C.; Gabaston, J.; Vaysse, C.; Bertrand, K.; et al. Circulating Human Serum Metabolites Derived from the Intake of a Saffron Extract (Safr’InsideTM) Protect Neurons from Oxidative Stress: Consideration for Depressive Disorders. Nutrients 2022, 14, 1511. [Google Scholar] [CrossRef]
- European Medicines Agency. Biologycal Method Validation M10. Sci. Med. Health 2019, 44, 6/7–20/40–41/49–57. [Google Scholar]
- Assimiadis, M.K.; Tarantilis, P.A.; Polissiou, M.G. UV-Vis, FT-Raman, and 1H NMR Spectroscopies of Cis-Trans Carotenoids from Saffron (Crocus sativus L.). Appl. Spectrosc. 1998, 52, 519–522. [Google Scholar] [CrossRef]
- Moras, B.; Loffredo, L.; Rey, S. Quality Assessment of Saffron (Crocus sativus L.) Extracts via UHPLC-DAD-MS Analysis and Detection of Adulteration Using Gardenia Fruit Extract (Gardenia Jasminoides Ellis). Food Chem. 2018, 257, 325–332. [Google Scholar] [CrossRef] [PubMed]
- Fusi, F.; Romano, G.; Speranza, G.; Agati, G. Photon- and Singlet-Oxygen-Induced Cis–Trans Isomerization of the Water-Soluble Carotenoid Crocin. Int. J. Mol. Sci. 2023, 24, 10783. [Google Scholar] [CrossRef] [PubMed]
- Umigai, N.; Murakami, K.; Ulit, M.V.; Antonio, L.S.; Shirotori, M.; Morikawa, H.; Nakano, T. The Pharmacokinetic Profile of Crocetin in Healthy Adult Human Volunteers after a Single Oral Administration. Phytomedicine 2011, 18, 575–578. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Fei, F.; Zhen, L.; Zhu, X.; Wang, J.; Li, S.; Geng, J.; Sun, R.; Yu, X.; Chen, T.; et al. Sensitive Analysis and Simultaneous Assessment of Pharmacokinetic Properties of Crocin and Crocetin after Oral Administration in Rats. J. Chromatogr. B 2017, 1044–1045, 1–7. [Google Scholar] [CrossRef]
- Zhu, A.; Lao, C.; Wang, Z.; Chen, Y.; Bai, C. Characterization of Crocetin-Monoglucuronide as a Neuron-Protective Metabolite of Crocin-1. Mol. Nutr. Food Res. 2019, 63, 1900024. [Google Scholar] [CrossRef] [PubMed]
- Miller, T.L.; Willett, S.L.; Moss, M.E.; Miller, J.; Belinka, B.A. Binding of Crocetin to Plasma Albumin. J. Pharm. Sci. 1982, 71, 173–177. [Google Scholar] [CrossRef]
- Butnariu, M. Methods of Analysis (Extraction, Separation, Identification and Quantification) of Carotenoids from Natural Products. J. Ecosyst. Ecography 2016, 6, 1000193. [Google Scholar] [CrossRef]
- Meléndez-Martínez, A.J.; Paulino, M.; Stinco, C.M.; Mapelli-Brahm, P.; Wang, X.D. Study of the Time-Course of Cis/Trans (Z/E) Isomerization of Lycopene, Phytoene, and Phytofluene from Tomato. J. Agric. Food Chem. 2014, 62, 12399–12406. [Google Scholar] [CrossRef]

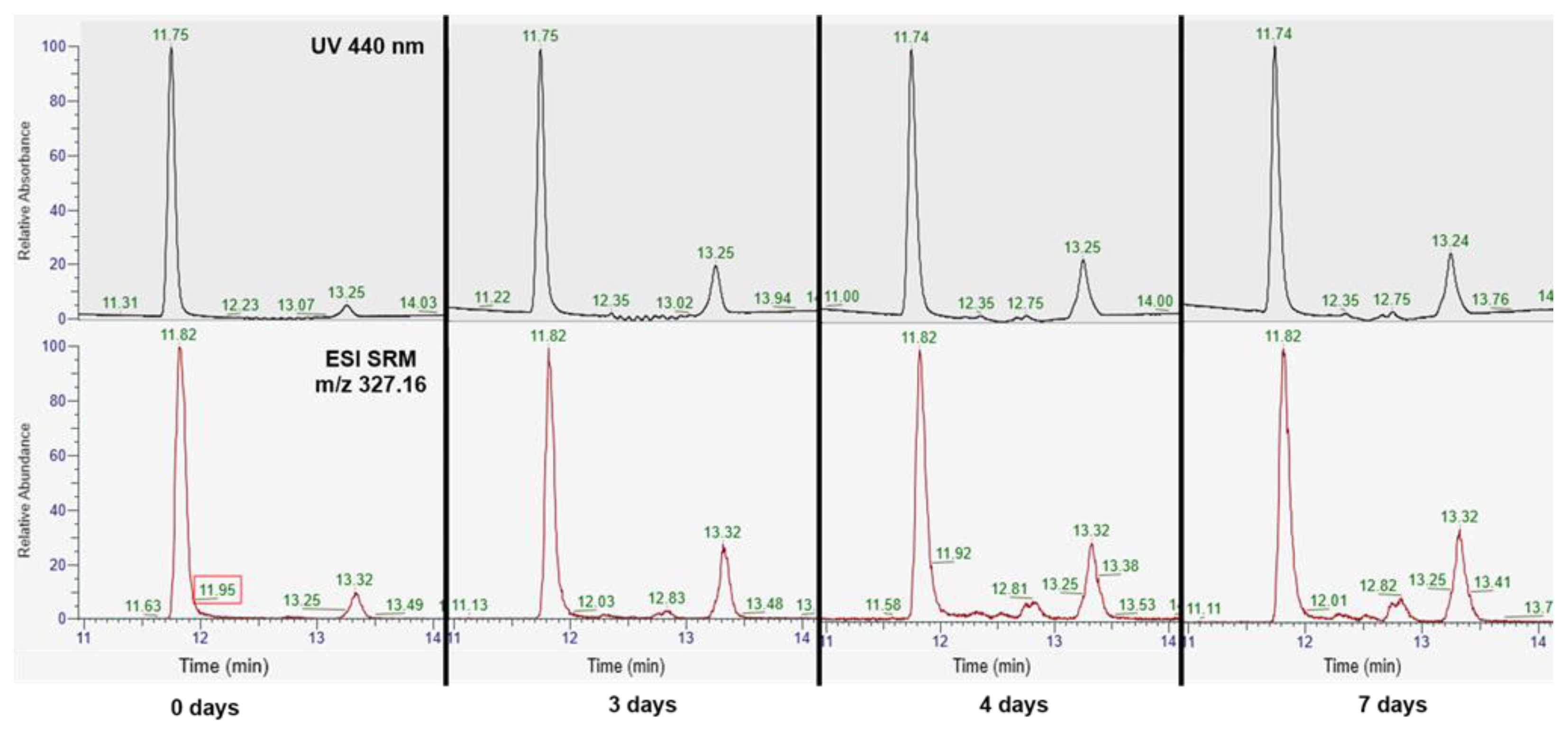

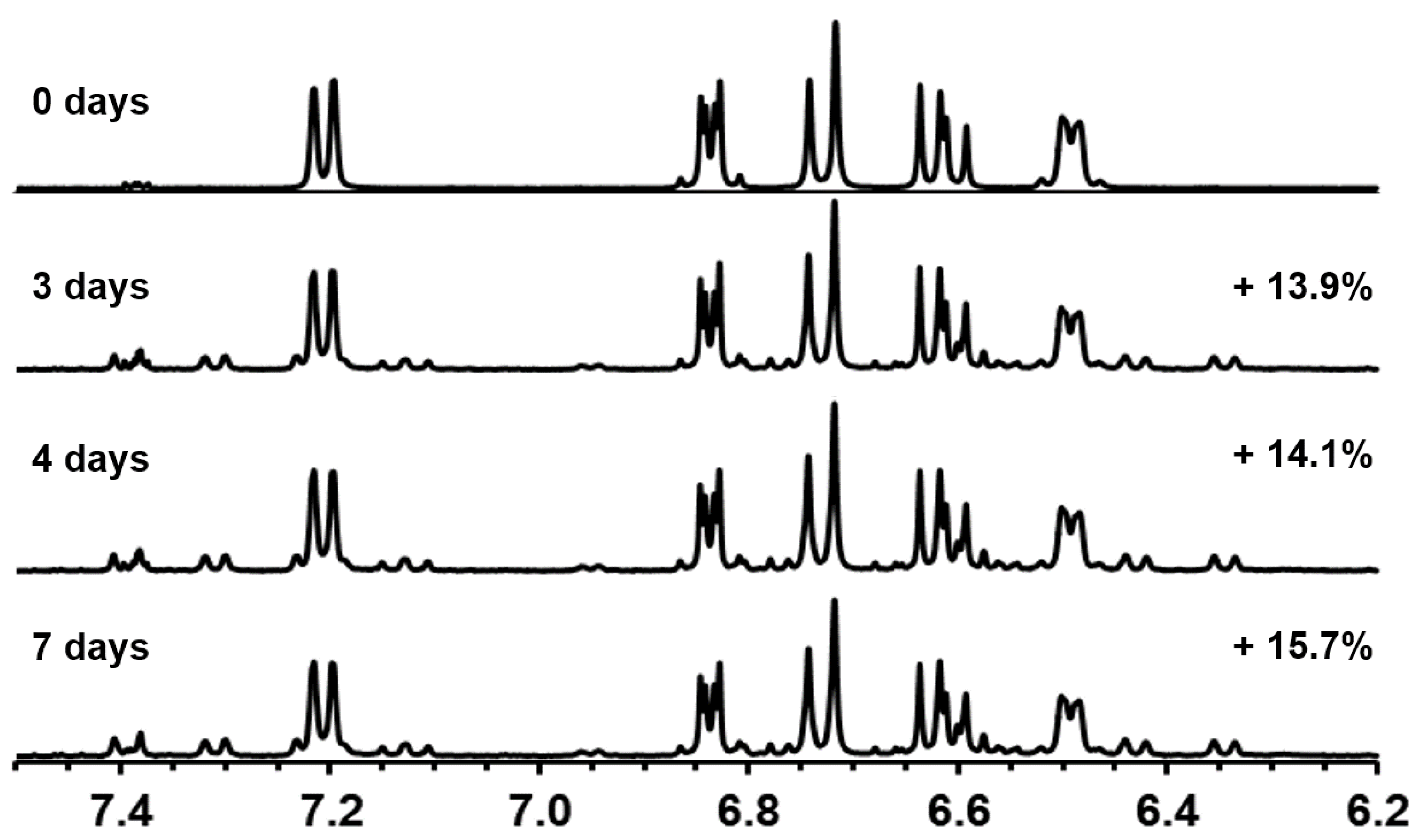
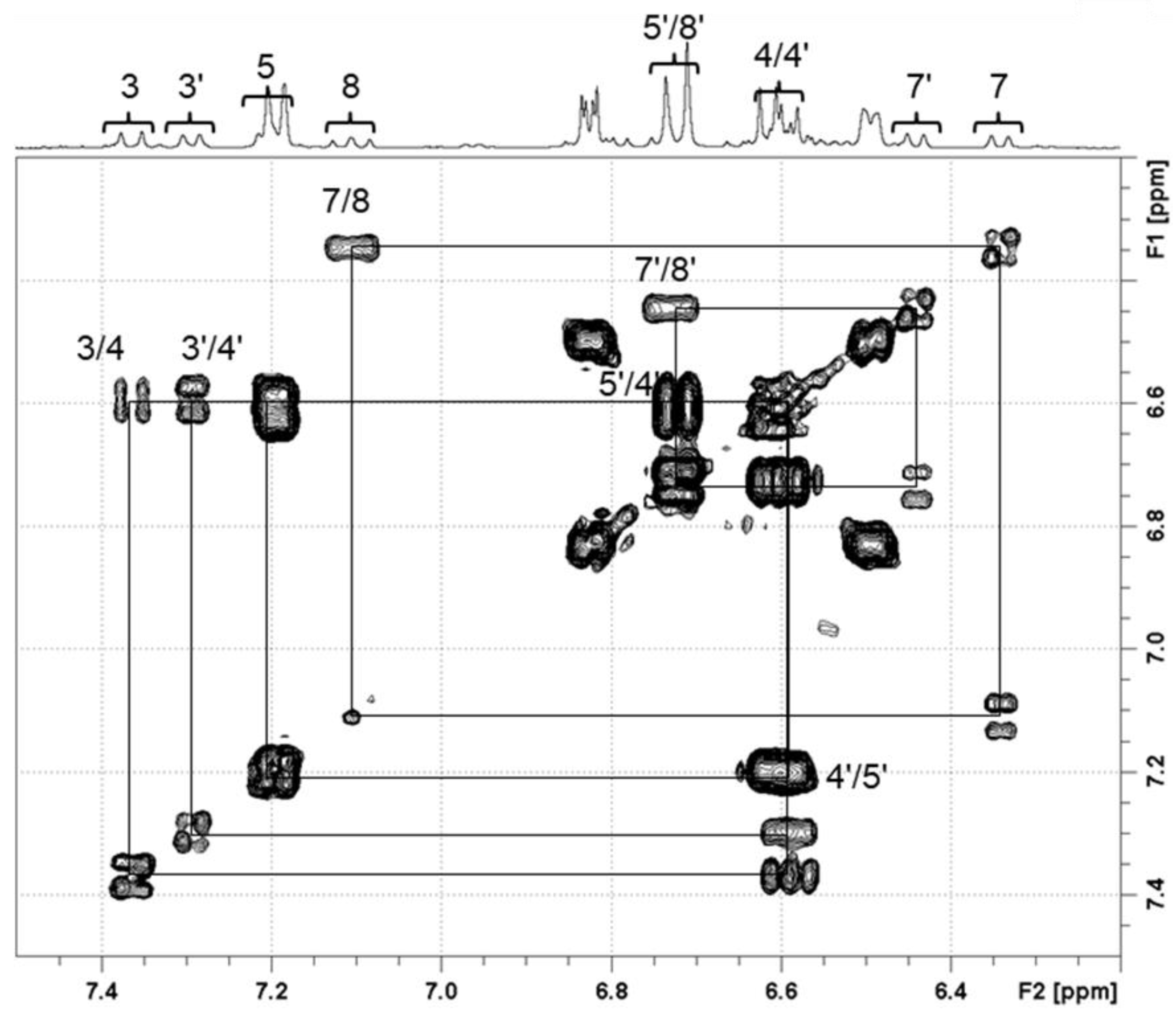
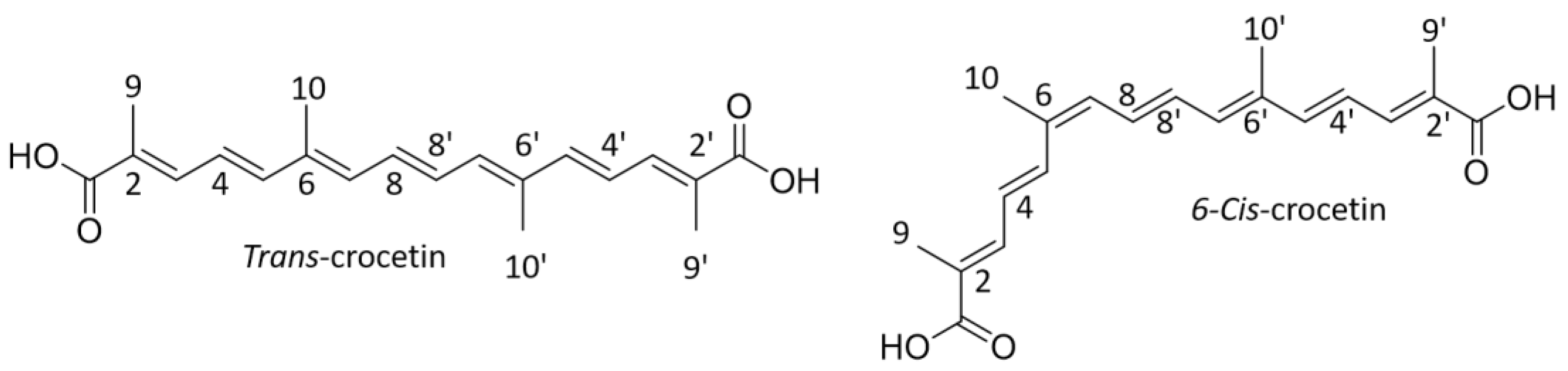


| Compounds | Tentative Identification | Retention Time (min) | Parent Ion m/z (Negative Mode) | Product Ions m/z (Negative Mode) | Maximum Wavelength (nm) |
|---|---|---|---|---|---|
| Peak 1 | Trans-crocetin | 11.75 | 327.16 | 239.14; 283.05 | 255; 440 |
| Peak 2 | Unknown (Supposed 6-cis-crocetin) | 13.25 | 327.16 | 239.14; 283.05 | 255; 320; 440 |
| trans-Isomer | cis-Isomer | |||
|---|---|---|---|---|
| Proton | δ (ppm) | J (Hz) | δ (ppm) | J (Hz) |
| 3 | 7.21 | d (11.4) | 7.39 | d (11.9) |
| 3′ | 7.31 | d (11.9) | ||
| 4 | 6.61 | dd (15.0, 11.4) | 6.58 | dd (11.9, 14.1) |
| 4′ | ||||
| 5 | 6.73 | d (15.0) | 7.19 | n.o. |
| 5′ | 6.60 | n.o. | ||
| 7 | 6.49 | dd (7.9, 3.0) | 6.33 | d (11.9) |
| 7′ | 6.43 | d (11.9) | ||
| 8 | 6.84 | dd (7.9, 3.0) | 7.10 | dd (11.9, 14.0) |
| 8′ | 6.72 | n.o. | ||
| 9 | 1.91 | s | 1.92 | s |
| 9′ | ||||
| 10 | 1.97 | s | 1.97 | s |
| 10′ | ||||
| Linearity and Sensitivity | |||||
|---|---|---|---|---|---|
| Linear Equation | Regression Coefficient (r2) | LOD (ng/mL) | LOQ (ng/mL) | ||
| y = 0.241 x − 0.031 | 0.9988 | 0.09 | 0.3 | ||
| Precision and accuracy | |||||
| Nominal concentration (ng/mL) | Concentration found (ng/mL) | Precision (%) | Accuracy (%) | ||
| Intra-day | Inter-day | Intra-day | Inter-day | ||
| 3.0 | 3.1 +/− 0.2 | 2.3 | 5.7 | −0.5 to 13.4 | 6.0 |
| 16.0 | 15.1 +/− 0.6 | 3.4 | 4.1 | −3.5 to −8.9 | −5.6 |
| 29.0 | 27.0 +/− 0.8 | 2.7 | 3.1 | −5.2 to −10.0 | −7.9 |
| Recovery and matrix effect | |||||
| Recovery | Matrix effect | ||||
| Nominal concentration (ng/mL) | Mean +/− SD | RSD | Nominal concentration (ng/mL) | Mean +/− SD | RSD |
| 3.0 | 106.0 +/− 6.0 | 5.7 | 1.4 | 93.4 ± 2.6 | 2.8 |
| 16.0 | 94.4 +/− 3.8 | 4.1 | 14.0 | 96.6 ± 1.1 | 1.1 |
| 29.0 | 92.1 +/− 2.9 | 3.1 | |||
| Stability | |||||
| Conditions | Spiked concentration (ng/mL) | Measured concentration (ng/mL) | Precision (RSD %) | Accuracy (RE %) | |
| Temperature (°C) | Time | ||||
| Room Temperature | 4 h | 14 | 13.2 ± 0.1 | 0.5% | −8.5% |
| 4 | 24 h | 14.3 ± 0.8 | 5.6% | −0.6% | |
| −20 | 1 month | 13.3 ± 0.5 | 3.6% | −7.3% | |
| 3 Freeze–thaw | 12.6 ± 0.3 | 2.0% | −12.4% | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vignault, A.; Vaysse, C.; Bertand, K.; Krisa, S.; Courtois, A.; Moras, B.; Richard, T.; Gaudout, D.; Pourtau, L. Characterization of Crocetin Isomers in Serum Samples via UHPLC-DAD-MS/MS and NMR after Saffron Extract (Safr’Inside™) Consumption. Metabolites 2024, 14, 190. https://doi.org/10.3390/metabo14040190
Vignault A, Vaysse C, Bertand K, Krisa S, Courtois A, Moras B, Richard T, Gaudout D, Pourtau L. Characterization of Crocetin Isomers in Serum Samples via UHPLC-DAD-MS/MS and NMR after Saffron Extract (Safr’Inside™) Consumption. Metabolites. 2024; 14(4):190. https://doi.org/10.3390/metabo14040190
Chicago/Turabian StyleVignault, Adeline, Carole Vaysse, Karène Bertand, Stéphanie Krisa, Arnaud Courtois, Benjamin Moras, Tristan Richard, David Gaudout, and Line Pourtau. 2024. "Characterization of Crocetin Isomers in Serum Samples via UHPLC-DAD-MS/MS and NMR after Saffron Extract (Safr’Inside™) Consumption" Metabolites 14, no. 4: 190. https://doi.org/10.3390/metabo14040190
APA StyleVignault, A., Vaysse, C., Bertand, K., Krisa, S., Courtois, A., Moras, B., Richard, T., Gaudout, D., & Pourtau, L. (2024). Characterization of Crocetin Isomers in Serum Samples via UHPLC-DAD-MS/MS and NMR after Saffron Extract (Safr’Inside™) Consumption. Metabolites, 14(4), 190. https://doi.org/10.3390/metabo14040190






