The Therapeutic Potential of Hemp Seed Oil in D-Galactose-Induced Aging Rat Model Was Determined through the Combined Assessment of 1H NMR Metabolomics and 16S rRNA Gene Sequencing
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Animal Experiments
2.3. Cognitive Function Assessment
2.3.1. Morris Water Maze
2.3.2. Open Field Test
2.4. Metabolomics Analysis Based on 1H NMR Spectroscopy
2.5. 16S rRNA-Based Microbial Community Analysis
2.6. Statistical Analysis
3. Results
3.1. Hemp Seed Oil Ameliorates D-Gal-Induced Aging in Rats
3.2. Hemp Seed Oil Treatment Alleviates Metabolic Disorders in Rats with D-Gal-Induced Aging
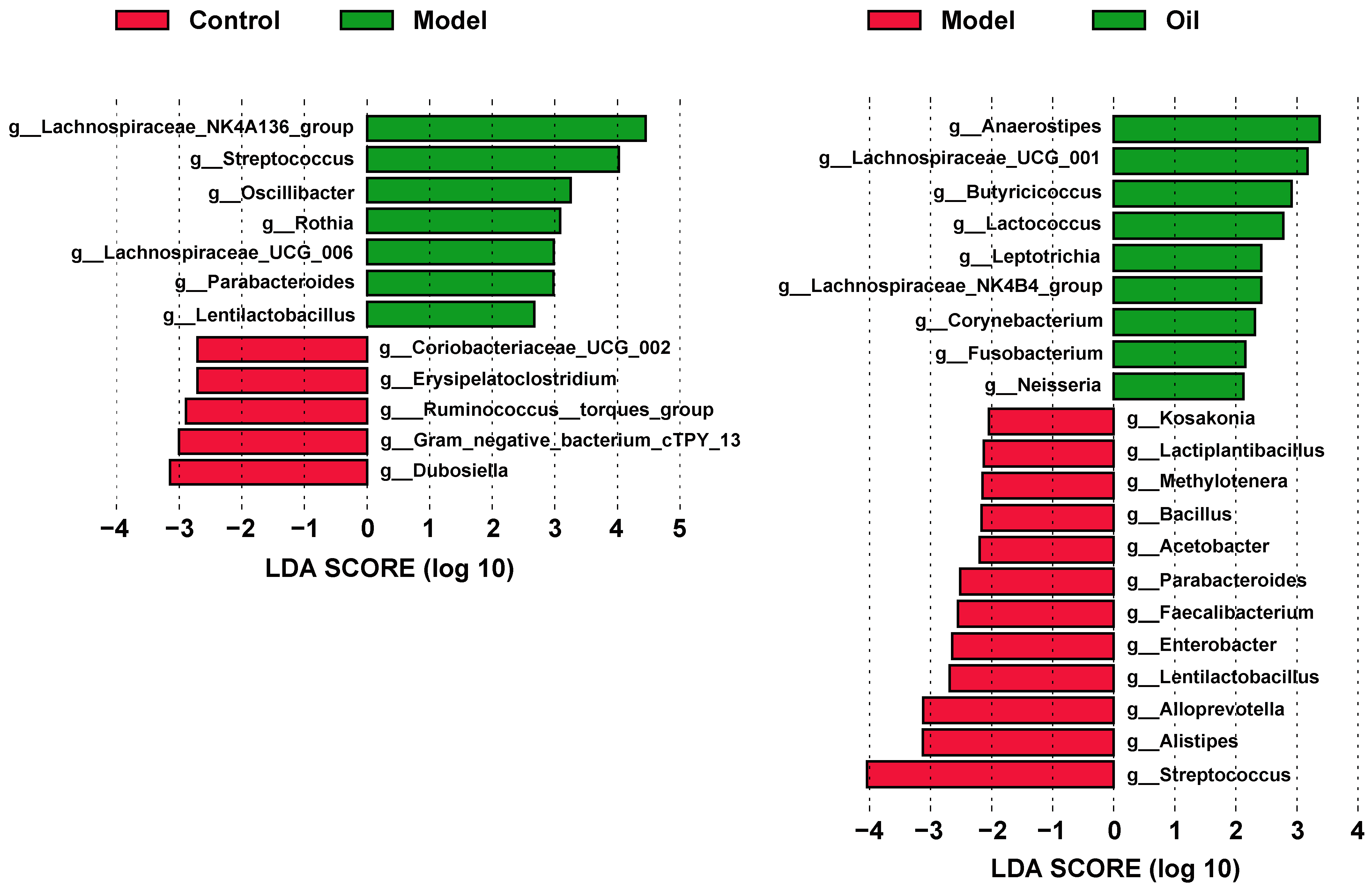
3.3. Hemp Seed Oil Treatment Modulates Gut Microbiota Composition in D-Gal-Induced Aged Rats
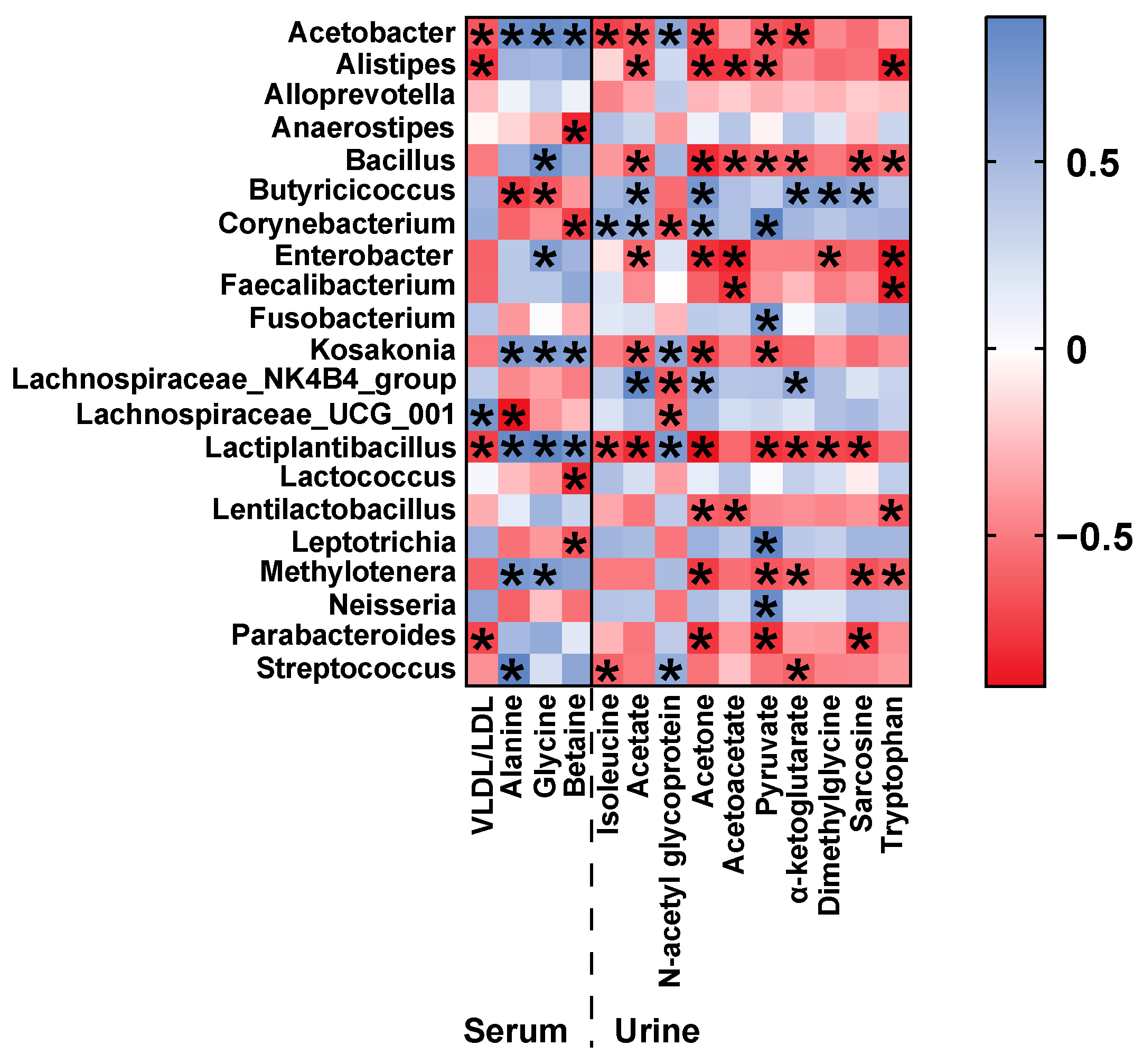
3.4. Correlation of Gut Microbiota and Metabolic Phenotype in D-Gal-Induced Aged Rats
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cai, Y.; Song, W.; Li, J.; Jing, Y.; Liang, C.; Zhang, L.; Zhang, X.; Zhang, W.; Liu, B.; An, Y.; et al. The landscape of aging. Sci. China Life Sci. 2022, 65, 2354–2454. [Google Scholar] [CrossRef] [PubMed]
- Devi, V.; Khanam, S. Comparative study of different extraction processes for hemp (Cannabis sativa) seed oil considering physical, chemical and industrial-scale economic aspects. J. Clean. Prod. 2019, 207, 645–657. [Google Scholar] [CrossRef]
- Gong, M.; Lu, H.; Li, L.; Feng, M.; Zou, Z. Integration of transcriptomics and metabonomics revealed the protective effects of hemp seed oil against methionine-choline-deficient diet-induced non-alcoholic steatohepatitis in mice. Food Funct. 2023, 14, 2096–2111. [Google Scholar] [CrossRef] [PubMed]
- Li, X.Y.; Liu, Y.H.; Wang, B.; Chen, C.Y.; Zhang, H.M.; Kang, J.X. Identification of a sustainable two-plant diet that effectively prevents age-related metabolic syndrome and extends lifespan in aged mice. J. Nutr. Biochem. 2018, 51, 16–26. [Google Scholar] [CrossRef] [PubMed]
- Xu, P.W.; Yuan, X.F.; Zhao, B. Bioactive polyphenols separated from hemp seed shells ameliorate H2O2-induced oxidative stress injury in human umbilical vein endothelial cells. J. Food Sci. 2023, 88, 537–551. [Google Scholar] [CrossRef] [PubMed]
- Puchades-Carrasco, L.; Pineda-Lucena, A. Metabolomics in pharmaceutical research and development. Curr. Opin. Biotechnol. 2015, 35, 73–77. [Google Scholar] [CrossRef] [PubMed]
- Balashova, E.E.; Maslov, D.L.; Trifonova, O.P.; Lokhov, P.G.; Archakov, A.I. Metabolome Profiling in Aging Studies. Biology 2022, 11, 1570. [Google Scholar] [CrossRef] [PubMed]
- Vaiserman, A.M.; Koliada, A.K.; Marotta, F. Gut microbiota: A player in aging and a target for anti-aging intervention. Ageing Res. Rev. 2017, 35, 36–45. [Google Scholar] [CrossRef] [PubMed]
- Tuikhar, N.; Keisam, S.; Labala, R.K.; Imrat; Ramakrishnan, P.; Arunkumar, M.C.; Ahmed, G.; Biagi, E.; Jeyaram, K. Comparative analysis of the gut microbiota in centenarians and young adults shows a common signature across genotypically non-related populations. Mech. Ageing Dev. 2019, 179, 23–35. [Google Scholar] [CrossRef] [PubMed]
- Biagi, E.; Nylund, L.; Candela, M.; Ostan, R.; Bucci, L.; Pini, E.; Nikkila, J.; Monti, D.; Satokari, R.; Franceschi, C.; et al. Through ageing, and beyond: Gut microbiota and inflammatory status in seniors and centenarians. PLoS ONE 2010, 5, e10667. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Mu, X.; Zeng, J.; Xu, C.; Liu, J.; Zhang, M.; Li, C.; Chen, J.; Li, T.; Wang, Y. Ginsenoside Rg1 prevents cognitive impairment and hippocampus senescence in a rat model of D-galactose-induced aging. PLoS ONE 2014, 9, e101291. [Google Scholar] [CrossRef] [PubMed]
- Farinon, B.; Molinari, R.; Costantini, L.; Merendino, N. The seed of industrial hemp (Cannabis sativa L.): Nutritional Quality and Potential Functionality for Human Health and Nutrition. Nutrients 2020, 12, 1935. [Google Scholar] [CrossRef] [PubMed]
- Reagan-Shaw, S.; Nihal, M.; Ahmad, N. Dose translation from animal to human studies revisited. FASEB J. 2008, 22, 659–661. [Google Scholar] [CrossRef]
- Vorhees, C.V.; Williams, M.T. Morris water maze: Procedures for assessing spatial and related forms of learning and memory. Nat. Protoc. 2006, 1, 848–858. [Google Scholar] [CrossRef] [PubMed]
- Pentkowski, N.S.; Rogge-Obando, K.K.; Donaldson, T.N.; Bouquin, S.J.; Clark, B.J. Anxiety and Alzheimer’s disease: Behavioral analysis and neural basis in rodent models of Alzheimer’s-related neuropathology. Neurosci. Biobehav. Rev. 2021, 127, 647–658. [Google Scholar] [CrossRef] [PubMed]
- Gong, M.J.; Han, B.; Wang, S.M.; Liang, S.W.; Zou, Z.J. Icariin reverses corticosterone-induced depression-like behavior, decrease in hippocampal brain-derived neurotrophic factor (BDNF) and metabolic network disturbances revealed by NMR-based metabonomics in rats. J. Pharm. Biomed. Anal. 2016, 123, 63–73. [Google Scholar] [CrossRef] [PubMed]
- Ding, L.; Hao, F.; Shi, Z.; Wang, Y.; Zhang, H.; Tang, H.; Dai, J. Systems biological responses to chronic perfluorododecanoic acid exposure by integrated metabonomic and transcriptomic studies. J. Proteome Res. 2009, 8, 2882–2891. [Google Scholar] [CrossRef] [PubMed]
- Segata, N.; Izard, J.; Waldron, L.; Gevers, D.; Miropolsky, L.; Garrett, W.S.; Huttenhower, C. Metagenomic biomarker discovery and explanation. Genome Biol. 2011, 12, R60. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Li, X.; Yang, S.; Li, Y.; Lin, X.; Xiu, M.; Li, X.; Liu, Y. Gastrodin extends the lifespan and protects against neurodegeneration in the Drosophila PINK1 model of Parkinson’s disease. Food Funct. 2021, 12, 7816–7824. [Google Scholar] [CrossRef] [PubMed]
- Longo, V.D.; Anderson, R.M. Nutrition, longevity and disease: From molecular mechanisms to interventions. Cell 2022, 185, 1455–1470. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Bai, F.; Wang, W.; Liu, Y.; Yuan, Q.; Qu, S.; Zhang, T.; Tian, G.; Li, S.; Li, D.; et al. Fibroblast growth factor 21 protects mouse brain against D-galactose induced aging via suppression of oxidative stress response and advanced glycation end products formation. Pharmacol. Biochem. Behav. 2015, 133, 122–131. [Google Scholar] [CrossRef]
- Alejandro, S.P. ER stress in cardiac aging, a current view on the D-galactose model. Exp. Gerontol. 2022, 169, 111953. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Yang, L.; Gao, L.; Du, G.; Qin, X.; Zhou, Y. The leaves of Scutellaria baicalensis Georgi attenuate brain aging in D-galactose-induced rats via regulating glutamate metabolism and Nrf2 signaling pathway. Exp. Gerontol. 2022, 170, 111978. [Google Scholar] [CrossRef] [PubMed]
- Gyanwali, B.; Lim, Z.X.; Soh, J.; Lim, C.; Guan, S.P.; Goh, J.; Maier, A.B.; Kennedy, B.K. Alpha-Ketoglutarate dietary supplementation to improve health in humans. Trends Endocrinol. Metab. 2022, 33, 136–146. [Google Scholar] [CrossRef] [PubMed]
- Chin, R.M.; Fu, X.; Pai, M.Y.; Vergnes, L.; Hwang, H.; Deng, G.; Diep, S.; Lomenick, B.; Meli, V.S.; Monsalve, G.C.; et al. The metabolite alpha-ketoglutarate extends lifespan by inhibiting ATP synthase and TOR. Nature 2014, 510, 397–401. [Google Scholar] [CrossRef]
- Zhou, F.Q. NAD(+), Senolytics, or Pyruvate for Healthy Aging? Nutr. Metab. Insights 2021, 14, 11786388211053407. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Ko, B.; Hensley, C.T.; Jiang, L.; Wasti, A.T.; Kim, J.; Sudderth, J.; Calvaruso, M.A.; Lumata, L.; Mitsche, M.; et al. Glutamine oxidation maintains the TCA cycle and cell survival during impaired mitochondrial pyruvate transport. Mol. Cell 2014, 56, 414–424. [Google Scholar] [CrossRef]
- Koh, A.; De Vadder, F.; Kovatcheva-Datchary, P.; Backhed, F. From Dietary Fiber to Host Physiology: Short-Chain Fatty Acids as Key Bacterial Metabolites. Cell 2016, 165, 1332–1345. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Jing, S.; Jiang, R.; Wang, C.; Zhang, C.; Chen, J.; Li, H. Metabolomics study of the therapeutic mechanism of Schisandra chinensis lignans on aging rats induced by d-galactose. Clin. Interv. Aging 2018, 13, 829–841. [Google Scholar] [CrossRef] [PubMed]
- Wu, G. Amino acids: Metabolism, functions, and nutrition. Amino Acids 2009, 37, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Wang, H.; Shenvi, S.; Hagen, T.M.; Liu, R.M. Glutathione metabolism during aging and in Alzheimer disease. Ann. N. Y. Acad. Sci. 2004, 1019, 346–349. [Google Scholar] [CrossRef]
- Trautman, M.E.; Richardson, N.E.; Lamming, D.W. Protein restriction and branched-chain amino acid restriction promote geroprotective shifts in metabolism. Aging Cell 2022, 21, e13626. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, T.; Hatabayashi, K.; Arita, M.; Yajima, N.; Takenaka, C.; Suzuki, T.; Takahashi, M.; Oshima, Y.; Hara, K.; Kagawa, K.; et al. Kynurenine signaling through the aryl hydrocarbon receptor maintains the undifferentiated state of human embryonic stem cells. Sci. Signal 2019, 12, eaaw3306. [Google Scholar] [CrossRef]
- Eckers, A.; Jakob, S.; Heiss, C.; Haarmann-Stemmann, T.; Goy, C.; Brinkmann, V.; Cortese-Krott, M.M.; Sansone, R.; Esser, C.; Ale-Agha, N.; et al. The aryl hydrocarbon receptor promotes aging phenotypes across species. Sci. Rep. 2016, 6, 19618. [Google Scholar] [CrossRef] [PubMed]
- Kalecky, K.; Ashcraft, P.; Bottiglieri, T. One-Carbon Metabolism in Alzheimer’s Disease and Parkinson’s Disease Brain Tissue. Nutrients 2022, 14, 599. [Google Scholar] [CrossRef] [PubMed]
- Go, E.K.; Jung, K.J.; Kim, J.M.; Lim, H.; Lim, H.K.; Yu, B.P.; Chung, H.Y. Betaine modulates age-related NF-kappaB by thiol-enhancing action. Biol. Pharm. Bull. 2007, 30, 2244–2249. [Google Scholar] [CrossRef] [PubMed]
- Go, E.K.; Jung, K.J.; Kim, J.Y.; Yu, B.P.; Chung, H.Y. Betaine suppresses proinflammatory signaling during aging: The involvement of nuclear factor-kappaB via nuclear factor-inducing kinase/IkappaB kinase and mitogen-activated protein kinases. J. Gerontol. A Biol. Sci. Med. Sci. 2005, 60, 1252–1264. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.H.; Li, J.J. Aging and dyslipidemia: A review of potential mechanisms. Ageing Res. Rev. 2015, 19, 43–52. [Google Scholar] [CrossRef] [PubMed]
- Agudelo-Ochoa, G.M.; Valdes-Duque, B.E.; Giraldo-Giraldo, N.A.; Jaillier-Ramirez, A.M.; Giraldo-Villa, A.; Acevedo-Castano, I.; Yepes-Molina, M.A.; Barbosa-Barbosa, J.; Benitez-Paez, A. Gut microbiota profiles in critically ill patients, potential biomarkers and risk variables for sepsis. Gut Microbes 2020, 12, 1707610. [Google Scholar] [CrossRef] [PubMed]
- Fatahi-Bafghi, M. Characterization of the Rothia spp. and their role in human clinical infections. Infect. Genet. Evol. 2021, 93, 104877. [Google Scholar] [CrossRef] [PubMed]
- Duan, H.; Li, J.; Fan, L. Agaricus bisporus Polysaccharides Ameliorates Behavioural Deficits in D-Galactose-Induced Aging Mice: Mediated by Gut Microbiota. Foods 2023, 12, 424. [Google Scholar] [CrossRef] [PubMed]
- Vaiserman, A.M.; Pasyukova, E.G. Epigenetic drugs: A novel anti-aging strategy? Front. Genet. 2012, 3, 224. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Xiao, C.; Wang, F.; Guo, X.; Zhou, Z.; Jiang, Y. Blueberry-Mulberry Extract Alleviates Cognitive Impairment, Regulates Gut Metabolites, and Inhibits Inflammation in Aged Mice. Foods 2023, 12, 860. [Google Scholar] [CrossRef] [PubMed]
- Mou, Y.; Du, Y.; Zhou, L.; Yue, J.; Hu, X.; Liu, Y.; Chen, S.; Lin, X.; Zhang, G.; Xiao, H.; et al. Gut Microbiota Interact with the Brain Through Systemic Chronic Inflammation: Implications on Neuroinflammation, Neurodegeneration, and Aging. Front. Immunol. 2022, 13, 796288. [Google Scholar] [CrossRef]

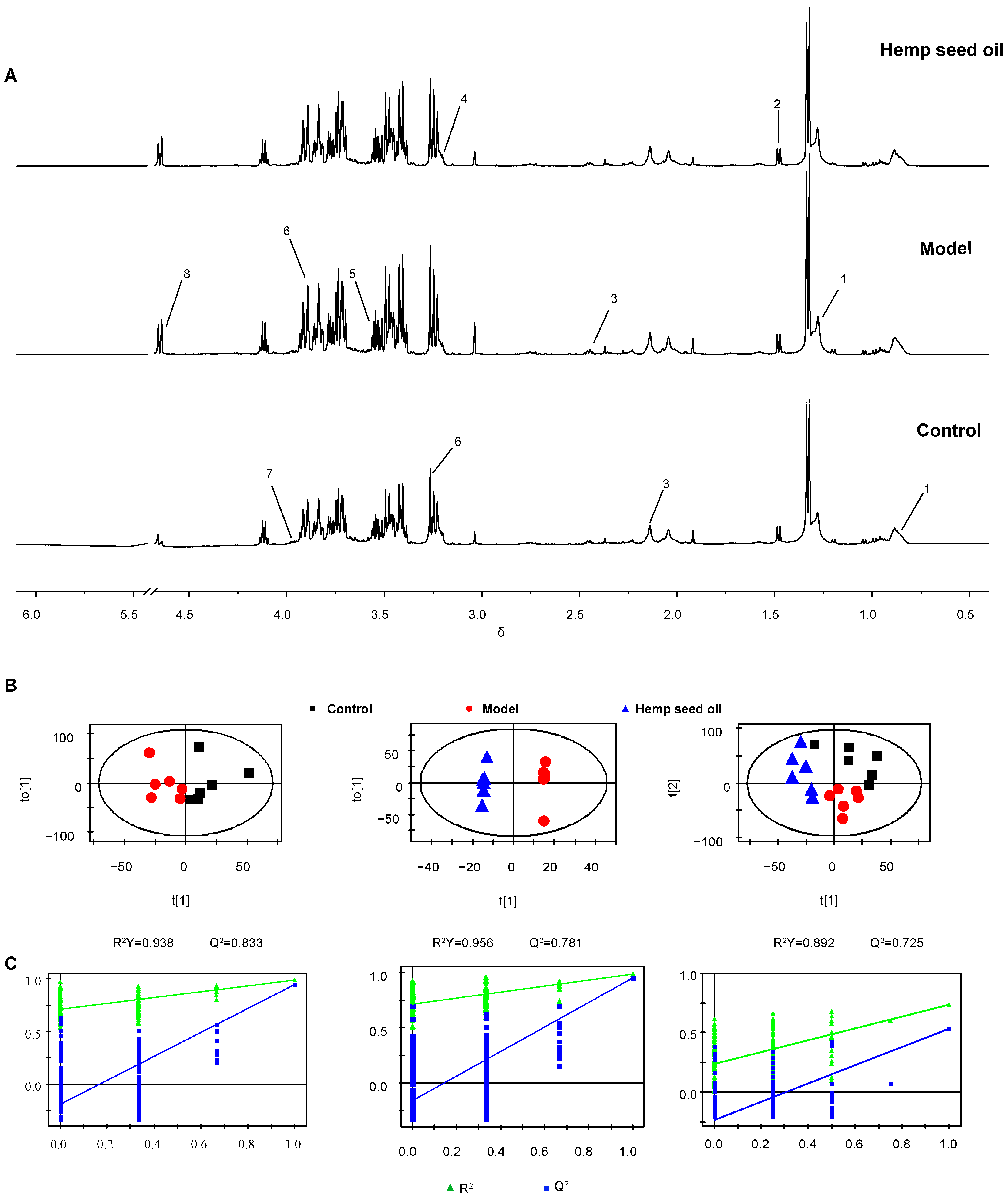

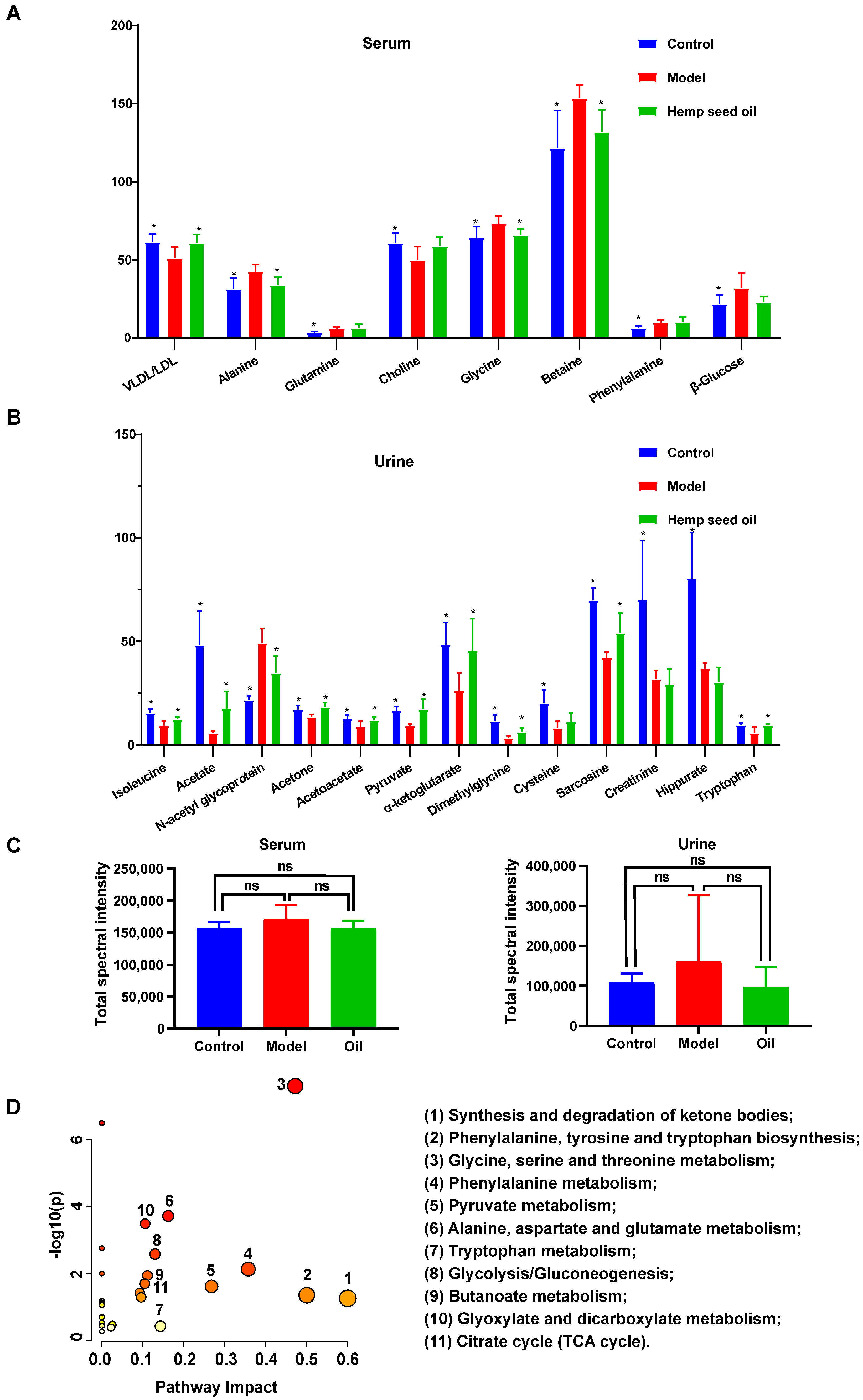
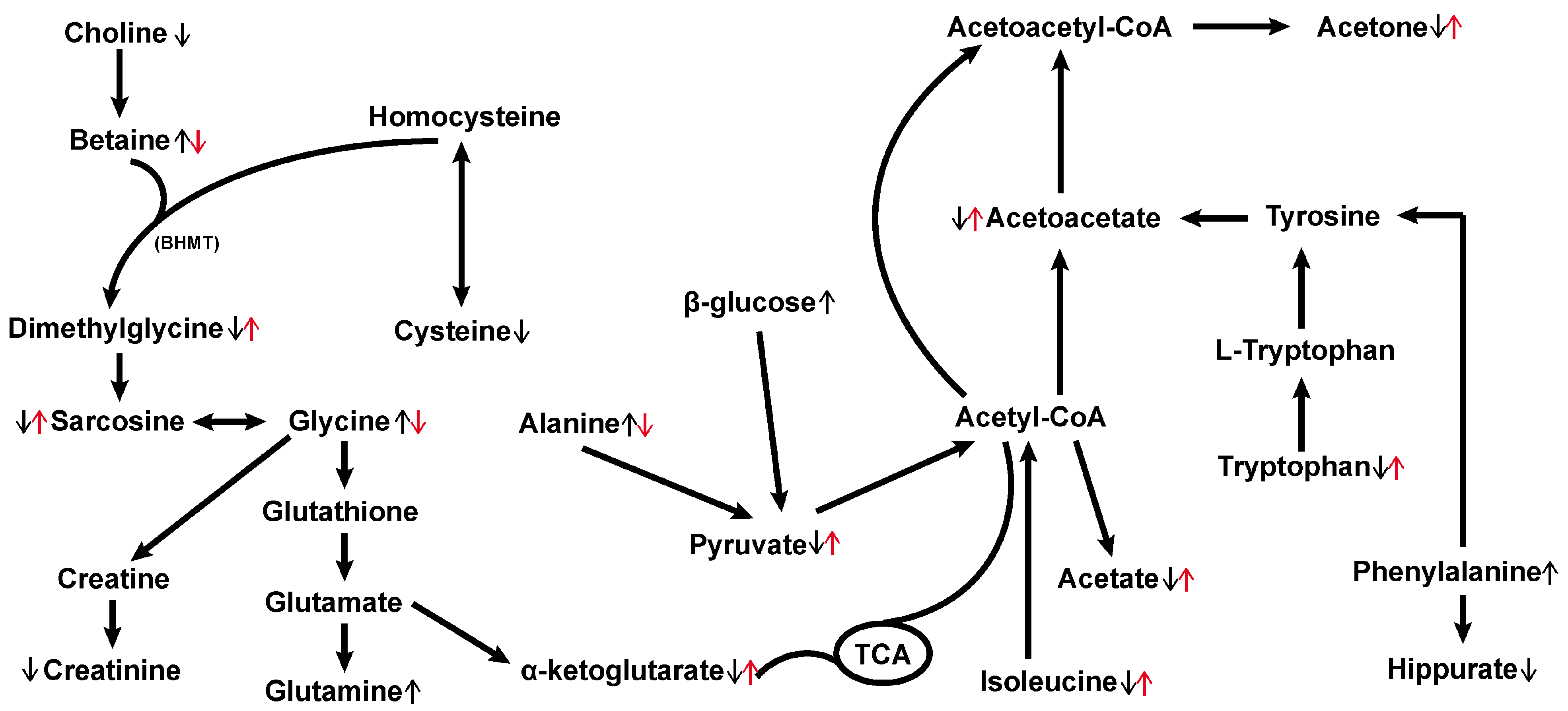

| Metabolite | Chemical Shift (ppm) a | VIP b | FC c | Model d | Hemp Seed Oil e |
|---|---|---|---|---|---|
| Serum | |||||
| VLDL/LDL | 0.87 (m), 1.28 (m) | 2.91 | 0.83 | ↓ * | ↑ * |
| Alanine | 1.48 (d) | 2.76 | 1.37 | ↑ * | ↓ * |
| Glutamine | 2.14 (m), 2.45 (m) | 1.25 | 1.84 | ↑ * | - |
| Choline | 3.20 (s) | 2.87 | 0.82 | ↓ * | - |
| Glycine | 3.55 (s) | 2.71 | 1.15 | ↑ * | ↓ * |
| Betaine | 3.27 (s), 3.90 (s) | 5.24 | 1.26 | ↑ * | ↓ * |
| Phenylalanine | 3.98 (dd) | 1.95 | 1.61 | ↑ * | - |
| β-glucose | 4.65 (d) | 2.71 | 1.47 | ↑ * | - |
| Urine | |||||
| Isoleucine | 0.94 (t) | 1.08 | 0.61 | ↓ * | ↑ * |
| Acetate | 1.92 (s) | 2.73 | 0.12 | ↓ * | ↑ * |
| N-Acetyl glycoprotein | 2.05 (s) | 2.42 | 2.24 | ↑ * | ↓ * |
| Acetone | 2.22 (s) | 0.79 | 0.79 | ↓ * | ↑ * |
| Acetoacetate | 2.30 (s) | 0.77 | 0.70 | ↓ * | ↑ * |
| Pyruvate | 2.38 (s) | 1.23 | 0.57 | ↓ * | ↑ * |
| α-Ketoglutarate | 2.44 (t), 3.01 (t) | 1.74 | 0.54 | ↓ * | ↑ * |
| Dimethylglycine | 2.93 (s) | 1.22 | 0.30 | ↓ * | ↑ * |
| Cysteine | 3.12 (m) | 1.47 | 0.40 | ↓ * | - |
| Sarcosine | 3.60 (s) | 2.45 | 0.60 | ↓ * | ↑ * |
| Creatinine | 4.05 (s) | 2.49 | 0.45 | ↓ * | - |
| Hippurate | 7.55 (t), 7.64 (t), 7.84 (d) | 2.88 | 0.46 | ↓ * | - |
| Tryptophan | 7.33 (s) | 0.77 | 0.59 | ↓ * | ↑ * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, H.; Li, L.; Zou, Z.; Han, B.; Gong, M. The Therapeutic Potential of Hemp Seed Oil in D-Galactose-Induced Aging Rat Model Was Determined through the Combined Assessment of 1H NMR Metabolomics and 16S rRNA Gene Sequencing. Metabolites 2024, 14, 304. https://doi.org/10.3390/metabo14060304
Lu H, Li L, Zou Z, Han B, Gong M. The Therapeutic Potential of Hemp Seed Oil in D-Galactose-Induced Aging Rat Model Was Determined through the Combined Assessment of 1H NMR Metabolomics and 16S rRNA Gene Sequencing. Metabolites. 2024; 14(6):304. https://doi.org/10.3390/metabo14060304
Chicago/Turabian StyleLu, Hailong, Lixi Li, Zhongjie Zou, Bin Han, and Mengjuan Gong. 2024. "The Therapeutic Potential of Hemp Seed Oil in D-Galactose-Induced Aging Rat Model Was Determined through the Combined Assessment of 1H NMR Metabolomics and 16S rRNA Gene Sequencing" Metabolites 14, no. 6: 304. https://doi.org/10.3390/metabo14060304
APA StyleLu, H., Li, L., Zou, Z., Han, B., & Gong, M. (2024). The Therapeutic Potential of Hemp Seed Oil in D-Galactose-Induced Aging Rat Model Was Determined through the Combined Assessment of 1H NMR Metabolomics and 16S rRNA Gene Sequencing. Metabolites, 14(6), 304. https://doi.org/10.3390/metabo14060304





