Discovery of Delirium Biomarkers through Minimally Invasive Serum Molecular Fingerprinting
Abstract
:1. Introduction
2. Materials and Methods
2.1. Population
2.2. Demographics and Other Clinical Characteristics
2.3. Collection of Biological Samples
2.4. FTIR Spectroscopy
2.5. Spectra Pre-Processing and Processing
2.6. Other Statistical Analysis
3. Results and Discussion
3.1. General Characterization of the Population
3.2. Serum Spectral Analysis
3.2.1. Univariate Spectral Analysis
3.2.2. Multivariate Spectral Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Girard, T.D.; Pandharipande, P.P.; Ely, E.W. Delirium in the Intensive Care Unit. Crit. Care 2008, 12, S3. [Google Scholar] [CrossRef] [PubMed]
- Stollings, J.L.; Kotfis, K.; Chanques, G.; Pun, B.T.; Pandharipande, P.P.; Ely, E.W. Delirium in Critical Illness: Clinical Manifestations, Outcomes, and Management. Intensive Care Med. 2021, 47, 1089–1103. [Google Scholar] [CrossRef] [PubMed]
- Cavallazzi, R.; Saad, M.; Marik, P.E. Delirium in the ICU: An Overview. Ann. Intensive Care 2012, 2, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Leslie, D.L.; Marcantonio, E.R.; Zhang, Y.; Leo-Summers, L.; Inouye, S.K. One-Year Health Care Costs Associated with Delirium in the Elderly. Arch. Intern. Med. 2008, 168, 27–32. [Google Scholar] [CrossRef] [PubMed]
- Inouye, S.K.; Westendorp, R.G.J.; Saczynski, J.S. Delirium in Elderly People. Lancet 2014, 383, 911–922. [Google Scholar] [CrossRef]
- Wilke, V.; Sulyok, M.; Stefanou, M.I.; Richter, V.; Bender, B.; Ernemann, U.; Ziemann, U.; Malek, N.; Kienzle, K.; Klein, C.; et al. Delirium in Hospitalized COVID-19 Patients: Predictors and Implications for Patient Outcome. PLoS ONE 2022, 17, e0278214. [Google Scholar] [CrossRef] [PubMed]
- Page, V.J.; Ely, E.W. Delirium in Critical Care, 1st ed.; Cambridge University Press: Cambridge, UK, 2011; ISBN 978-0-521-13253-4. [Google Scholar]
- Otani, K.; Fukushima, H.; Matsuishi, K. COVID-19 Delirium and Encephalopathy: Pathophysiology Assumed in the First 3 Years of the Ongoing Pandemic. Brain Disord. 2023, 10, 100074. [Google Scholar] [CrossRef] [PubMed]
- Giménez-Esparza Vich, C.; Alcántara Carmona, S.; García Sánchez, M. Delirium in COVID-19. Practical Aspects of a Frequent Association. Med. Intensiva 2022, 46, 336–340. [Google Scholar] [CrossRef]
- Pun, B.T.; Badenes, R.; Heras La Calle, G.; Orun, O.M.; Chen, W.; Raman, R.; Simpson, B.G.K.; Wilson-Linville, S.; Hinojal Olmedillo, B.; Vallejo de la Cueva, A.; et al. Prevalence and Risk Factors for Delirium in Critically Ill Patients with COVID-19 (COVID-D): A Multicentre Cohort Study. Lancet Respir. Med. 2021, 9, 239–250. [Google Scholar] [CrossRef]
- Kotfis, K.; Williams Roberson, S.; Wilson, J.E.; Dabrowski, W.; Pun, B.T.; Ely, E.W. COVID-19: ICU Delirium Management during SARS-CoV-2 Pandemic. Crit. Care 2020, 24, 176. [Google Scholar] [CrossRef]
- Garcez, F.B.; Aliberti, M.J.R.; Poco, P.C.E.; Hiratsuka, M.; Takahashi, S.d.F.; Coelho, V.A.; Salotto, D.B.; Moreira, M.L.V.; Jacob-Filho, W.; Avelino-Silva, T.J. Delirium and Adverse Outcomes in Hospitalized Patients with COVID-19. J. Am. Geriatr. Soc. 2020, 68, 2440–2446. [Google Scholar] [CrossRef] [PubMed]
- Gravante, F.; Giannarelli, D.; Pucci, A.; Gagliardi, A.M.; Mitello, L.; Montagna, A.; Latina, R. Prevalence and Risk Factors of Delirium in the Intensive Care Unit: An Observational Study. Nurs. Crit. Care 2021, 26, 156–165. [Google Scholar] [CrossRef] [PubMed]
- Helms, J.; Kremer, S.; Merdji, H.; Schenck, M.; Severac, F.; Clere-Jehl, R.; Studer, A.; Radosavljevic, M.; Kummerlen, C.; Monnier, A.; et al. Delirium and Encephalopathy in Severe COVID-19: A Cohort Analysis of ICU Patients. Crit. Care 2020, 24, 491. [Google Scholar] [CrossRef]
- Wilcox, M.E.; Shankar-Hari, M.; McAuley, D.F. Delirium in COVID-19: Can We Make the Unknowns Knowns? Intensive Care Med. 2021, 47, 1144–1147. [Google Scholar]
- Dias, R.; Caldas, J.P.; Silva-Pinto, A.; Costa, A.; Sarmento, A.; Santos, L. Delirium Severity in Critical Patients with COVID-19 from an Infectious Disease Intensive Care Unit. Int. J. Infect. Dis. 2022, 118, 109–115. [Google Scholar] [CrossRef]
- Rogers, J.P.; Chesney, E.; Oliver, D.; Pollak, T.A.; McGuire, P.; Fusar-Poli, P.; Zandi, M.S.; Lewis, G.; David, A.S. Psychiatric and Neuropsychiatric Presentations Associated with Severe Coronavirus Infections: A Systematic Review and Meta-Analysis with Comparison to the COVID-19 Pandemic. Lancet Psychiatry 2020, 7, 611–627. [Google Scholar] [CrossRef]
- Westphal, G.A.; Fernandes, R.P.; Pereira, A.B.; Moerschberger, M.S.; Pereira, M.R.; Gonçalves, A.R.R. Incidence of Delirium in Critically Ill Patients with and Without COVID-19. J. Intensive Care Med. 2023, 38, 751–759. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.H.; Lindroth, H.; Perkins, A.J.; Jamil, Y.; Wang, S.; Roberts, S.; Farber, M.; Rahman, O.; Gao, S.; Marcantonio, E.R.; et al. Delirium Incidence, Duration, and Severity in Critically Ill Patients with Coronavirus Disease 2019. Crit Care Explor. 2020, 2, E0290. [Google Scholar] [CrossRef] [PubMed]
- Wilson, J.E.; Mart, M.F.; Cunningham, C.; Shehabi, Y.; Girard, T.D.; MacLullich, A.M.J.; Slooter, A.J.C.; Ely, E.W. Delirium. Nat. Rev. 2020, 6, 90. [Google Scholar] [CrossRef]
- Hawkins, M.; Sockalingam, S.; Bonato, S.; Rajaratnam, T.; Ravindran, M.; Gosse, P.; Sheehan, K.A. A Rapid Review of the Pathoetiology, Presentation, and Management of Delirium in Adults with COVID-19. J. Psychosom. Res. 2021, 141, 110350. [Google Scholar] [CrossRef]
- Ragheb, J.; McKinney, A.; Zierau, M.; Brooks, J.; Hill-Caruthers, M.; Iskander, M.; Ahmed, Y.; Lobo, R.; Mentz, G.; Vlisides, P.E. Delirium and Neuropsychological Outcomes in Critically Ill Patients with COVID-19: A Cohort Study. BMJ Open 2021, 11, e050045. [Google Scholar] [CrossRef] [PubMed]
- Shao, S.C.; Lai, C.C.; Chen, Y.H.; Chen, Y.C.; Hung, M.J.; Liao, S.C. Prevalence, Incidence and Mortality of Delirium in Patients with COVID-19: A Systematic Review and Meta-Analysis. Age Ageing 2021, 50, 1445–1453. [Google Scholar] [CrossRef] [PubMed]
- Kotfis, K.; van Diem-Zaal, I.; Roberson, S.W.; Sietnicki, M.; van den Boogaard, M.; Shehabi, Y.; Ely, E.W. The Future of Intensive Care: Delirium Should No Longer Be an Issue. Crit. Care 2022, 26, 200. [Google Scholar] [CrossRef] [PubMed]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 5th ed.; American Psychiatric Association: Washington, DC, USA, 2013; ISBN 978-0-89042-554-1. [Google Scholar]
- Morandi, A.; Pandharipande, P.; Trabucchi, M.; Rozzini, R.; Mistraletti, G.; Trompeo, A.C.; Gregoretti, C.; Gattinoni, L.; Ranieri, M.V.; Brochard, L.; et al. Understanding International Differences in Terminology for Delirium and Other Types of Acute Brain Dysfunction in Critically Ill Patients. Intensive Care Med. 2008, 34, 1907–1915. [Google Scholar] [CrossRef] [PubMed]
- Slooter, A.J.C.; Otte, W.M.; Devlin, J.W.; Arora, R.C.; Bleck, T.P.; Claassen, J.; Duprey, M.S.; Ely, E.W.; Kaplan, P.W.; Latronico, N.; et al. Updated Nomenclature of Delirium and Acute Encephalopathy: Statement of Ten Societies. Intensive Care Med. 2020, 46, 1020–1022. [Google Scholar] [CrossRef] [PubMed]
- Dunne, S.S.; Coffey, J.C.; Konje, S.; Gasior, S.; Clancy, C.C.; Gulati, G.; Meagher, D.; Dunne, C.P. Biomarkers in Delirium: A Systematic Review. J. Psychosom. Res. 2021, 147, 110530. [Google Scholar] [CrossRef] [PubMed]
- Ely, E.W.; Bernard, G.R.; Speroff, T.; Gautam, S.; Dittus, R.; May, L.; Truman, B.; Gordon, S.; Margolin, R.; Inouye, S.K.; et al. Delirium in Mechanically Ventilated Patients: Validity and Reliability of the Confusion Assessment Method for the Intensive Care Unit (CAM-ICU). JAMA 2001, 286, 2703–2710. [Google Scholar] [CrossRef] [PubMed]
- Wass, S.; Webster, P.J.; Nair, B.R. Delirium in the Elderly: A Review. Oman Med. J. 2008, 23, 150. [Google Scholar] [PubMed]
- Bellelli, G.; Brathwaite, J.S.; Mazzola, P. Delirium: A Marker of Vulnerability in Older People. Front. Aging Neurosci. 2021, 13, 626127. [Google Scholar] [CrossRef]
- Salluh, J.I.F.; Wang, H.; Schneider, E.B.; Nagaraja, N.; Yenokyan, G.; Damluji, A.; Serafim, R.B.; Stevens, R.D. Outcome of Delirium in Critically Ill Patients: Systematic Review and Meta-Analysis. BMJ 2015, 350, h2538. [Google Scholar] [CrossRef]
- Khachaturian, A.S.; Hayden, K.M.; Devlin, J.W.; Fleisher, L.A.; Lock, S.L.; Cunningham, C.; Oh, E.S.; Fong, T.G.; Fick, D.M.; Marcantonio, E.R.; et al. International Drive to Illuminate Delirium: A Developing Public Health Blueprint for Action. Alzheimer’s Dement. 2020, 16, 711–725. [Google Scholar] [CrossRef] [PubMed]
- Dziegielewski, C.; Skead, C.; Canturk, T.; Webber, C.; Fernando, S.M.; Thompson, L.H.; Foster, M.; Ristovic, V.; Lawlor, P.G.; Chaudhuri, D.; et al. Delirium and Associated Length of Stay and Costs in Critically Ill Patients. Crit. Care Res. Pract. 2021, 2021, 6612187. [Google Scholar] [CrossRef] [PubMed]
- Xie, G.H.; Fang, X.M. Importance of Recognizing and Managing Delirium in Intensive Care Unit. Chin. J. Traumatol. 2009, 12, 370–374. [Google Scholar] [PubMed]
- Hall, R.J.; Watne, L.O.; Cunningham, E.L.; Zetterberg, H.; Shenkin, S.D.; Wyller, T.B.; MacLullich, A.M.J. CSF Biomarkers in Delirium: A Systematic Review. Int. J. Geriatr. Psychiatry 2018, 33, 1479–1500. [Google Scholar] [CrossRef] [PubMed]
- Wood, M.D.; Boyd, J.G.; Wood, N.; Frank, J.; Girard, T.D.; Ross-White, A.; Chopra, A.; Foster, D.; Griesdale, D.E.G. The Use of Near-Infrared Spectroscopy and/or Transcranial Doppler as Non-Invasive Markers of Cerebral Perfusion in Adult Sepsis Patients with Delirium: A Systematic Review. J. Intensive Care Med. 2022, 37, 408–422. [Google Scholar] [CrossRef] [PubMed]
- Lozano-Vicario, L.; García-Hermoso, A.; Cedeno-Veloz, B.A.; Fernández-Irigoyen, J.; Santamaría, E.; Romero-Ortuno, R.; Zambom-Ferraresi, F.; Sáez de Asteasu, M.L.; Muñoz-Vázquez, Á.J.; Izquierdo, M.; et al. Biomarkers of Delirium Risk in Older Adults: A Systematic Review and Meta-Analysis. Front. Aging Neurosci. 2023, 15, 1174644. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Zhang, W.; Liu, J.; Song, Y.; Liu, T.; Li, Z.; Wang, X.; Yang, N.; Li, Y.; Han, D.; et al. Metabolomic and Lipidomic Profiling of Preoperative CSF in Elderly Hip Fracture Patients with Postoperative Delirium. Front. Aging Neurosci. 2020, 12, 570210. [Google Scholar] [CrossRef] [PubMed]
- Tripp, B.A.; Dillon, S.T.; Yuan, M.; Asara, J.M.; Vasunilashorn, S.M.; Fong, T.G.; Metzger, E.D.; Inouye, S.K.; Xie, Z.; Ngo, L.H.; et al. Targeted Metabolomics Analysis of Postoperative Delirium. Sci. Rep. 2021, 11, 1521. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Zhang, Y.; Jia, P.; Wang, W.; Zhou, Q.; Sun, L.; Zhao, A.; Zhang, X.; Wang, X.; Li, Y.; et al. Preoperative Serum Metabolites Are Associated with Postoperative Delirium in Elderly Hip-Fracture Patients. J. Gerontol. Ser. A 2017, 72, 1689–1696. [Google Scholar] [CrossRef]
- Correia, M.; Lopes, J.; Silva, R.; Rosa, I.M.; Henriques, A.G.; Delgadillo, I.; Cruz e Silva, O.A.B.; Nunes, A. FTIR Spectroscopy—A Potential Tool to Identify Metabolic Changes in Dementia Patients. J. Alzheimers Neurodegener. Dis. 2016, 2, 007. [Google Scholar] [CrossRef]
- Huang, H.; Han, J.; Li, Y.; Yang, Y.; Shen, J.; Fu, Q.; Chen, Y. Early Serum Metabolism Profile of Post-Operative Delirium in Elderly Patients Following Cardiac Surgery with Cardiopulmonary Bypass. Front. Aging Neurosci. 2022, 14, 857902. [Google Scholar] [CrossRef] [PubMed]
- See, K.C. Personalizing Care for Critically Ill Adults Using Omics: A Concise Review of Potential Clinical Applications. Cells 2023, 12, 541. [Google Scholar] [CrossRef] [PubMed]
- Fonseca, T.A.H.; Von Rekowski, C.P.; Araújo, R.; Oliveira, M.C.; Justino, G.C.; Bento, L.; Calado, C.R.C. The Impact of the Serum Extraction Protocol on Metabolomic Profiling Using UPLC-MS/MS and FTIR Spectroscopy. ACS Omega 2023, 8, 20755–20766. [Google Scholar] [CrossRef] [PubMed]
- Araújo, R.; Ramalhete, L.; Ribeiro, E.; Calado, C. Plasma versus Serum Analysis by FTIR Spectroscopy to Capture the Human Physiological State. BioTech 2022, 11, 56. [Google Scholar] [CrossRef] [PubMed]
- Baker, M.J.; Trevisan, J.; Bassan, P.; Bhargava, R.; Butler, H.J.; Dorling, K.M.; Fielden, P.R.; Fogarty, S.W.; Fullwood, N.J.; Heys, K.A.; et al. Using Fourier Transform IR Spectroscopy to Analyze Biological Materials. Nat. Protoc. 2014, 9, 1771–1791. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro Da Cunha, B.; Fonseca, L.P.; Calado, C.R.C. Simultaneous Elucidation of Antibiotic Mechanism of Action and Potency with High-Throughput Fourier-Transform Infrared (FTIR) Spectroscopy and Machine Learning. Appl. Microbiol. Biotechnol. 2021, 105, 1269–1286. [Google Scholar] [CrossRef] [PubMed]
- Ramalhete, L.; Vieira, M.B.; Araújo, R.; Vigia, E.; Aires, I.; Ferreira, A.; Calado, C.R.C. Predicting Cellular Rejection of Renal Allograft Based on the Serum Proteomic Fingerprint. Int. J. Mol. Sci. 2024, 25, 3844. [Google Scholar] [CrossRef]
- Sessler, C.N.; Gosnell, M.S.; Grap, M.J.; Brophy, G.M.; O’Neal, P.V.; Keane, K.A.; Tesoro, E.P.; Elswick, R.K. The Richmond Agitation–Sedation Scale. Am. J. Respir. Crit. Care Med. 2002, 166, 1338–1344. [Google Scholar] [CrossRef] [PubMed]
- Demšar, J.; Erjavec, A.; Hočevar, T.; Milutinovič, M.; Možina, M.; Toplak, M.; Umek, L.; Zbontar, J.; Zupan, B. Orange: Data Mining Toolbox in Python. J. Mach. Learn. Res. 2013, 14, 2349–2353. [Google Scholar]
- Alzoubi, E.; Shaheen, F.; Yousef, K. Delirium Incidence, Predictors and Outcomes in the Intensive Care Unit: A Prospective Cohort Study. Int. J. Nurs. Pract. 2024, 30, e13154. [Google Scholar] [CrossRef]
- Mesa-Herrera, F.; Taoro-González, L.; Valdés-Baizabal, C.; Diaz, M.; Marín, R. Lipid and Lipid Raft Alteration in Aging and Neurodegenerative Diseases: A Window for the Development of New Biomarkers. Int. J. Mol. Sci. 2019, 20, 3810. [Google Scholar] [PubMed]
- Estes, R.E.; Lin, B.; Khera, A.; Davis, M.Y. Lipid Metabolism Influence on Neurodegenerative Disease Progression: Is the Vehicle as Important as the Cargo? Front. Mol. Neurosci. 2021, 14, 788695. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.; Wang, X.; Zhang, L.; Fang, Y.; Zheng, Q.; Liu, X.; Yu, W.; Chen, S.; Ying, J.; Hua, F. Lipid Metabolism and Storage in Neuroglia: Role in Brain Development and Neurodegenerative Diseases. Cell Biosci. 2022, 12, 106. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.; Wong, L.C.; Boland, S. Lipids as Emerging Biomarkers in Neurodegenerative Diseases. Int. J. Mol. Sci. 2024, 25, 131. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Peng, X.; Lin, X.; Deng, X.; Liu, F.; Tao, H.; Dong, R.; Wang, B.; Bi, Y. Potential Value of Serum Lipid in the Identication of Postoperative Delirium Undergoing Knee/Hip Arthroplasty: The Perioperative Neurocognitive Disorder and Biomarker Lifestyle Study. Front. Psychiatry 2022, 13, 870317. [Google Scholar] [CrossRef] [PubMed]
- Korevaar, J.C.; Van Munster, B.C.; De Rooij, S.E. Risk Factors for Delirium in Acutely Admitted Elderly Patients: A Prospective Cohort Study. BMC Geriatr. 2005, 5, 6. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Li, Y.; Zhang, Y.; Fang, S.; Xu, X.; Zhao, A.; Zhang, J.; Li, J.V.; Ma, D.; Jia, W.; et al. Post-Operative Delirium Associated with Metabolic Alterations Following Hemi-Arthroplasty in Older Patients. Age Ageing 2019, 49, 88–95. [Google Scholar] [CrossRef] [PubMed]
- Cermenati, G.; Mitro, N.; Audano, M.; Melcangi, R.C.; Crestani, M.; De Fabiani, E.; Caruso, D. Lipids in the Nervous System: From Biochemistry and Molecular Biology to Patho-Physiology. Biochim. Biophys. Acta 2015, 1851, 51–60. [Google Scholar] [CrossRef] [PubMed]
- Tracey, T.J.; Steyn, F.J.; Wolvetang, E.J.; Ngo, S.T. Neuronal Lipid Metabolism: Multiple Pathways Driving Functional Outcomes in Health and Disease. Front. Mol. Neurosci. 2018, 11, 10. [Google Scholar] [CrossRef]
- Hiller, J.K.; Jangmo, A.; Tesli, M.S.; Jaholkowski, P.P.; Hoseth, E.Z.; Steen, N.E.; Haram, M. Lipid Biomarker Research in Bipolar Disorder: A Scoping Review of Trends, Challenges, and Future Directions. Biol. Psychiatry Glob. Open Sci. 2023, 3, 594–604. [Google Scholar] [CrossRef]
- Schneider, M.; Levant, B.; Reichel, M.; Gulbins, E.; Kornhuber, J.; Müller, C.P. Lipids in Psychiatric Disorders and Preventive Medicine. Neurosci. Biobehav. Rev. 2017, 76, 336–362. [Google Scholar] [CrossRef] [PubMed]
- Müller, C.P.; Reichel, M.; Mühle, C.; Rhein, C.; Gulbins, E.; Kornhuber, J. Brain Membrane Lipids in Major Depression and Anxiety Disorders. Biochim. Biophys. Acta 2015, 1851, 1052–1065. [Google Scholar] [CrossRef] [PubMed]
- Bazinet, R.P.; Layé, S. Polyunsaturated Fatty Acids and Their Metabolites in Brain Function and Disease. Nat. Rev. Neurosci. 2014, 15, 771–785. [Google Scholar] [CrossRef] [PubMed]
- Van Munster, B.C.; Aronica, E.; Zwinderman, A.H.; Eikelenboom, P.; Cunningham, C.; De Rooij, S.E.J.A. Neuroinflammation in Delirium: A Postmortem Case-Control Study. Rejuvenation Res. 2011, 14, 615–622. [Google Scholar] [CrossRef] [PubMed]
- Cerejeira, J.; Firmino, H.; Vaz-Serra, A.; Mukaetova-Ladinska, E.B. The Neuroinflammatory Hypothesis of Delirium. Acta Neuropathol. 2010, 119, 737–754. [Google Scholar] [CrossRef] [PubMed]
- Cunningham, C.; MacLullich, A.M.J. At the Extreme End of the Psychoneuroimmunological Spectrum: Delirium as a Maladaptive Sickness Behaviour Response. Brain Behav. Immun. 2013, 28, 1–13. [Google Scholar] [PubMed]
- Olney, J.W. Excitotoxicity, Apoptosis and Neuropsychiatric Disorders. Curr. Opin. Pharmacol. 2003, 3, 101–109. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Garcia, G.E.; Huang, W.; Constantini, S.; Kobeissy, F.H. The Involvement of Secondary Neuronal Damage in the Development of Neuropsychiatric Disorders Following Brain Insults. Front. Neurol. 2014, 5. [Google Scholar] [CrossRef] [PubMed]
- Barber, C.N.; Raben, D.M. Lipid Metabolism Crosstalk in the Brain: Glia and Neurons. Front. Cell. Neurosci. 2019, 13. [Google Scholar] [CrossRef]
- Bellisola, G.; Sorio, C. Infrared Spectroscopy and Microscopy in Cancer Research and Diagnosis. Am. J. Cancer Res. 2012, 2, 1–21. [Google Scholar]
- Wiredu, K.; Aduse-Poku, E.; Shaefi, S.; Gerber, S.A. Proteomics for the Discovery of Clinical Delirium Biomarkers: A Systematic Review of Major Studies. Anesth. Analg. 2023, 136, 422–432. [Google Scholar] [CrossRef] [PubMed]
- Ospina, J.P.; IV, F.K.; Madva, E.; Celano, C.M. Epidemiology, Mechanisms, Diagnosis, and Treatment of Delirium: A Narrative Review. Clin. Med. Ther. 2018, 1, 3. [Google Scholar] [CrossRef]
- Ritter, C.; Tomasi, C.D.; Dal-Pizzol, F.; Pinto, B.B.; Dyson, A.; de Miranda, A.S.; Comim, C.M.; Soares, M.; Teixeira, A.L.; Quevedo, J.; et al. Inflammation Biomarkers and Delirium in Critically Ill Patients. Crit. Care 2014, 18, R106. [Google Scholar] [CrossRef] [PubMed]
- Capri, M.; Yani, S.L.; Chattat, R.; Fortuna, D.; Bucci, L.; Lanzarini, C.; Morsiani, C.; Catena, F.; Ansaloni, L.; Adversi, M.; et al. Pre-Operative, High IL-6 Blood Level Is a Risk Factor of Post-Operative Delirium Onset in Old Patients. Front. Endocrinol. 2014, 5, 173. [Google Scholar] [CrossRef] [PubMed]
- Vasunilashorn, S.M.; Ngo, L.; Inouye, S.K.; Libermann, T.A.; Jones, R.N.; Alsop, D.C.; Guess, J.; Jastrzebski, S.; McElhaney, J.E.; Kuchel, G.A.; et al. Cytokines and Postoperative Delirium in Older Patients Undergoing Major Elective Surgery. J. Gerontol. A Biol. Sci. Med. Sci. 2015, 70, 1289–1295. [Google Scholar] [CrossRef] [PubMed]
- Westhoff, D.; Witlox, J.; Koenderman, L.; Kalisvaart, K.J.; de Jonghe, J.F.M.; van Stijn, M.F.M.; Houdijk, A.P.J.; Hoogland, I.C.M.; MacLullich, A.M.J.; van Westerloo, D.J.; et al. Preoperative Cerebrospinal Fluid Cytokine Levels and the Risk of Postoperative Delirium in Elderly Hip Fracture Patients. J. Neuroinflamm. 2013, 10, 889. [Google Scholar] [CrossRef]
- Liu, P.; Li, Y.W.; Wang, X.S.; Zou, X.; Zhang, D.Z.; Wang, D.X.; Li, S.Z. High Serum Interleukin-6 Level Is Associated with Increased Risk of Delirium in Elderly Patients after Noncardiac Surgery: A Prospective Cohort Study. Chin. Med. J. 2013, 126, 3621–3627. [Google Scholar]
- Han, Y.; Chen, W.; Song, Y.; Yuan, Y.; Li, Z.; Zhou, Y.; Liu, T.; Han, D.; Mi, X.; Li, M.; et al. Proteomic Analysis of Preoperative CSF Reveals Risk Biomarkers of Postoperative Delirium. Front. Psychiatry 2020, 11, 170. [Google Scholar] [CrossRef] [PubMed]
- Dillon, S.T.; Vasunilashorn, S.M.; Otu, H.H.; Ngo, L.; Fong, T.; Gu, X.; Cavallari, M.; Touroutoglou, A.; Shafi, M.; Inouye, S.K.; et al. Aptamer-Based Proteomics Measuring Preoperative Cerebrospinal Fluid Protein Alterations Associated with Postoperative Delirium. Biomolecules 2023, 13, 1395. [Google Scholar] [CrossRef]
- Ruhnau, J.; Müller, J.; Nowak, S.; Strack, S.; Sperlich, D.; Pohl, A.; Dilz, J.; Saar, A.; Veser, Y.; Behr, F.; et al. Serum Biomarkers of a Pro-Neuroinflammatory State May Define the Pre-Operative Risk for Postoperative Delirium in Spine Surgery. Int. J. Mol. Sci. 2023, 24, 335. [Google Scholar] [CrossRef]
- Peters van Ton, A.M.; Verbeek, M.M.; Alkema, W.; Pickkers, P.; Abdo, W.F. Downregulation of Synapse-Associated Protein Expression and Loss of Homeostatic Microglial Control in Cerebrospinal Fluid of Infectious Patients with Delirium and Patients with Alzheimer’s Disease. Brain Behav. Immun. 2020, 89, 656–667. [Google Scholar] [CrossRef] [PubMed]
- Marcantonio, E.R.; Rudolph, J.L.; Culley, D.; Crosby, G.; Alsop, D.; Inouye, S.K. Serum Biomarkers for Delirium. J. Gerontol. A Biol. Sci. Med. Sci. 2006, 61, 1281–1286. [Google Scholar] [CrossRef] [PubMed]


| Delirium Patients (n = 26) | Non-Delirium Patients (n = 108) | p-Value | Statistic Test Used | |
|---|---|---|---|---|
| Age (years) (mean) | 55.38 ± 14.82 | 57.00 ± 16.46 | 0.469 | Student’s t-test |
| Male gender (n) | 22 | 67 | 0.029 | Fisher’s exact test |
| BMI (mean) | 27.42 ± 4.60 | 31.16 ± 23.88 | 0.408 | Mann–Whitney U |
| Presence of comorbidities (n) | 23 | 86 | 0.299 | Fisher’s exact test |
| Arterial Hypertension (n) | 12 | 46 | 0.427 | Chi-square |
| Diabetes Mellitus (n) | 7 | 26 | 0.449 | Chi-square |
| Hospital death (n) | 6 | 25 | 0.994 | Chi-square |
| ICU death (n) | 4 | 24 | 0.441 | Fisher’s exact test |
| N. of days of ICU stay (mean) | 25.42 ± 19.70 | 8.56 ± 9.25 | <0.001 | Mann–Whitney U |
| Use of IMV (n) | 26 | 58 | <0.001 | Fisher’s exact test |
| Use of ECMO (n) | 5 | 9 | 0.103 | Chi-square |
| Oxazepam (n) | 12 | 5 | <0.001 | Fisher’s exact test |
| Midazolam (n) | 24 | 37 | <0.001 | Fisher’s exact test |
| Delirium Patients (n = 26) | Non-Delirium Patients (n = 26) | p-Value | Statistic Test Used | |
|---|---|---|---|---|
| Age (years) (mean) | 55.38 ± 14.82 | 57.65 ± 15.87 | 0.806 | Student’s t-test |
| Male gender (n) | 22 | 20 | 0.726 | Fisher’s exact test |
| BMI (mean) | 27.42 ± 4.59 | 29.76 ± 6.44 | 0.189 | Mann–Whitney U |
| Presence of comorbidities (n) | 23 | 24 | 1.000 | Fisher’s exact test |
| Arterial Hypertension (n) | 14 | 12 | 0.782 | Chi-square |
| Diabetes Mellitus (n) | 8 | 8 | 1.000 | Chi-square |
| Hospital death (n) | 6 | 9 | 0.540 | Chi-square |
| ICU death (n) | 4 | 9 | 0.199 | Fisher’s exact test |
| N. of days of ICU stay (mean) | 25.46 ± 19.70 | 19.73 ± 13.00 | 0.081 | Mann–Whitney U |
| Use of IMV (n) | 26 | 25 | 1.000 | Fisher’s exact test |
| Use of ECMO (n) | 5 | 5 | 1.000 | Chi-square |
| Oxazepam (n) | 12 | 4 | 0.034 | Fisher’s exact test |
| Midazolam (n) | 24 | 23 | 1.000 | Fisher’s exact test |
| Delirium | Non-Delirium | ||||||
|---|---|---|---|---|---|---|---|
| Bands (cm−1) | Average | Standard Deviation | Average | Standard Deviation | p-Value | Vibrational Mode | Functional Group/Biocompound |
| 1470 | −0.046 | 0.008 | −0.041 | 0.008 | 0.046 | δas(CH3) δas(CH3) | Lipid, protein |
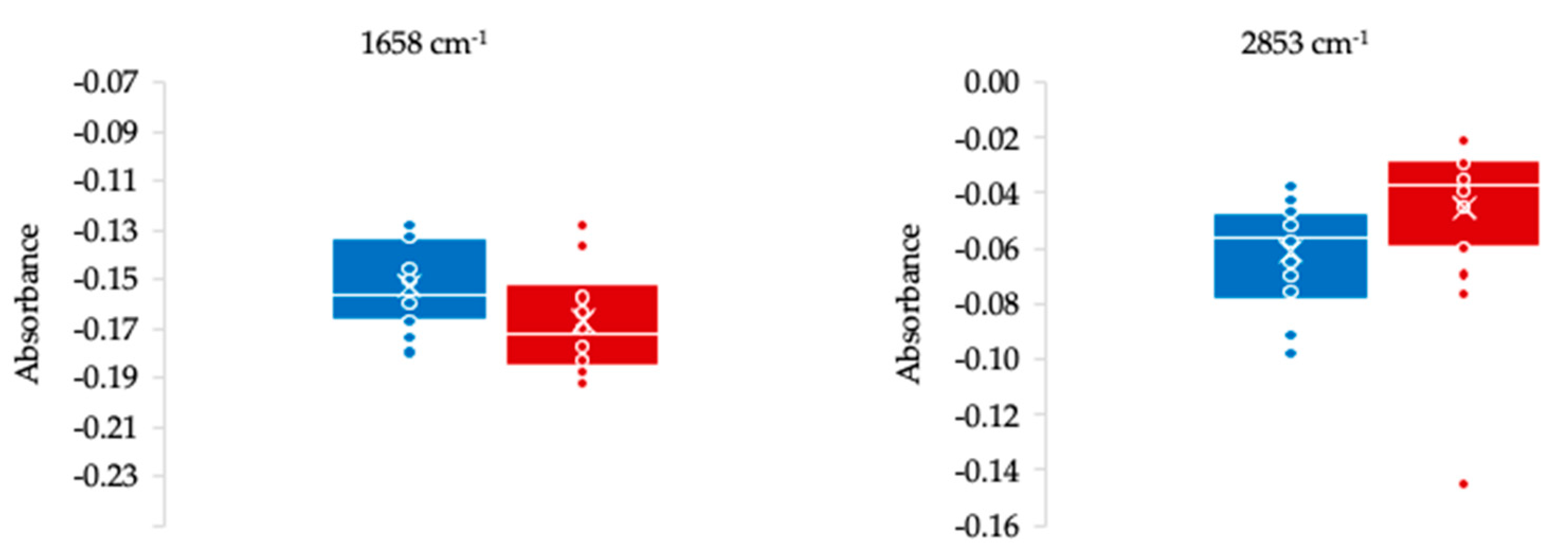
| 1658 | −0.152 | 0.017 | −0.167 | 0.022 | 0.012 | 80% ν(CO), 20% ν(CN) | Amide I peptide, protein |
| 2853 | −0.061 | 0.020 | −0.045 | 0.028 | 0.025 | νs(CH2) | Lipids |
| 2926 | −0.055 | 0.020 | −0.041 | 0.022 | 0.027 | νas(CH2) | Lipids |
| 2961 | −0.037 | 0.005 | −0.034 | 0.004 | 0.043 | νas(CH3) | Lipids |
| Spectra Pre-Processing | Regions (cm−1) | AUC | Sensitivity | Specificity | Accuracy | Precision |
|---|---|---|---|---|---|---|
| Baseline correction | 400–4000 | 0.678 | 0.538 | 0.538 | 0.538 | 0.538 |
| Normalized and baseline | 0.639 | 0.462 | 0.615 | 0.538 | 0.545 | |
| Second derivative | 800–1800 and 2800–3400 | 0.583 | 0.423 | 0.462 | 0.442 | 0.440 |
| Normalized second derivative | 0.589 | 0.731 | 0.577 | 0.654 | 0.633 | |
| Baseline correction | 438, 922 and 418 | 0.856 | 0.692 | 0.769 | 0.731 | 0.750 |
| Normalized and baseline | 841 and 3962 | 0.767 | 0.692 | 0.731 | 0.712 | 0.720 |
| Second derivative | 2868, 1587, 1149, 855, 1061, 1630 and 1792 | 0.900 | 0.846 | 0.885 | 0.865 | 0.880 |
| Normalized second derivative | 2912, 1149, 942, 1546, 1268, 987 and 1041 | 0.989 | 0.923 | 0.923 | 0.923 | 0.923 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Viegas, A.; Araújo, R.; Ramalhete, L.; Von Rekowski, C.; Fonseca, T.A.H.; Bento, L.; Calado, C.R.C. Discovery of Delirium Biomarkers through Minimally Invasive Serum Molecular Fingerprinting. Metabolites 2024, 14, 301. https://doi.org/10.3390/metabo14060301
Viegas A, Araújo R, Ramalhete L, Von Rekowski C, Fonseca TAH, Bento L, Calado CRC. Discovery of Delirium Biomarkers through Minimally Invasive Serum Molecular Fingerprinting. Metabolites. 2024; 14(6):301. https://doi.org/10.3390/metabo14060301
Chicago/Turabian StyleViegas, Ana, Rúben Araújo, Luís Ramalhete, Cristiana Von Rekowski, Tiago A. H. Fonseca, Luís Bento, and Cecília R. C. Calado. 2024. "Discovery of Delirium Biomarkers through Minimally Invasive Serum Molecular Fingerprinting" Metabolites 14, no. 6: 301. https://doi.org/10.3390/metabo14060301






