Mapping the Metabolic Characteristics and Perturbation of Adult Casper Zebrafish by Ambient Mass Spectrometry Imaging
Abstract
1. Introduction
2. Materials and Methods
2.1. Zebrafish and Reagents
2.2. Sample Preparation
2.3. AFADESI-MSI Analysis
2.4. LC-MS/MS Analysis
2.5. Data Processing
3. Results
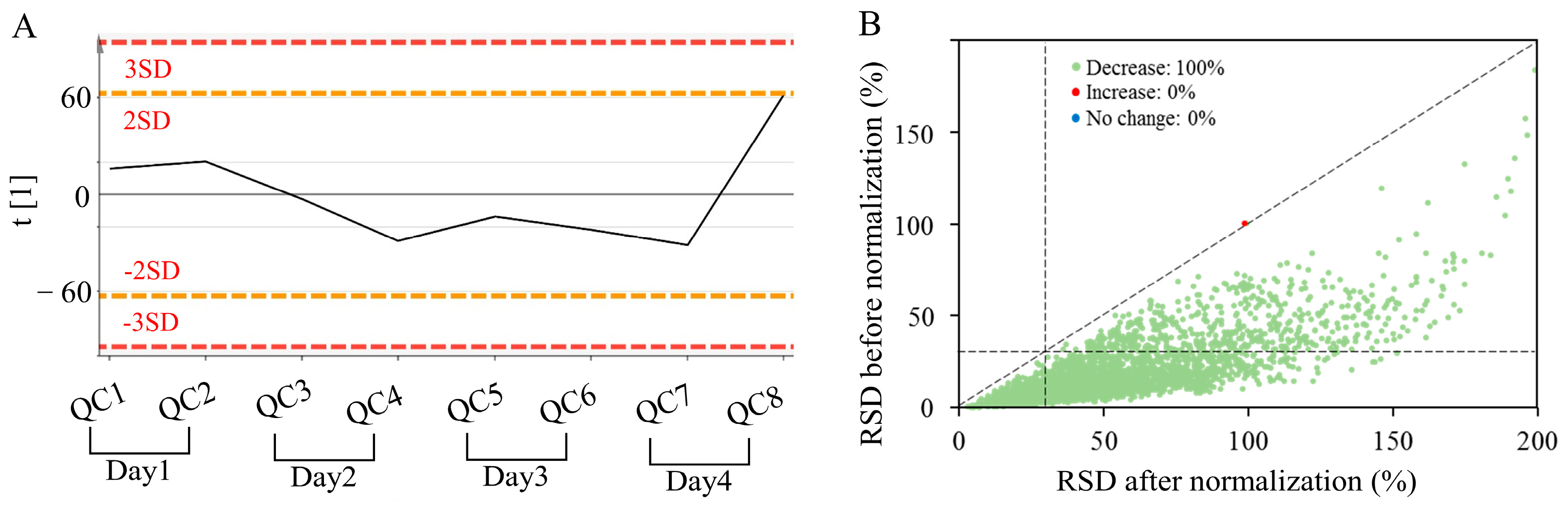
3.1. Data Quality Control
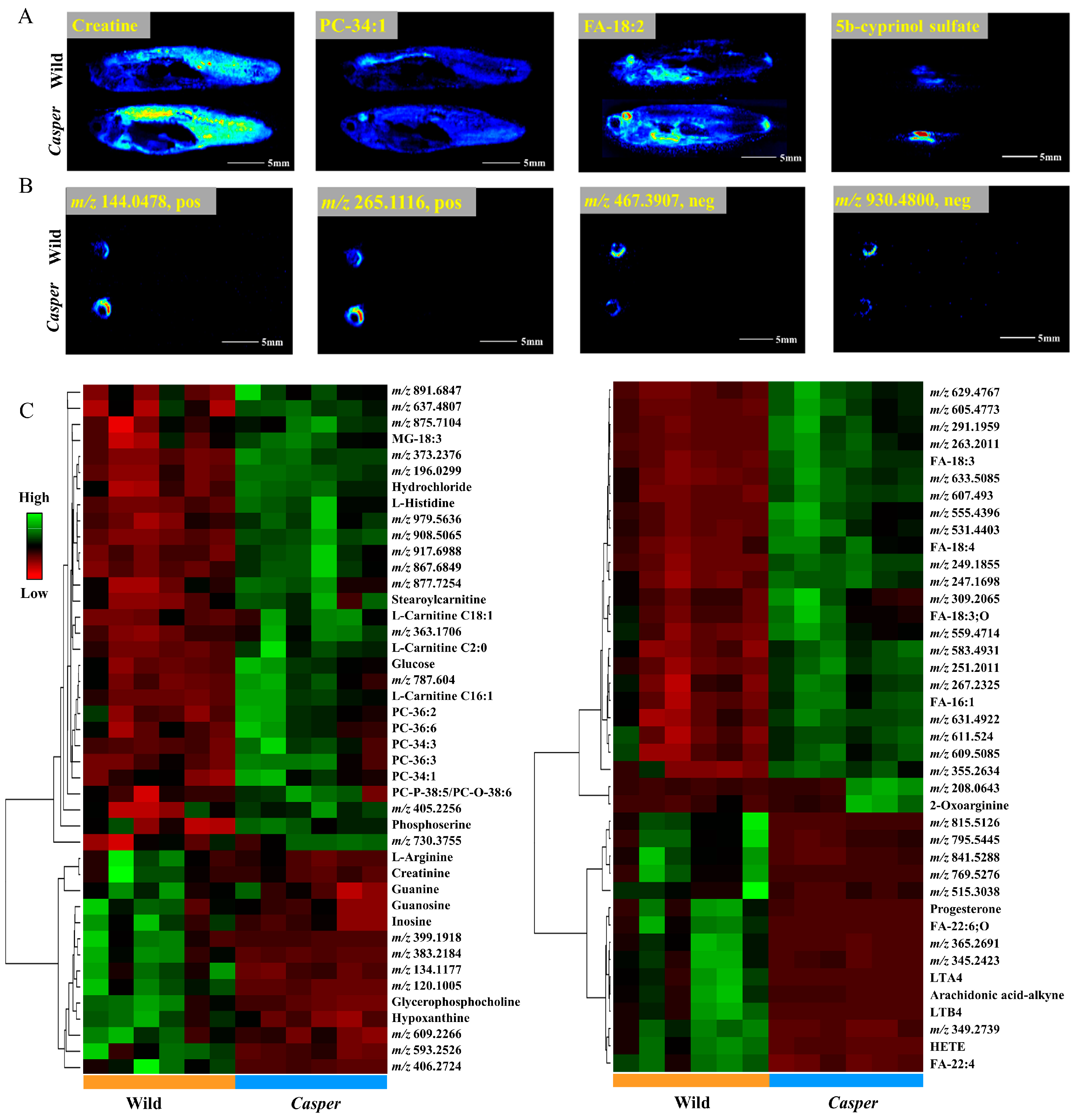
3.2. Spatial Metabolome of Casper Zebrafish
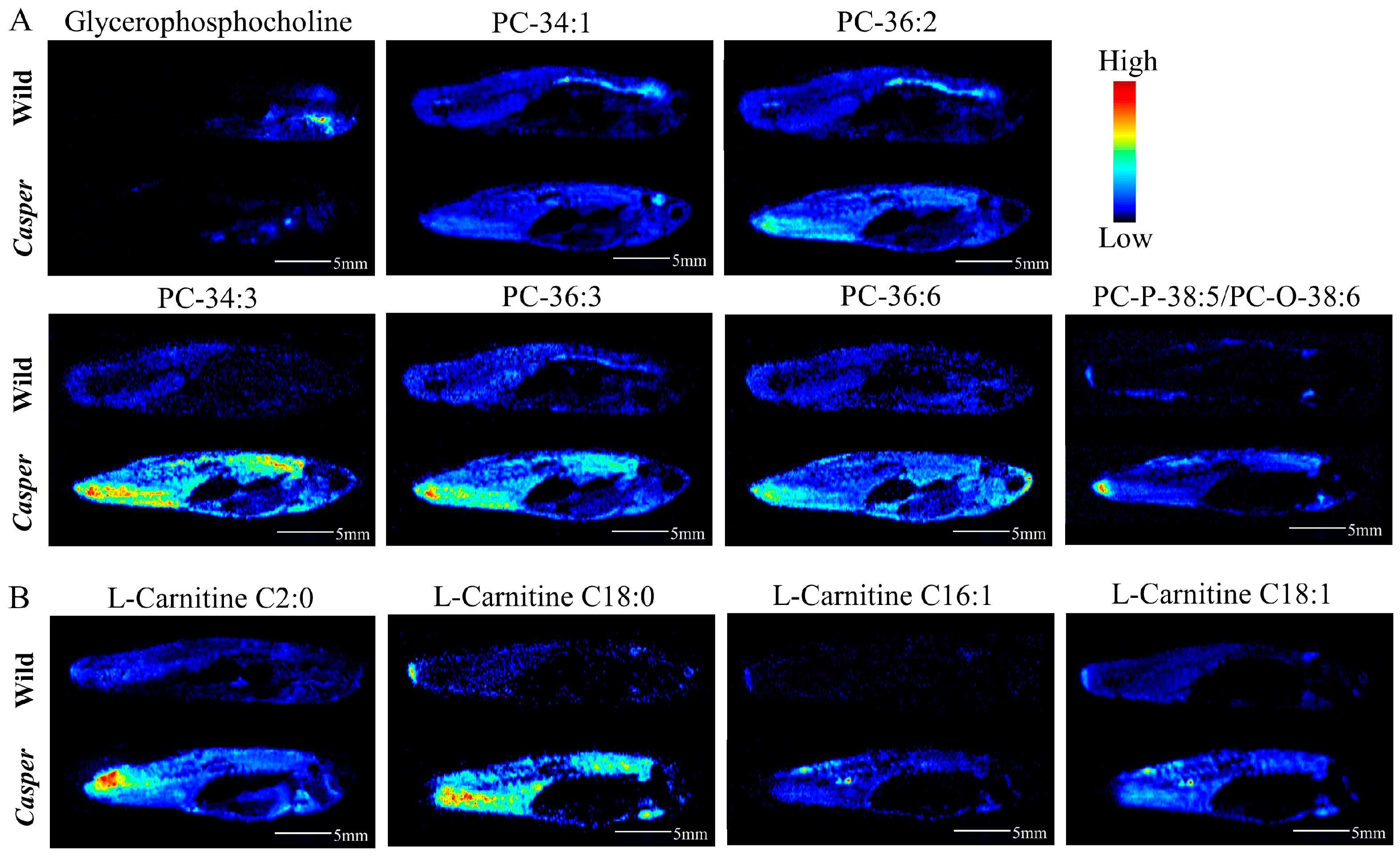
3.3. Spatial Metabolome Differentials between Casper and Wild-Type Zebrafish
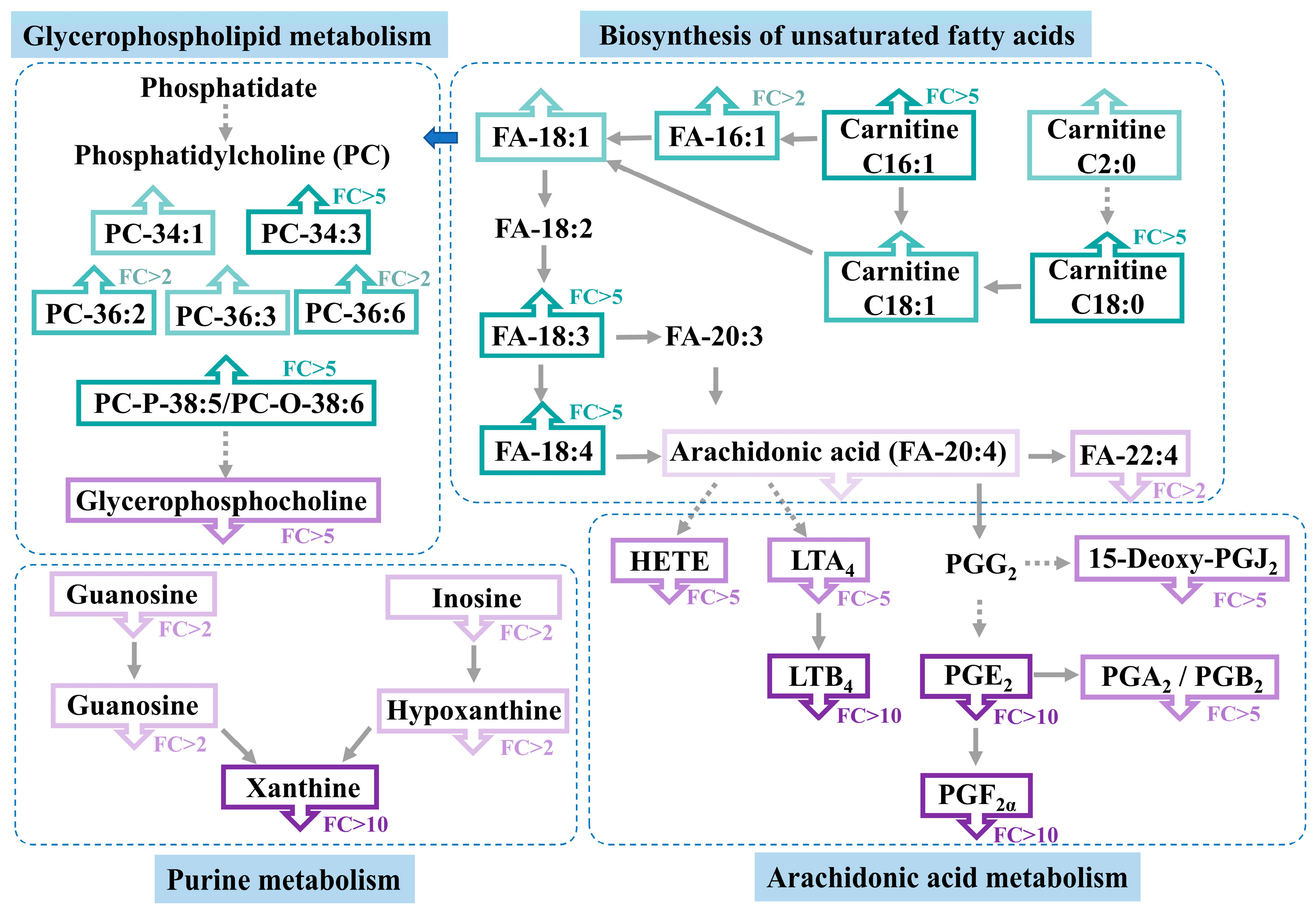
3.4. Altered Metabolic Pathway Analysis in Casper Zebrafish
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Astone, M.; Dankert, E.N.; Alam, S.K.; Hoeppner, L.H. Fishing for cures: The alLURE of using zebrafish to develop precision oncology therapies. NPJ. Precis. Oncol. 2017, 1, 39. [Google Scholar] [CrossRef]
- Heckler, K.; Kroll, J. Zebrafish as a Model for the Study of Microvascular Complications of Diabetes and Their Mechanisms. Int. J. Mol. Sci. 2017, 18, 2002. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Yang, Z.; Huang, Z.; Li, L.; Huang, J.; Chen, J.; Ni, R.; Luo, L.; He, J. Rngtt governs biliary-derived liver regeneration initiation by transcriptional regulation of mTORC1 and Dnmt1 in zebrafish. Hepatology 2023, 78, 167–178. [Google Scholar] [CrossRef] [PubMed]
- Jessie, V.H.; Lies, D.G.; Eline, D.; Lieve, M. The zebrafish as a gerontology model in nervous system aging, disease, and repair. Ageing Res. Rev. 2015, 24, 358–368. [Google Scholar]
- White, R.; Rose, K.; Zon, L. Zebrafish cancer: The state of the art and the path forward. Nat. Rev. Cancer 2013, 13, 624–636. [Google Scholar] [CrossRef] [PubMed]
- Tang, Q.; Moore, J.C.; Ignatius, M.S.; Tenente, I.M.; Hayes, M.N.; Garcia, E.G.; Yordán, N.T.; Bourque, C.; He, S.; Blackburn, J.S.; et al. Imaging tumour cell heterogeneity following cell transplantation into optically clear immune-deficient zebrafish. Nat. Commun. 2016, 7, 10358. [Google Scholar] [CrossRef]
- Silja, H.; Kajan, R.; Erin, L.; Emily, K.; Isabella, K.; Nathaniel, R.C.; Elizabeth, P.; Amy, M.; Charles, K.; Ellen, V.R.; et al. A quantitative system for studying metastasis using transparent zebrafish. Cancer Res. 2015, 75, 4272–4282. [Google Scholar]
- Yan, C.; Do, D.; Yang, Q.Q.; Brunson, D.C.; Rawls, J.F.; Langenau, D.M. Single-cell imaging of human cancer xenografts using adult immunodeficient zebrafish. Nat. Protoc. 2020, 15, 3105–3128. [Google Scholar] [CrossRef]
- Staal, F.J.T.; Spaink, H.P.; Fibbe, W.E. Visualizing Human Hematopoietic Stem Cell Trafficking In Vivo Using a Zebrafish Xenograft Model. Stem Cells Dev. 2016, 25, 360–365. [Google Scholar] [CrossRef]
- Kettleborough, R.N.W.; Busch-Nentwich, E.M.; Harvey, S.A.; Dooley, C.M.; Bruijn, E.D.; Eeden, F.V.; Sealy, I.; White, R.J.; Herd, C.; Nijman, I.J.; et al. A systematic genome-wide analysis of zebrafish protein-coding gene function. Nature 2013, 496, 494–497. [Google Scholar] [CrossRef]
- Lucitt, M.B.; Price, T.S.; Pizarro, A.; Wu, W.C.; Yocum, A.K.; Seiler, C.; Pack, M.A.; Blair, I.A.; Fitzgerald, G.A.; Grosser, T. Analysis of the zebrafish proteome during embryonic development. Mol. Cell. Proteom. 2008, 7, 981–994. [Google Scholar] [CrossRef] [PubMed]
- Shaik, A.A.; Wee, S.; Li, R.H.X.; Li, Z.; Carney, T.J.; Mathavan, S.; Gunaratne, J. Functional mapping of the zebrafish early embryo proteome and transcriptome. J. Proteome Res. 2014, 13, 5536–5550. [Google Scholar] [CrossRef] [PubMed]
- Bian, C.; Chen, W.; Ruan, Z.; Hu, Z.; Huang, Y.; Lv, Y.; Xu, T.; Li, J.; Shi, Q.; Ge, W. Genome and Transcriptome Sequencing of casper and roy Zebrafish Mutants Provides Novel Genetic Clues for Iridophore Loss. Int. J. Mol. Sci. 2020, 21, 2385. [Google Scholar] [CrossRef] [PubMed]
- Stutts, W.L.; Knuth, M.M.; Ekelöf, M.; Mahapatra, D.; Kullman, S.W.; Muddiman, D.C. Methods for Cryosectioning and Mass Spectrometry Imaging of Whole-Body Zebrafish. J. Am. Soc. Mass Spectrom. 2020, 31, 768–772. [Google Scholar] [CrossRef] [PubMed]
- Liang, X.P.; Cao, S.X.; Xie, P.S.; Hu, X.D.; Lin, Y.S.; Liang, J.H.; Zhang, S.Q.; Xian, B.; Cao, H.; Luan, T.Q.; et al. Three-Dimensional Imaging of Whole-Body Zebrafish Revealed Lipid Disorders Associated with Niemann-Pick Disease Type C1. Anal. Chem. 2021, 93, 8178–8187. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Xie, Q.; Du, M.; Huang, Y.; Chen, Y.; Chen, D.; Xu, Y.; Xu, H. Sample preparation optimization of insects and zebrafish for whole-body mass spectrometry imaging. Anal. Bioanal. Chem. 2022, 414, 4777–4790. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.L.; Li, T.; Song, X.; Huang, L.; Zang, Q.; Xu, J.; Bi, N.; Jiao, G.; Hao, Y.; Chen, Y. Spatially resolved metabolomics to discover tumor-associated metabolic alterations. Proc. Natl. Acad. Sci. USA 2019, 116, 52–57. [Google Scholar] [CrossRef]
- Sun, Y.; Zhou, Z. Spatially Resolved Metabolomics Method for Mapping the Global Molecular Landscape of Whole-Body Zebrafish (Danio rerio) Using Ambient Mass Spectrometry Imaging. Anal. Chem. 2023, 95, 9164–9172. [Google Scholar] [CrossRef] [PubMed]
- White, R.M.; Sessa, A.; Burke, C.; Bowman, T.; LeBlanc, J.; Ceol, C.; Bourque, C.; Dovey, M.; Goessling, W.; Burns, C.E.; et al. Transparent adult zebrafish as a tool for in vivo transplantation analysis. Cell Stem Cell 2008, 2, 183–189. [Google Scholar] [CrossRef]
- Wang, H.; Xu, J.; Chen, Y.; Zhang, R.; He, J.; Wang, Z.; Zan, Q.; Wei, J.; Song, Y.; Abliz, Z. Optimization and Evaluation Strategy of Esophageal Tissue Preparation Protocols for Metabolomics by LC–MS. Anal. Chem. 2016, 88, 3459–3464. [Google Scholar] [CrossRef]
- He, J.; Sun, C.; Li, T.; Luo, Z.; Huang, L.; Song, X.; Li, X.; Abliz, Z. A Sensitive and Wide Coverage Ambient Mass Spectrometry Imaging Method for Functional Metabolites Based Molecular Histology. Adv. Sci. 2018, 5, 1800250. [Google Scholar] [CrossRef] [PubMed]
- Pang, X.C.; Gao, S.S.; Ga, M.; Zhang, J.; Luo, Z.; Chen, Y.; Zhang, R.; He, J. Mapping Metabolic Networks in the Brain by Ambient Mass Spectrometry Imaging and Metabolomics. Anal. Chem. 2021, 93, 6746–6754. [Google Scholar] [CrossRef] [PubMed]
- Huo, M.L.; Wang, Z.H.; Fu, W.Q.; Tian, L.; Li, W.; Zhou, Z.; Chen, Y.; Wei, J.; Abliz, Z. Spatially Resolved Metabolomics Based on Air-Flow-Assisted Desorption Electrospray Ionization-Mass Spectrometry Imaging Reveals Region-Specific Metabolic Alterations in Diabetic Encephalopathy. J. Proteome Res. 2021, 20, 3567–3579. [Google Scholar] [CrossRef] [PubMed]
- Shen, X.T.; Gong, X.Y.; Cai, Y.; Guo, Y.P.; Tu, J.; Li, H.; Zhang, T.; Wang, J.L.; Xue, F.Z.; Zhu, Z.J. Normalization and integration of large-scale metabolomics data using support vector regression. Metabolomics 2016, 12, 89. [Google Scholar] [CrossRef]
- Beirl, A.J.; Linbo, T.H.; Cobb, M.J.; Cooper, C.D. oca2 regulation of chromatophore differentiation and number is cell type specific in zebrafish. Pigm. Cell Melanoma Res. 2013, 27, 178–189. [Google Scholar] [CrossRef] [PubMed]
- Gur, D.; Palmer, B.A.; Leshem, B.; Oron, D.; Fratzl, P.; Weiner, S.; Addadi, L. The mechanism of color change in the Neon tetra fish: A light-induced tunable photonic crystal Array. Angew. Chem. Int. Ed. 2015, 54, 12426–12430. [Google Scholar] [CrossRef] [PubMed]
- Salis, P.; Lorin, T.; Laudet, V.; Frédérich, B. Magic traits in magic fish: Understanding color pattern evolution using reef fish. Trends Genet. 2019; 35, 265–278. [Google Scholar]
- Jordan, T.M.; Partridge, J.C.; Roberts, N.W. Disordered animal multilayer reflectors and the localization of light. J. R. Soc. Interface 2014, 11, 20140948. [Google Scholar] [CrossRef]
- Ligon, R.A.; McCartney, K.L. Biochemical regulation of pigment motility in vertebrate chromatophores: A review of physiological color change mechanisms. Curr. Zool. 2016, 62, 237–252. [Google Scholar] [CrossRef]
- Gur, D.; Nicolas, J.D.; Brumfeld, V.; Bar-Elli, O.; Oron, D.; Levkowitz, G. The Dual Functional Reflecting Iris of the Zebrafish. Adv. Sci. 2018, 5, 1800338. [Google Scholar] [CrossRef]
- Zhu, X.W.; Hao, R.J.; Tian, C.X.; Zhang, J.P.; Zhu, C.H.; Li, G.L. Integrative Transcriptomics and Metabolomics Analysis of Body Color Formation in the Leopard Coral Grouper (Plectropomus leopardus). Front. Mar. Sci. 2021, 8, 726102. [Google Scholar] [CrossRef]
- Zhao, H.J.; Xu, J.K.; Yan, Z.H.; Ren, H.Q.; Zhang, Y. Microplastics enhance the developmental toxicity of synthetic phenolic antioxidants by disturbing the thyroid function and metabolism in developing zebrafish. Environ. Int. 2020, 140, 10570. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, Z.; Sun, Y.; Yang, J.; Abliz, Z. Mapping the Metabolic Characteristics and Perturbation of Adult Casper Zebrafish by Ambient Mass Spectrometry Imaging. Metabolites 2024, 14, 204. https://doi.org/10.3390/metabo14040204
Zhou Z, Sun Y, Yang J, Abliz Z. Mapping the Metabolic Characteristics and Perturbation of Adult Casper Zebrafish by Ambient Mass Spectrometry Imaging. Metabolites. 2024; 14(4):204. https://doi.org/10.3390/metabo14040204
Chicago/Turabian StyleZhou, Zhi, Yue Sun, Ji Yang, and Zeper Abliz. 2024. "Mapping the Metabolic Characteristics and Perturbation of Adult Casper Zebrafish by Ambient Mass Spectrometry Imaging" Metabolites 14, no. 4: 204. https://doi.org/10.3390/metabo14040204
APA StyleZhou, Z., Sun, Y., Yang, J., & Abliz, Z. (2024). Mapping the Metabolic Characteristics and Perturbation of Adult Casper Zebrafish by Ambient Mass Spectrometry Imaging. Metabolites, 14(4), 204. https://doi.org/10.3390/metabo14040204




