Nuclear Magnetic Resonance (NMR) Metabolomics: Current Applications in Equine Health Assessment
Abstract
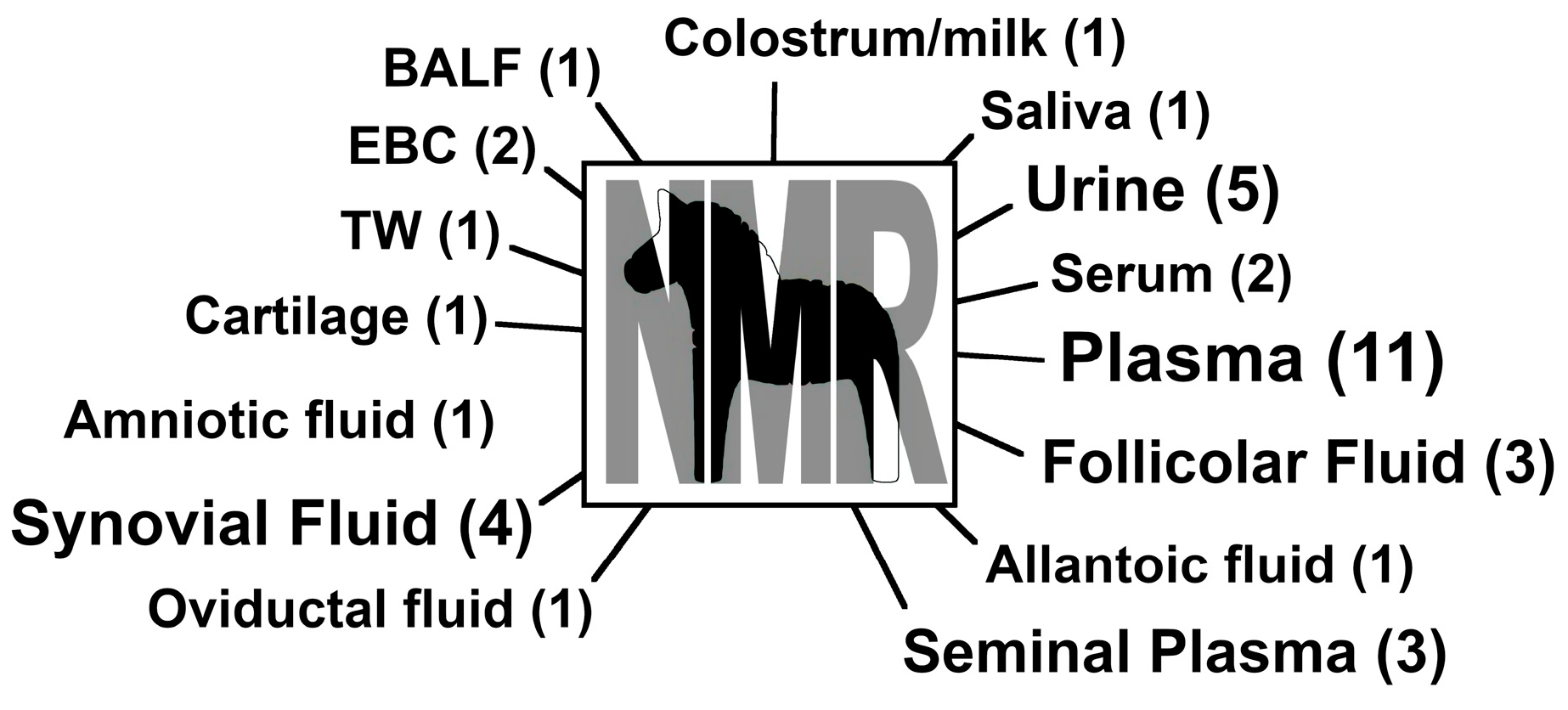
:1. Introduction
2. Methods
3. Results
3.1. Metabolomics in Respiratory Diseases
3.2. Metabolomics in the Musculoskeletal System
3.3. Metabolomics in Sport Medicine
3.4. Metabolomics in Reproduction
3.5. Metabolomics in Nutrition
4. Discussion and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhang, A.; Sun, H.; Wang, P.; Han, Y.; Wang, X. Modern Analytical Techniques in Metabolomics Analysis. Analyst 2012, 137, 293–300. [Google Scholar] [CrossRef] [PubMed]
- Nicholson, J.K.; Lindon, J.C. Systems Biology Metabonomics. Nature 2008, 455, 1054–1056. [Google Scholar] [CrossRef]
- Rivera-Velez, S.M.; Navas, J.; Villarino, N.F. Applying Metabolomics to Veterinary Pharmacology and Therapeutics. J. Vet. Pharmacol. Ther. 2021, 44, 855–869. [Google Scholar] [CrossRef] [PubMed]
- Laus, F.; Gialletti, R.; Bazzano, M.; Laghi, L.; Dini, F.; Marchegiani, A. Synovial Fluid Metabolome Can Differentiate between Healthy Joints and Joints Affected by Osteoarthritis in Horses. Metabolites 2023, 13, 913. [Google Scholar] [CrossRef] [PubMed]
- Seyfinejad, B.; Nemutlu, E.; Taghizadieh, A.; Khoubnasabjafari, M.; Ozkan, S.A.; Jouyban, A. Biomarkers in Exhaled Breath Condensate as Fingerprints of Asthma, Chronic Obstructive Pulmonary Disease and Asthma–Chronic Obstructive Pulmonary Disease Overlap: A Critical Review. Biomark. Med. 2024, 17, 811–837. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Chen, B.; Fang, Z.; Leng, Y.-F.; Wang, D.-W.; Chen, F.-Q.; Xu, X.; Sun, Z.-L. Metabolomics in the Development and Progression of Rheumatoid Arthritis: A Systematic Review. Jt. Bone Spine 2020, 87, 425–430. [Google Scholar] [CrossRef] [PubMed]
- Ribbenstedt, A.; Ziarrusta, H.; Benskin, J.P. Development, Characterization and Comparisons of Targeted and Non-Targeted Metabolomics Methods. PLoS ONE 2018, 13, e0207082. [Google Scholar] [CrossRef] [PubMed]
- Klein, D.J.; Anthony, T.G.; McKeever, K.H. Metabolomics in Equine Sport and Exercise. J. Anim. Physiol. Anim. Nutr. 2021, 105, 140–148. [Google Scholar] [CrossRef] [PubMed]
- Stringer, K.A.; McKay, R.T.; Karnovsky, A.; Quémerais, B.; Lacy, P. Metabolomics and Its Application to Acute Lung Diseases. Front. Immunol. 2016, 7, 159205. [Google Scholar] [CrossRef]
- Chan, E.C.Y.; Pasikanti, K.K.; Nicholson, J.K. Global Urinary Metabolic Profiling Procedures Using Gas Chromatography–Mass Spectrometry. Nat. Protoc. 2011, 6, 1483–1499. [Google Scholar] [CrossRef]
- Li, B.; He, X.; Jia, W.; Li, H. Novel Applications of Metabolomics in Personalized Medicine: A Mini-Review. Molecules 2017, 22, 1173. [Google Scholar] [CrossRef] [PubMed]
- Wishart, D.S. Emerging Applications of Metabolomics in Drug Discovery and Precision Medicine. Nat. Rev. Drug Discov. 2016, 15, 473–484. [Google Scholar] [CrossRef] [PubMed]
- Laghi, L.; Picone, G.; Capozzi, F. Nuclear Magnetic Resonance for Foodomics beyond Food Analysis. Trends Anal. Chem. 2014, 59, 93–102. [Google Scholar] [CrossRef]
- Park, J.A.; Pham, D.; Yalamanchili, S.; Twardus, S.; Suzuki, K. Developing Technologies and Areas of Interest in Lung Cancer Screening Adjuncts. J. Thorac. Dis. 2024, 16, 1552–1564. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Gu, Y.; Chu, Q.; Wang, X.; Ding, Y.; Qin, X.; Liu, T.; Wang, S.; Liu, X.; Wang, B.; et al. Gut Microbiota and Metabolites as Predictors of Biologics Response in Inflammatory Bowel Disease: A Comprehensive Systematic Review. Microbiol. Res. 2024, 282, 127660. [Google Scholar] [CrossRef] [PubMed]
- Bradburn, E.; Conde-Agudelo, A.; Roberts, N.W.; Villar, J.; Papageorghiou, A.T. Accuracy of Prenatal and Postnatal Biomarkers for Estimating Gestational Age: A Systematic Review and Meta-Analysis. eClinicalMedicine 2024, 70, 102498. [Google Scholar] [CrossRef] [PubMed]
- Nikulkova, M.; Abdrabou, W.; Carlton, J.M.; Idaghdour, Y. Exploiting Integrative Metabolomics to Study Host–Parasite Interactions in Plasmodium Infections. Trends Parasitol. 2024, 40, 313–323. [Google Scholar] [CrossRef] [PubMed]
- Kaszak, I.; Witkowska-Piłaszewicz, O.; Domrazek, K.; Jurka, P. The Novel Diagnostic Techniques and Biomarkers of Canine Mammary Tumors. Vet. Sci. 2022, 9, 526. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, A.; Kar, I.; Patra, A.K. Understanding Anthelmintic Resistance in Livestock Using “Omics” Approaches. Environ. Sci. Pollut. Res. 2023, 30, 125439–125463. [Google Scholar] [CrossRef]
- Barosova, R.; Baranovicova, E.; Hanusrichterova, J.; Mokra, D. Metabolomics in Animal Models of Bronchial Asthma and Its Translational Importance for Clinics. Int. J. Mol. Sci. 2023, 25, 459. [Google Scholar] [CrossRef]
- Moitra, S.; Bandyopadhyay, A.; Lacy, P. Metabolomics of Respiratory Diseases. In Handbook of Experimental Pharmacology; Springer: Cham, Switzerland, 2023; Volume 277, pp. 339–365. [Google Scholar] [CrossRef]
- Bazzano, M.; Laghi, L.; Zhu, C.; Magi, G.E.; Tesei, B.; Laus, F. Respiratory Metabolites in Bronchoalveolar Lavage Fluid (BALF) and Exhaled Breath Condensate (EBC) Can Differentiate Horses Affected by Severe Equine Asthma from Healthy Horses. BMC Vet. Res. 2020, 16, 233. [Google Scholar] [CrossRef] [PubMed]
- Bazzano, M.; Laghi, L.; Zhu, C.; Magi, G.E.; Serri, E.; Spaterna, A.; Tesei, B.; Laus, F. Metabolomics of Tracheal Wash Samples and Exhaled Breath Condensates in Healthy Horses and Horses Affected by Equine Asthma. J. Breath. Res. 2018, 12, 46015. [Google Scholar] [CrossRef] [PubMed]
- Bizzarri, M.; Fuso, A.; Dinicola, S.; Cucina, A.; Bevilacqua, A. Pharmacodynamics and Pharmacokinetics of Inositol(s) in Health and Disease. Expert. Opin. Drug Metab. Toxicol. 2016, 12, 1181–1196. [Google Scholar] [CrossRef] [PubMed]
- Gilmore, A.P.; Burridge, K. Regulation of Vinculin Binding to Talin and Actin by Phosphatidyl-Inositol-4-5-Bisphosphate. Nature 1996, 381, 531–535. [Google Scholar] [CrossRef] [PubMed]
- Hallman, M.; Spragg, R.; Harrell, J.H.; Moser, K.M.; Gluck, L. Evidence of Lung Surfactant Abnormality in Respiratory Failure. Study of Bronchoalveolar Lavage Phospholipids, Surface Activity, Phospholipase Activity, and Plasma Myoinositol. J. Clin. Investig. 1982, 70, 673–683. [Google Scholar] [CrossRef] [PubMed]
- Howlett, A.; Ohlsson, A.; Plakkal, N. Inositol in Preterm Infants at Risk for or Having Respiratory Distress Syndrome. Cochrane Database Syst. Rev. 2019, 2019, CD000366. [Google Scholar] [CrossRef] [PubMed]
- Montuschi, P.; Paris, D.; Melck, D.; Lucidi, V.; Ciabattoni, G.; Raia, V.; Calabrese, C.; Bush, A.; Barnes, P.J.; Motta, A. NMR Spectroscopy Metabolomic Profiling of Exhaled Breath Condensate in Patients with Stable and Unstable Cystic Fibrosis. Thorax 2012, 67, 222–228. [Google Scholar] [CrossRef] [PubMed]
- Lacitignola, L.; Fanizzi, F.P.; Francioso, E.; Crovace, A. 1H NMR Investigation of Normal and Osteo-Arthritic Synovial Fluid in the Horse. Vet. Comp. Orthop. Traumatol. 2008, 21, 85–88. [Google Scholar] [CrossRef]
- Anderson, J.R.; Phelan, M.M.; Clegg, P.D.; Peffers, M.J.; Rubio-Martinez, L.M. Synovial Fluid Metabolites Differentiate between Septic and Nonseptic Joint Pathologies. J. Proteome Res. 2018, 17, 2735–2743. [Google Scholar] [CrossRef]
- Keller, M.D.; Pollitt, C.C.; Marx, U.C. Nuclear Magnetic Resonance-Based Metabonomic Study of Early Time Point Laminitis in an Oligofructose-Overload Model. Equine Vet. J. 2011, 43, 737–743. [Google Scholar] [CrossRef]
- Graham, R.J.T.Y.; Anderson, J.R.; Phelan, M.M.; Cillan-Garcia, E.; Bladon, B.M.; Taylor, S.E. Metabolomic Analysis of Synovial Fluid from Thoroughbred Racehorses Diagnosed with Palmar Osteochondral Disease Using Magnetic Resonance Imaging. Equine Vet. J. 2020, 52, 384–390. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Che, N.; Xu, L.; Zhang, Q.; Wang, Q.; Tan, W.; Zhang, M. LC-MS-Based Serum Metabolomics Reveals a Distinctive Signature in Patients with Rheumatoid Arthritis. Clin. Rheumatol. 2018, 37, 1493–1502. [Google Scholar] [CrossRef]
- Takahashi, S.; Saegusa, J.; Sendo, S.; Okano, T.; Akashi, K.; Irino, Y.; Morinobu, A. Glutaminase 1 Plays a Key Role in the Cell Growth of Fibroblast-like Synoviocytes in Rheumatoid Arthritis. Arthritis Res. Ther. 2017, 19, 76. [Google Scholar] [CrossRef] [PubMed]
- Le Moyec, L.; Robert, C.; Triba, M.N.; Billat, V.L.; Mata, X.; Schibler, L.; Barrey, E. Protein Catabolism and High Lipid Metabolism Associated with Long-Distance Exercise Are Revealed by Plasma NMR Metabolomics in Endurance Horses. PLoS ONE 2014, 9, e90730. [Google Scholar] [CrossRef] [PubMed]
- Le Moyec, L.; Robert, C.; Triba, M.N.; Bouchemal, N.; Mach, N.; Rivière, J.; Zalachas-Rebours, E.; Barrey, E. A First Step toward Unraveling the Energy Metabolism in Endurance Horses: Comparison of Plasma Nuclear Magnetic Resonance Metabolomic Profiles before and after Different Endurance Race Distances. Front. Mol. Biosci. 2019, 6, 448755. [Google Scholar] [CrossRef]
- Luck, M.M.; Le Moyec, L.; Barrey, E.; Triba, M.N.; Bouchemal, N.; Savarin, P.; Robert, C. Energetics of Endurance Exercise in Young Horses Determined by Nuclear Magnetic Resonance Metabolomics. Front. Physiol. 2015, 6, 138314. [Google Scholar] [CrossRef]
- Park, J.W.; Kim, K.H.; Kim, S.; So, J.R.; Cho, B.W.; Song, K.D. Comparative Metabolomic Analysis in Horses and Functional Analysis of Branched Chain (Alpha) Keto Acid Dehydrogenase Complex in Equine Myoblasts under Exercise Stress. J. Anim. Sci. Technol. 2022, 64, 800–811. [Google Scholar] [CrossRef] [PubMed]
- Bazzano, M.; Laghi, L.; Zhu, C.; Lotito, E.; Sgariglia, S.; Tesei, B.; Laus, F. Exercise Induced Changes in Salivary and Serum Metabolome in Trained Standardbred, Assessed by 1H-NMR. Metabolites 2020, 10, 298. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.; Faillace, V.; Laus, F.; Bazzano, M.; Laghi, L. Characterization of Trotter Horses Urine Metabolome by Means of Proton Nuclear Magnetic Resonance Spectroscopy. Metabolomics 2018, 14, 106. [Google Scholar] [CrossRef]
- Laghi, L.; Zhu, C.; Campagna, G.; Rossi, G.; Bazzano, M.; Laus, F. Probiotic Supplementation in Trained Trotter Horses: Effect on Blood Clinical Pathology Data and Urine Metabolomic Assessed in Field. J. Appl. Physiol. 2018, 125, 654–660. [Google Scholar] [CrossRef]
- Jang, H.J.; Kim, D.M.; Kim, K.B.; Park, J.W.; Choi, J.Y.; Hyeog Oh, J.; Song, K.D.; Kim, S.; Cho, B.W. Analysis of Metabolomic Patterns in Thoroughbreds before and after Exercise. Asian-Australas. J. Anim. Sci. 2017, 30, 1633. [Google Scholar] [CrossRef]
- Mach, N.; Ramayo-Caldas, Y.; Clark, A.; Moroldo, M.; Robert, C.; Barrey, E.; López, J.M.; Le Moyec, L. Understanding the Response to Endurance Exercise Using a Systems Biology Approach: Combining Blood Metabolomics, Transcriptomics and MiRNomics in Horses. BMC Genom. 2017, 18, 187. [Google Scholar] [CrossRef] [PubMed]
- Ueda, T.; Tozaki, T.; Nozawa, S.; Kinoshita, K.; Gawahara, H. Identification of Metabolomic Changes in Horse Plasma after Racing by Liquid Chromatography-High Resolution Mass Spectrometry as a Strategy for Doping Testing. J. Equine Sci. 2019, 30, 55–61. [Google Scholar] [CrossRef] [PubMed]
- Berton, R.; Conceição, M.S.; Libardi, C.A.; Canevarolo, R.R.; Gáspari, A.F.; Chacon-Mikahil, M.P.T.; Zeri, A.C.; Cavaglieri, C.R. Metabolic Time-Course Response after Resistance Exercise: A Metabolomics Approach. J. Sports Sci. 2017, 35, 1211–1218. [Google Scholar] [CrossRef] [PubMed]
- Sutton, E.E.; Coll, M.R.; Deuster, P.A. Ingestion of Tyrosine: Effects on Endurance, Muscle Strength, and Anaerobic Performance. Int. J. Sport. Nutr. Exerc. Metab. 2005, 15, 173–185. [Google Scholar] [CrossRef] [PubMed]
- Kalaiselvi, G.; Tirumurugaan, K.G.; Vijayarani, K.; Dhinakar Raj, G.; Baranidharan, G.R.; Sumedha, B. Livestock Metabolomics—A overview. Health Sci. 2019, 11, 1972–1980. [Google Scholar]
- Gérard, N.; Loiseau, S.; Duchamp, G.; Seguin, F. Analysis of the Variations of Follicular Fluid Composition during Follicular Growth and Maturation in the Mare Using Proton Nuclear Magnetic Resonance (1H NMR). Reproduction 2002, 124, 241–248. [Google Scholar] [CrossRef] [PubMed]
- Gérard, N.; Fahiminiya, S.; Grupen, C.G.; Nadal-Desbarats, L. Reproductive Physiology and Ovarian Folliculogenesis Examined via 1H-NMR Metabolomics Signatures: A Comparative Study of Large and Small Follicles in Three Mammalian Species (Bos Taurus, Sus Scrofa Domesticus and Equus Ferus Caballus). Omics J. Integr. Biol. 2014, 19, 31–40. [Google Scholar] [CrossRef] [PubMed]
- Fernández-hernández, P.; Sánchez-calabuig, M.J.; García-marín, L.J.; Bragado, M.J.; Gutiérrez-adán, A.; Millet, Ó.; Bruzzone, C.; González-fernández, L.; Macías-garcía, B. Study of the Metabolomics of Equine Preovulatory Follicular Fluid: A Way to Improve Current In Vitro Maturation Media. Animals 2020, 10, 883. [Google Scholar] [CrossRef] [PubMed]
- Magistrini, M.; Seguin, F.; Beau, P.; Akoka, S.; Le Pape, A.; Palmer, E. 1H Nuclear Magnetic Resonance Analysis of Stallion Genital Tract Fluids and Seminal Plasma: Contribution of the Accessory Sex Glands to the Ejaculate. Biol. Reprod. 1995, 52, 599–607. [Google Scholar] [CrossRef]
- Bazzano, M.; Zhu, C.; Laus, F.; Di Giambattista, A.; Laghi, L. Exploring the Metabolome of Seminal Plasma in Two Different Horse Types Light versus Draft Stallions Enhanced Reader. Reprod. Domest. Anim. 2022, 58, 109–116. [Google Scholar] [CrossRef] [PubMed]
- Catalán, J.; Yánez-Ortiz, I.; Martínez-Rodero, I.; Mateo-Otero, Y.; Nolis, P.; Yeste, M.; Miró, J. Comparison of the Metabolite Profile of Donkey and Horse Seminal Plasma and Its Relationship with Sperm Viability and Motility. Res. Vet. Sci. 2023, 165, 105046. [Google Scholar] [CrossRef] [PubMed]
- González-Fernández, L.; Sánchez-Calabuig, M.J.; Calle-Guisado, V.; García-Marín, L.J.; Bragado, M.J.; Fernández-Hernández, P.; Gutiérrez-Adán, A.; Macías-García, B. Stage-Specific Metabolomic Changes in Equine Oviductal Fluid: New Insights into the Equine Fertilization Environment. Theriogenology 2020, 143, 35–43. [Google Scholar] [CrossRef] [PubMed]
- Beachler, T.M.; Scott Bailey, C.; Gracz, H.S.; Morgan, D.R.; Von Dollen, K.A.; Ellis, K.E.; Gadsby, J.E.; Lyle, S.K. Metabolomic Profile of Allantoic and Amniotic Fluid in Late-Term Gestational Mares Characterized by 1H-Nuclear Magnetic Resonance Spectroscopy. J. Equine Vet. Sci. 2020, 94, 103235. [Google Scholar] [CrossRef] [PubMed]
- Beachler, T.M.; Gracz, H.S.; Morgan, D.R.; Bembenek Bailey, S.A.; Borst, L.; Ellis, K.E.; Von Dollen, K.A.; Lyle, S.K.; Nebel, A.; Andrews, N.C.; et al. Plasma Metabolomic Profiling of Healthy Pregnant Mares and Mares with Experimentally Induced Placentitis. Equine Vet. J. 2021, 53, 85–93. [Google Scholar] [CrossRef] [PubMed]
- Frédé, F.; Courant, F.; Antignac, J.-P.; Monteau, F.; Le Bizec, B. Metabolomics as a Potential New Approach for Investigating Human Reproductive Disorders. J. Proteome Res. 2013, 12, 2914–2920. [Google Scholar] [CrossRef] [PubMed]
- Engel, K.M.; Baumann, S.; Rolle-Kampczyk, U.; Schiller, J.; von Bergen, M.; Grunewald, S. Metabolomic Profiling Reveals Correlations between Spermiogram Parameters and the Metabolites Present in Human Spermatozoa and Seminal Plasma. PLoS ONE 2019, 14, e0211679. [Google Scholar] [CrossRef] [PubMed]
- Paiva, C.; Amaral, A.; Rodriguez, M.; Canyellas, N.; Correig, X.; Ballescà, J.L.; Ramalho-Santos, J.; Oliva, R. Identification of Endogenous Metabolites in Human Sperm Cells Using Proton Nuclear Magnetic Resonance (1H-NMR) Spectroscopy and Gas Chromatography-Mass Spectrometry (GC-MS). Andrology 2015, 3, 496–505. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.X.; Wu, Y.; Chen, H.G.; Duan, P.; Wang, L.; Shen, H.Q.; Lu, W.Q.; Sun, B.; Wang, Q.; Zhang, B.; et al. Seminal Plasma Metabolome in Relation to Semen Quality and Urinary Phthalate Metabolites among Chinese Adult Men. Environ. Int. 2019, 129, 354–363. [Google Scholar] [CrossRef]
- Kosior, M.A.; Pagano, N.; Staropoli, A.; De Canditiis, C.; Longobardi, V.; Zullo, G.; Vinale, F.; Gasparrini, B. 142 Metabolomic Analysis of Fresh and Frozen Bovine Seminal Plasma: A Preliminary Study. Reprod. Fertil. Dev. 2020, 32, 197. [Google Scholar] [CrossRef]
- Kumar, A.; Kroetsch, T.; Blondin, P.; Anzar, M. Fertility-Associated Metabolites in Bull Seminal Plasma and Blood Serum: 1 H Nuclear Magnetic Resonance Analysis. Mol. Reprod. Dev. 2015, 82, 123–131. [Google Scholar] [CrossRef] [PubMed]
- Sousa, S.D.; Lucini, L.; Ajmone-Marsan, P.; van Tilburg, M.F.; Moura, A.A.; Arlindo Moura, C.A. Untargeted Metabolomic Profiling of Accessory Sex Gland Fluid from Morada Nova Rams. Mol. Reprod. Dev. 2020, 87, 409–418. [Google Scholar] [CrossRef]
- Mateo-Otero, Y.; Fernández-López, P.; Delgado-Bermúdez, A.; Nolis, P.; Roca, J.; Miró, J.; Barranco, I.; Yeste, M. Metabolomic fingerprinting of pig seminal plasma identifies in vivo fertility biomarkers. J. Anim. Sci. Biotechnol. 2021, 12, 113. [Google Scholar] [CrossRef] [PubMed]
- Tenori, L.; Santucci, C.; Meoni, G.; Morrocchi, V.; Matteucci, G.; Luchinat, C. NMR Metabolomic Fingerprinting Distinguishes Milk from Different Farms. Food Res. Int. 2018, 113, 131–139. [Google Scholar] [CrossRef] [PubMed]
- Antequera, T.; Caballero, D.; Grassi, S.; Uttaro, B.; Perez-Palacios, T. Evaluation of Fresh Meat Quality by Hyperspectral Imaging (HSI), Nuclear Magnetic Resonance (NMR) and Magnetic Resonance Imaging (MRI): A Review. Meat Sci. 2021, 172, 108340. [Google Scholar] [CrossRef] [PubMed]
- Ashokan, M.; Rana, E.; Sneha, K.; Namith, C.; Naveen Kumar, G.S.; Azharuddin, N.; Elango, K.; Jeyakumar, S.; Ramesha, K.P. Metabolomics—A Powerful Tool in Livestock Research. Anim. Biotechnol. 2023, 34, 3237–3249. [Google Scholar] [CrossRef] [PubMed]
- Leng, J.; Mcnally, S.; Walton, G.; Swann, J.; Proudman, C.; Argo, C.; Emery, S.; La Ragione, R.; Eustace, R. Hay versus Haylage: Forage Type Influences the Equine Urinary Metabonome and Faecal Microbiota. Equine Vet. J. 2021, 54, 614–625. [Google Scholar] [CrossRef] [PubMed]
- De Castro Duarte, P.; Ribeiro, R.M.; Machado, A.R.; Faleiros, R.R.; Pimenta, L.P.; de Souza Filho, J.D. Metabolic Profile Changes in Mangalarga Marchador Horses Subjected to A Hypercaloric Diet Evaluated by Proton NMR Spectroscopy. J. Equine Vet. Sci. 2021, 103, 103684. [Google Scholar] [CrossRef] [PubMed]
- Laus, F.; Laghi, L.; Bazzano, M.; Cifone, M.G.; Cinque, B.; Yang, Y.; Marchegiani, A. Donkey Colostrum and Milk: How Dietary Probiotics Can Affect Metabolomic Profile, Alkaline Sphingomyelinase and Alkaline Phosphatase Activity. Metabolites 2023, 13, 622. [Google Scholar] [CrossRef]
- Frank, N. Equine Metabolic Syndrome. Vet. Clin. N. Am. Equine Pract. 2011, 27, 73–92. [Google Scholar] [CrossRef]

| Respiratory System | |||
|---|---|---|---|
| Study | Type of Sample | Study Population | Summary of Main Results |
| [22] | BALF EBC | 6 horses with severe asthma 6 healthy horses | Increase in BALF of asthmatic horses: formate, isopropanol. Decrease in BALF of asthmatic horses: myo-inositol, glycerol. Increase in EBC of asthmatic horses: ethanol, methanol. |
| [23] | TW EBC | 6 horses with asthma 6 healthy horses | Increase in TW of asthmatic horses: histamine, glutamate, valine, leucine, isoleucine. Decrease in TW of asthmatic horses: ascorbate, O-phosphocholine, methylamine, dimethylamine, propylene glycol. No differences between groups for EBC. |
| Musculoskeletal System | |||
|---|---|---|---|
| Study | Type of Sample | Study Population | Summary of Main Results |
| [4] | Synovial Fluid | 11 horses with osteoarthritis 8 healthy | Increased in sick horses: 1,3-dihydroxyacetone, 2-hydroxyisobutyrate, creatinine. Decreased in sick horses: 2-hydroxyisovalerate, 3-hydroxybutyrate, 3-methyl-2-oxovalerate, arginine, asparagine, glutamine, glycine, methionine, phenylalanine, tryptophan, tyrosine. |
| [29] | Synovial fluid | 11 horses with osteoarthritis 8 healthy | Increased in affected horse: alanine, acetate, N-acetyle, pyruvate, citrate, creatine, creatinine, choline, glycerol, lactate, glucose. |
| [30] | Plasma | 5 horses before and after laminitis induction using oligofructose | Increased after laminitis induction: oligofructose, lactate, 3-hydroxy-butyrate, glycine, alanine, glutamine. Decreased after laminitis induction: acetate, citric acid, LDLs. |
| [31] | Synovial Fluid | 7 horses with septic joint disease 4 horses with nonseptic joint disease | Higher levels in septic: glycylproline. Higher levels in nonseptic: acetate, alanine, citrate, creatine phosphate, creatinine, glucose, glutamate, glutamine, glycine, phenylalanine, pyruvate, valine. |
| [32] | Synovial fluid | 15 horses affected by palmar osteochondral disease 14 healthy | No statistically significant differences but reduced concentration of glucose and lactate in sick horses. |
| Exercise | |||
|---|---|---|---|
| Study | Type of Sample | Study Population | Summary of Main Results |
| [35] | Plasma | 29 horses before and after 160 km endurance race | Increased after exercise: lactate, creatine, urea, several amino acids such as valine, leucine, tyrosine. Decreased after exercise: fatty acid chains of lipids, glucosamine. |
| [36] | Plasma | 40 horses before and after 90, 120, and 160 km endurance races | Beta hydroxybutyrate, lactate, acetate, acetoacetate, glutamate, glutamine, creatine, urea, and some other metabolites resulted to be different in function of race distance. |
| [37] | Plasma | Horses (young and experienced) before and after endurance race | Increased after exercise: -hydroxybutyrate, glycerol, choline, lactate, fumarate, creatine, creatinine, phenylalanine, tyrosine, glutamate, 2-hydroxy-3-methylvalerate. Decreased after exercise: Glucose. Some metabolites resulted to be different among young vs. experienced. |
| [38] | Muscle biopsy and plasma | 3 Thoroughbred and 3 Jeju horses before and after exercise | Higher concentrations of aspartate, leucine, isoleucine, and lysine in the skeletal muscle of Jeju horses than in Thoroughbred horses. Thoroughbred horses had higher levels of alanine and methionine before exercise, whereas postexercise, lysine levels were increased. |
| [39] | Serum Saliva | 12 Standardbred horses before/after full speed exercise | SERUM: Increased after exercise: alanine, citrate, fumarate, glycerol, lactate, leucine, pyruvate, succinate. Decreased after exercise: 3-hydroxybutyrate, 2-hydroxyisobutyrate, acetoacetate, acetone, asparagine, aspartate, creatine, dimethyl sulfone, dimethylglycine, glutamine, histidine, mannose, methanol, myo-inositol, proline, threonine, trimethylamine, valine. SALIVA: Increased after exercise: creatine, ornithine, phenylalanine, sarcosine, tyrosine. Decreased after exercise: 4-aminobutyrate, betaine, fumarate, galactose, malate, malonate, methanol, pyruvate, succinate. |
| [40] | Urine | 10 trotter horse before and after training section | Increased after exercise: 3-indoxylsulfate, threonine. Decreased after exercise: quinic acid. |
| [41] | Urine | 10 Standardbred horses before and after exercise/probiotic supplementation | Increased after exercise because of probiotic supplementation: dimethyl sulfone, pantothenate, taurine. Decreased after exercise because of probiotic supplementation: 2-hydroxyisovalerate, trans-aconitate, citrate, P-cresol sulfate, glycine. |
| [42] | Plasma, muscle, urine | 3 Thoroughbred after 30 min exercise bout | Alteration of 35 metabolites related to amino acid and energy metabolism. |
| [43] | Plasma | 10 Arabian horses before and after a 160 km endurance competition | 11 metabolites linked to energetic metabolism resulted in being different. |
| [44] | Plasma | 30 samples before and after jockeyed race * | Levels of inosine, xanthosine, uric acid, and allantoin, which are induced by extensive exercise, were significantly increased after racing activity in comparison with resting. |
| Study | Type of Sample | Study Population | Summary of Main Results |
|---|---|---|---|
| [48] | Follicular fluid at various physiological stages of follicle development plasma | Pony mares * | The intrafollicular contents of alanine and lipoproteins decreased in dominant follicles during growth. Strong correlation between the intrafollicular content of alanine and circulating estradiol. |
| [49] | Follicular fluid from large and small follicles | 5 samples from large and 5 samples from small follicles of cow, pigs, and mares | Several differences between large and small follicles for equine, including amino acids, creatine, trimethylamine, and glucose, that resulted in being higher in large follicles. Great differences between cow and mares, indicating species-specific differences in follicular metabolism. |
| [50] | Preovulatory follicular fluid | 6 mares | A total of 9 of the 22 metabolites identified are not currently included in the most commonly used media for equine in vitro maturation of oocytes. |
| [51] | Seminal Plasma | 3 stallions | 9 metabolites from ampulla and bulbourethral glands. |
| [52] | Seminal Plasma | 6 American Quarter Horse (AQH) and 6 Italian Draft Horse (IDH) stallions | Higher in IDH compared to AQH: 2-hydroxyisobutyrate, 3-hydroxybutyrate, cystine, citrate, glucose, fumarate, hippurate sarcosine, and tyrosine. Lower in IDH compared to AQH: isopropanol, isovalerate. |
| [53] | Seminal Plasma | 18 donkeys and 18 horses | 18 metabolites (amino acids, amino acid derivates. and alcohols) resulted in being different between horses and donkeys. |
| [54] | Oviductal fluid | 5 samples per- and post-ovulatory | A total of 18 metabolites were identified with the highest concentrations for lactate, myoinositol, creatine, alanine, and carnitine. Fumarate and glycine were higher in post-ovulatory samples. |
| [55] | Fetal fluids | 7 mares between 270 and 295 days of gestation | Allantoic fluid contained a higher concentration of betaine, creatine, creatinine, citrate, histidine, nitrophenol, tryptophan, and methylhistidine and a lower concentration of lactate compared with amniotic fluid. |
| [56] | Plasma | 10 mares before and after placentitis induction. Five control mares | Four hours post-inoculation, a significant increase was detected in alanine, phenylalanine, histidine, pyruvate, citrate, glucose, creatine, glycolate, hippurate, lactate, and 3-hydroxyisobutyrate. On day 4, a significant reduction in alanine, phenylalanine, histidine, tyrosine, pyruvate, citrate, glycolate, lactate, and dimethylsulfone was seen in infected mares compared with the controls. |
| Study | Type of Sample | Study Population | Summary of Main Results |
|---|---|---|---|
| [68] | Urine Feces | 20 ponies divided into two groups: hay vs. haylage feeding | The urinary excretion of hippurate was greater in the hay-fed ponies, while ethyl-glucoside excretion was higher in the haylage-fed ponies. |
| [69] | Serum | Nine Mangalarga Marchador horses before, during, and after a 5-month hypercaloric diet | Choline resulted to be higher in horse after receiving hypercaloric diet. |
| [70] | Colostrum and milk | 20 jennies before and after probiotics supplementation | Higher concentration in milk after probiotics supplementation: phenylglycine, glucose-1-phosphate, glucose, dimethylamine, 2-oxoisocaproate, glutamine, butyrate, isovalerate, caprylate. Lower concentration in milk after probiotics supplementation: 4-pyridoxate, lactose, O-phosphocholine, ethanol. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Laus, F.; Bazzano, M.; Spaterna, A.; Laghi, L.; Marchegiani, A. Nuclear Magnetic Resonance (NMR) Metabolomics: Current Applications in Equine Health Assessment. Metabolites 2024, 14, 269. https://doi.org/10.3390/metabo14050269
Laus F, Bazzano M, Spaterna A, Laghi L, Marchegiani A. Nuclear Magnetic Resonance (NMR) Metabolomics: Current Applications in Equine Health Assessment. Metabolites. 2024; 14(5):269. https://doi.org/10.3390/metabo14050269
Chicago/Turabian StyleLaus, Fulvio, Marilena Bazzano, Andrea Spaterna, Luca Laghi, and Andrea Marchegiani. 2024. "Nuclear Magnetic Resonance (NMR) Metabolomics: Current Applications in Equine Health Assessment" Metabolites 14, no. 5: 269. https://doi.org/10.3390/metabo14050269
APA StyleLaus, F., Bazzano, M., Spaterna, A., Laghi, L., & Marchegiani, A. (2024). Nuclear Magnetic Resonance (NMR) Metabolomics: Current Applications in Equine Health Assessment. Metabolites, 14(5), 269. https://doi.org/10.3390/metabo14050269






