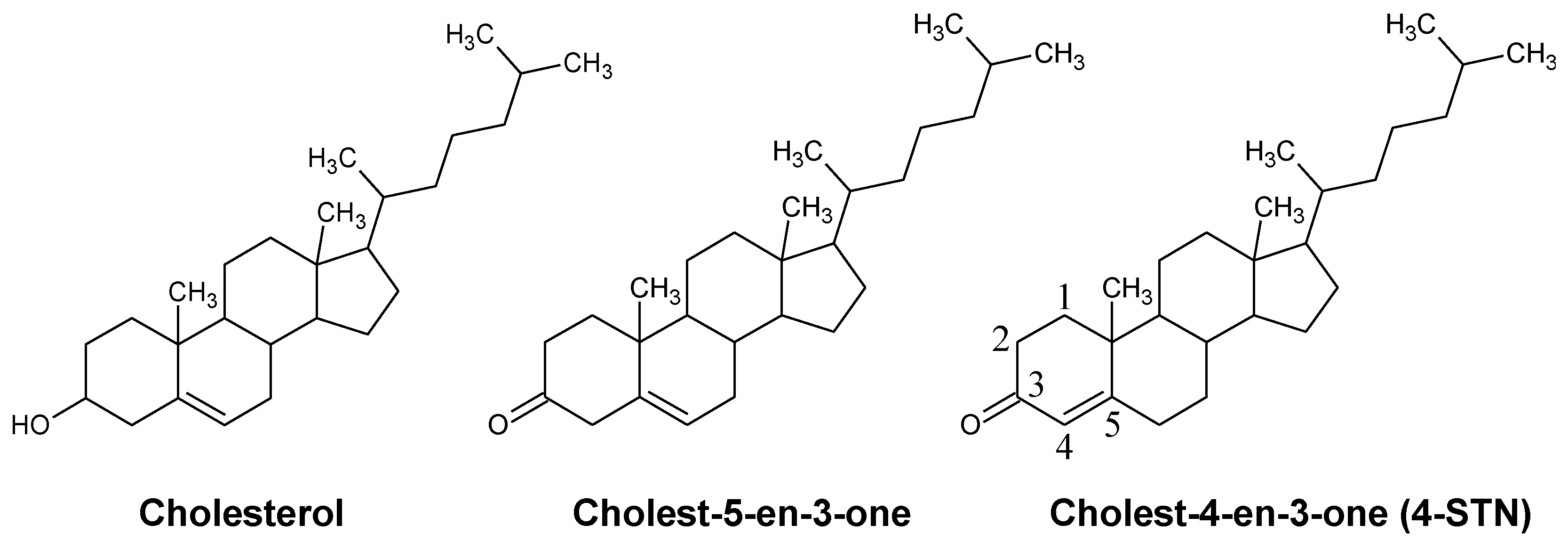
Dietary Cholest-4-en-3-one, a Cholesterol Metabolite of Gut Microbiota, Alleviates Hyperlipidemia, Hepatic Cholesterol Accumulation, and Hyperinsulinemia in Obese, Diabetic db/db Mice
Abstract
:1. Introduction
2. Materials and methods
2.1. Animals and Diets
2.2. Respiratory Gas Analysis
2.3. Measurement of Plasma Biochemical Parameters
2.4. Measurement of Triglyceride, Cholesterol, and Glycogen Contents in the Liver
2.5. Measurement of Triglyceride and Free Fatty Acids Contents in Feces
2.6. Measurement of Steroid Contents in the Liver, Plasma, and Feces
2.7. Measurement of mRNA Levels in the Liver and Epididymal WAT
2.8. Statistical Analysis
3. Results
3.1. Effects of Dietary 4-Cholestenone on Nutrients Oxidation in db/db Mice
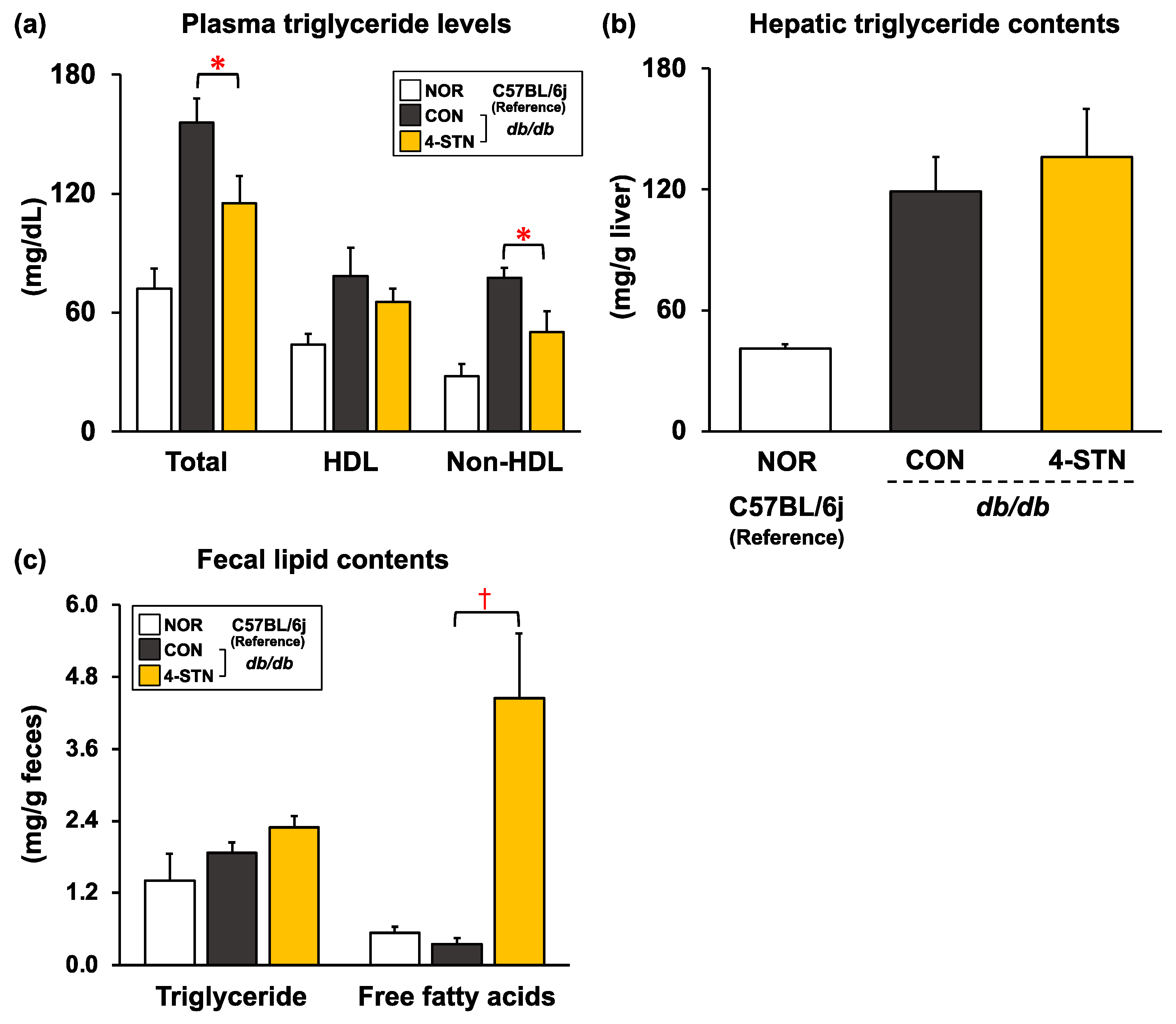
3.2. Effects of Dietary 4-Cholestenone on Morphometric Variables, and Biochemical Parameters in Plasma, the Liver, and Feces of db/db Mice
3.3. Effects of Dietary 4-Cholestenone on Cholesterol Precursor and Metabolite Levels in the Plasma and Liver of db/db Mice
3.4. Effects of Dietary 4-STN on mRNA Levels in Epididymal WAT and the Liver of db/db Mice
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Róg, T.; Pasenkiewicz-Gierula, M.; Vattulainen, I.; Karttunen, M. Ordering effects of cholesterol and its analogues. Biochim. Biophys. Acta 2009, 1788, 97–121. [Google Scholar] [CrossRef] [PubMed]
- Polyzos, S.A.; Targher, G. Role of glucocorticoids in metabolic dysfunction-associated steatotic liver disease. Curr. Obes. Rep. 2024. online ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Hysa, E.; Vojinovic, T.; Gotelli, E.; Alessandri, E.; Pizzorni, C.; Paolino, S.; Sulli, A.; Smith, V.; Cutolo, M. The dichotomy of glucocorticosteroid treatment in immune-inflammatory rheumatic diseases: An evidence-based perspective and insights from clinical practice. Reumatologia 2023, 61, 283–293. [Google Scholar] [CrossRef] [PubMed]
- Laitinen, K.; Gylling, H. Dose-dependent LDL-cholesterol lowering effect by plant stanol ester consumption: Clinical evidence. Lipids Health Dis. 2012, 11, 140. [Google Scholar] [CrossRef] [PubMed]
- Cusack, L.K.; Fernandez, M.L.; Volek, J.S. The food matrix and sterol characteristics affect the plasma cholesterol lowering of phytosterol/phytostanol. Adv. Nutr. 2013, 4, 633–643. [Google Scholar] [CrossRef] [PubMed]
- Devaraj, S.; Jialal, I.; Vega-López, S. Plant sterol-fortified orange juice effectively lowers cholesterol levels in mildly hypercholesterolemic healthy individuals. Arterioscler. Thromb. Vasc. Biol. 2004, 24, e25–e28. [Google Scholar] [CrossRef]
- Bogie, J.; Hoeks, C.; Schepers, M.; Tiane, A.; Cuypers, A.; Leijten, F.; Chintapakorn, Y.; Suttiyut, T.; Pornpakakul, S.; Struik, D.; et al. Dietary Sargassum fusiforme improves memory and reduces amyloid plaque load in an Alzheimer’s disease mouse model. Sci. Rep. 2019, 9, 4908. [Google Scholar] [CrossRef]
- Olkkonen, V.M.; Gylling, H.; Ikonen, E. Plant sterols, cholesterol precursors and oxysterols: Minute concentrations-Major physiological effects. J. Steroid Biochem. Mol. Biol. 2017, 169, 4–9. [Google Scholar] [CrossRef] [PubMed]
- Evtyugin, D.D.; Evtuguin, D.V.; Casal, S.; Domingues, M.R. Advances and Challenges in Plant Sterol Research: Fundamentals, Analysis, Applications and Production. Molecules 2023, 28, 6526. [Google Scholar] [CrossRef]
- Bordet, T.; Berna, P.; Abitbol, J.L.; Pruss, R.M. Olesoxime (TRO19622): A Novel Mitochondrial-Targeted Neuroprotective Compound. Pharmaceuticals 2010, 3, 345–368. [Google Scholar] [CrossRef]
- Deng, C.; Pan, J.; Zhu, H.; Chen, Z.Y. Effect of gut microbiota on blood cholesterol: A review on mechanisms. Foods 2023, 12, 4308. [Google Scholar] [CrossRef] [PubMed]
- Fredrickson, D.S.; Steinberg, D. Inhibitors of cholesterol biosynthesis and the problem of hypercholesterolemia. Ann. N. Y. Acad. Sci. 1956, 64, 579–589. [Google Scholar] [CrossRef] [PubMed]
- Packie, R.M.; Kandutsch, A.A. Rates of sterol synthesis and hydroxymethylglutaryl coenzyme A reductase levels, and the effects of cholest-4-en-3-one on these parameters, in the livers of inbred strains of mice. Biochem. Genet. 1970, 4, 203–214. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, K. Anti-obesity effect of cholest-4-en-3-one, an intestinal catabolite of cholesterol, on mice. J. Nutr. Sci. Vitaminol. 1993, 39, 537–543. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, K.; Shimizu, T.; Nakata, T. The cholesterol metabolite cholest-4-en-3-one and its 3-oxo derivatives suppress body weight gain, body fat accumulation and serum lipid concentration in mice. Bioorg. Med. Chem. Lett. 1998, 8, 2133–2138. [Google Scholar] [CrossRef] [PubMed]
- Nagao, K.; Inoue, N.; Suzuki, K.; Shimizu, T.; Yanagita, T. The cholesterol Metabolite Cholest-5-en-3-One Alleviates Hyperglycemia and hyperinsulinemia in Obese (db/db) Mice. Metabolites 2021, 12, 26. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Charlat, O.; Tartaglia, L.A.; Woolf, E.A.; Weng, X.; Ellis, S.J.; Lakey, N.D.; Culpepper, J.; Moore, K.J.; Breitbart, R.E.; et al. Evidence that the diabetes gene encodes the leptin receptor: Identification of a mutation in the leptin receptor gene in db/db mice. Cell 1996, 84, 491–495. [Google Scholar] [CrossRef] [PubMed]
- Lee, G.H.; Proenca, R.; Montez, J.M.; Carroll, K.M.; Darvishzadeh, J.G.; Lee, J.I.; Friedman, J.M. Abnormal splicing of the leptin receptor in diabetic mice. Nature 1996, 379, 632–635. [Google Scholar] [CrossRef] [PubMed]
- American Institute of Nutrition. Report of the American Institute of Nutrition ad hoc Committee on Standards for Nutritional Studies. J. Nutr. 1977, 107, 1340–1348. [Google Scholar] [CrossRef]
- Niibo, M.; Shirouchi, B.; Umegatani, M.; Morita, Y.; Ogawa, A.; Sakai, F.; Kadooka, Y.; Sato, M. Probiotic Lactobacillus gasseri SBT2055 improves insulin secretion in a diabetic rat model. J. Dairy Sci. 2019, 102, 997–1006. [Google Scholar] [CrossRef]
- Shirouchi, B.; Fukuda, A.; Akasaka, T. Unlike glycerophosphocholine or choline chloride, dietary phosphatidylcholine does not increase plasma trimethylamine- N-oxide levels in Sprague-Dawley rats. Metabolites 2022, 12, 64. [Google Scholar] [CrossRef]
- Carr, T.P.; Andresen, C.J.; Rudel, L.L. Enzymatic determination of triglyceride, free cholesterol, and total cholesterol in tissue lipid extracts. Clin. Biochem. 1993, 26, 39–42. [Google Scholar] [CrossRef]
- Rouser, G.; Siakotos, A.N.; Fleischer, S. Quantitative analysis of phospholipids by thin-layer chromatography and phosphorus analysis of spots. Lipids 1966, 1, 85–86. [Google Scholar] [CrossRef]
- Lo, S.; Russell, J.C.; Taylor, A.W. Determination of glycogen in small tissue samples. Br. J. Nutr. 2017, 118, 423–430. [Google Scholar] [CrossRef]
- Jeejeebhoy, K.N.; Ahmad, S.; Kozak, G. Determination of fecal fats containing both medium and long chain triglycerides and fatty acids. Clin. Biochem. 1970, 3, 157–163. [Google Scholar] [CrossRef]
- Shirouchi, B.; Nagao, K.; Furuya, K.; Inoue, N.; Inafuku, M.; Nasu, M.; Otsubo, K.; Koga, S.; Matsumoto, H.; Yanagita, T. Effect of dietary phosphatidylinositol on cholesterol metabolism in Zucker (fa/fa) rats. J. Oleo Sci. 2009, 58, 111–115. [Google Scholar] [CrossRef]
- Miettinen, T.A. Serum squalene and methyl sterols as indicators of cholesterol synthesis in vivo. Life Sci. 1969, 8, 713–721. [Google Scholar] [CrossRef]
- Brown, A.J.; Ikonen, E.; Olkkonen, V.M. Cholesterol precursors: More than mere markers of biosynthesis. Curr. Opin. Lipidol. 2014, 25, 133–139. [Google Scholar] [CrossRef]
- Shirouchi, B.; Kawahara, Y.; Kutsuna, Y.; Higuchi, M.; Okumura, M.; Mitsuta, S.; Nagao, N.; Tanaka, K. Oral Administration of Chaetoceros gracilis—A Marine Microalga—Alleviates Hepatic Lipid Accumulation in Rats Fed a High-Sucrose and Cholesterol-Containing Diet. Metabolites 2023, 13, 436. [Google Scholar] [CrossRef]
- Pfaffl, M.W. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001, 29, e45. [Google Scholar] [CrossRef]
- Kanda, Y. Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transplant. 2013, 48, 452–458. [Google Scholar] [CrossRef]
- Eckel, R.H.; Grundy, S.M.; Zimmet, P.Z. The metabolic syndrome. Lancet 2005, 365, 1415–1428. [Google Scholar] [CrossRef]
- Nagao, K.; Wang, Y.M.; Inoue, N.; Han, S.Y.; Buang, Y.; Noda, T.; Kouda, N.; Okamatsu, H.; Yanagita, T. The 10trans, 12cis isomer of conjugated linoleic acid promotes energy metabolism in OLETF rats. Nutrition 2003, 19, 652–656. [Google Scholar] [CrossRef]
- Nagao, K.; Jinnouchi, T.; Kai, S.; Yanagita, T. Effect of dietary resveratrol on the metabolic profile of nutrients in obese OLETF rats. Lipids Health Dis. 2013, 12, 8. [Google Scholar] [CrossRef]
- Shirouchi, B.; Nagao, K.; Umegatani, M.; Shiraishi, A.; Morita, Y.; Kai, S.; Yanagita, T.; Ogawa, A.; Kadooka, Y.; Sato, M. Probiotic Lactrobacillus gasseri SBT2055 improves glucose tolerance and reduces body weight gain in rats by stimulating energy expenditure. Br. J. Nutr. 2016, 116, 451–458. [Google Scholar] [CrossRef]
- Nagao, K.; Jinnouchi, T.; Kai, S.; Yanagita, T. Pterostilbene, a demethylated analog of resveratrol, promotes energy metabolism in obese rats. J. Nutr. Biochem. 2017, 43, 151–155. [Google Scholar] [CrossRef]
- Takeyama, A.; Nagata, Y.; Shirouchi, B.; Nonaka, C.; Aoki, H.; Haraguchi, T.; Sato, M.; Tamaya, K.; Yamamoto, H.; Tanaka, K. Dietary Sparassis crispa reduces body fat mass and hepatic lipid levels by enhancing energy expenditure and suppressing lipogenesis in rats. J. Oleo Sci. 2018, 67, 1137–1147. [Google Scholar] [CrossRef]
- Herman-Edelstein, M.; Scherzer, P.; Tobar, A.; Levi, M.; Gafter, U. Altered renal lipid metabolism and renal lipid accumulation in human diabetic nephropathy. J. Lipid Res. 2014, 55, 561–572. [Google Scholar] [CrossRef]
- Stadler, K.; Goldberg, I.J.; Susztak, K. The evolving understanding of the contribution of lipid metabolism to diabetic kidney disease. Curr. Diab. Rep. 2015, 15, 40. [Google Scholar] [CrossRef]
- Athyros, V.G.; Doumas, M.; Imprialos, K.P.; Stavropoulos, K.; Georgianou, E.; Katsimardou, A.; Karagiannis, A. Diabetes and lipid metabolism. Hormones 2018, 17, 61–67. [Google Scholar] [CrossRef]
- Rideout, T.C.; Marinangeli, C.P.F.; Harding, S.V. Triglyceride-lowering response to plant sterol and stanol consumption. J. AOAC Int. 2015, 98, 707–715. [Google Scholar] [CrossRef]
- Tomoyori, H.; Kawata, Y.; Higuchi, T.; Ichi, I.; Sato, H.; Sato, M.; Ikeda, I.; Imaizumi, K. Phytosterol oxidation products are absorbed in the intestinal lymphatics in rats but do not accelerate atherosclerosis in apolipoprotein E-deficient mice. J. Nutr. 2004, 134, 1690–1696. [Google Scholar] [CrossRef]
- Ikeda, I.; Konno, R.; Shimizu, T.; Ide, T.; Takahashi, N.; Kawada, T.; Nagao, K.; Inoue, N.; Yanagita, T.; Hamada, T.; et al. Campest-5-en-3-one, an oxidized derivative of campesterol, activates PPARalpha, promotes energy consumption and reduces visceral fat deposition in rats. Biochim. Biophys. Acta 2006, 1760, 800–807. [Google Scholar] [CrossRef]
- Wang, X.; Guan, L.; Zhao, Y.; Lei, L.; Liu, Y.; Ma, K.Y.; Wang, L.; Man, S.W.; Wang, J.; Huang, Y.; et al. Plasma cholesterol-lowering activity of dietary dihydrocholesterol in hypercholesterolemia hamsters. Atherosclerosis 2015, 242, 77–86. [Google Scholar] [CrossRef]
- Saisho, Y. Postprandial C-peptide to glucose ratio as a marker of β cell function: Implication for the management of type 2 diabetes. Int. J. Mol. Sci. 2016, 17, 744. [Google Scholar] [CrossRef]
- Suzuki, K.; Tanaka, M.; Konno, R.; Kaneko, Y. Effects of 5-campestenone (24-methylcholest-5-en-3-one) on the type 2 diabetes mellitus model animal C57BL/KsJ-db/db mice. Horm. Metab. Res. 2002, 34, 121–126. [Google Scholar] [CrossRef]
- Konno, R.; Kaneko, Y.; Suzuki, K.; Matsui, Y. Effect of 5-Campestenone (24-methylcholest-5-en-3-one) on Zucker diabetic fatty rats as a type 2 diabetes mellitus model. Horm. Metab. Res. 2005, 37, 79–83. [Google Scholar] [CrossRef]
- Xu, H.; Barnes, G.T.; Yang, Q.; Tan, G.; Yang, D.; Chou, C.J.; Sole, J.; Nichols, A.; Ross, J.S.; Tartaglia, L.A.; et al. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J. Clin. Investig. 2003, 112, 1821–1830. [Google Scholar] [CrossRef]
- Song, Y.; Liu, J.; Zhao, K.; Gao, L.; Zhao, J. Cholesterol-induced toxicity: An integrated view of the role of cholesterol in multiple diseases. Cell Metab. 2021, 33, 1911–1925. [Google Scholar] [CrossRef]
- Harold, F.M.; Abraham, S.; Chaikoff, I.L. Metabolism of delta4-cholestenone in the rat. J. Biol. Chem. 1956, 221, 435–447. [Google Scholar] [CrossRef]


| Control Diet | 4-STN Diet | |
|---|---|---|
| Ingredients | (g/kg Diet) | |
| Sucrose | 479 | 476.5 |
| Casein | 200 | 200 |
| β-Cornstarch | 150 | 150 |
| Cellulose | 50 | 50 |
| Corn oil | 70 | 70 |
| 4-STN | --- | 2.5 |
| Mineral mixture (AIN-76) | 35 | 35 |
| Vitamin mixture (AIN-76) | 10 | 10 |
| DL-Methionine | 3 | 3 |
| Choline bitartrate | 2 | 2 |
| Cholesterol | 1 | 1 |
| C57BL/6J | db/db | ||
|---|---|---|---|
| NOR Group | CON Group | 4-STN Group | |
| Initial B.W. (g) | 21.0 ± 0.2 | 30.1 ± 0.4 | 30.0 ± 0.4 |
| Final B.W. (g) | 25.2 ± 0.3 | 36.2 ± 1.3 | 35.5 ± 0.9 |
| Food intake (g/day) | 2.97 ± 0.05 | 4.32 ± 0.19 | 4.29 ± 0.15 |
| Food efficiency (mg B.W. gain/g food intake) | |||
| 51.0 ± 3.7 | 48.3 ± 11.6 | 45.5 ± 6.5 | |
| Organ weight (g/100 g B.W.) | |||
| Liver | 4.06 ± 0.07 | 6.18 ± 0.41 | 5.95 ± 0.47 |
| Pancreas | 0.329 ± 0.020 | 0.253 ± 0.013 | 0.226 ± 0.010 |
| Kidney | 1.34 ± 0.02 | 1.08 ± 0.02 | 1.05 ± 0.04 |
| Quadriceps muscle | 1.52 ± 0.06 | 0.455 ± 0.014 | 0.478 ± 0.017 |
| White adipose tissue weight (g/100 g B.W.) | |||
| Perirenal | 0.749 ± 0.072 | 2.16 ± 0.12 | 2.13 ± 0.09 |
| Epididymal | 2.00 ± 0.08 | 5.16 ± 0.13 | 4.86 ± 0.13 |
| Mesenteric | 0.969 ± 0.084 | 3.08 ± 0.09 | 3.25 ± 0.10 |
| Brown adipose tissue weight (g/100 g B.W.) | |||
| 0.545 ± 0.048 | 1.14 ± 0.13 | 1.25 ± 0.07 | |
| Feces weight (g/6 days) | 1.60 ± 0.04 | 1.97 ± 0.25 | 2.10 ± 0.34 |
| Plasma biochemical parameters | |||
| Atherogenic index # | 0.115 ± 0.018 | 0.300 ± 0.050 | 0.274 ± 0.041 |
| Phospholipid (mg/dL) | 221 ± 6 | 323 ± 16 | 275 ± 17 (p = 0.067) |
| FFAs (mmol/L) | 0.930 ± 0.066 | 1.41 ± 0.11 | 1.55 ± 0.09 |
| ALT (IU/L) | 4.69 ± 0.36 | 25.4 ± 1.5 | 24.2 ± 3.1 |
| Adiponectin (µg/mL) | 16.4 ± 0.3 | 8.00 ± 0.42 | 7.02 ± 0.36 |
| Leptin (ng/mL) | 1.67 ± 0.33 | 42.4 ± 9.4 | 53.0 ± 8.6 |
| Hepatic biochemical parameters (mg/g liver) | |||
| Phospholipid | 24.3 ± 0.6 | 20.2 ± 0.3 | 19.9 ± 1.4 |
| Glycogen | 5.03 ± 1.76 | 21.1 ± 3.4 | 16.7 ± 5.3 |
| C57BL/6J | db/db | ||
|---|---|---|---|
| NOR Group | CON Group | 4-STN Group | |
| Epididymal WAT | (arbitrary unit) | ||
| Genes related to inflammatory response | |||
| Ccl2 | 100 ± 18 | 165 ± 23 | 191 ± 18 |
| Il6 | 100 ± 22 | 133 ± 19 | 189 ± 44 |
| Genes related to insulin signaling | |||
| Irs1 | 100 ± 6 | 40.5 ± 3.3 | 32.2 ± 1.0 (p = 0.056) |
| Irs2 | 100 ± 9 | 36.6 ± 6.8 | 42.8 ± 6.0 |
| Liver | |||
| Genes related to inflammatory response | |||
| Tnf | 100 ± 14 | 167 ± 67 | 131 ± 30 |
| Ccl2 | 100 ± 8 | 325 ± 110 | 335 ± 34 |
| Genes related to ER stress response | |||
| Xbp1 | 100 ± 7 | 145 ± 57 | 102 ± 23 |
| Mapk8 | 100 ± 18 | 87.2 ± 10.2 | 112 ± 15 |
| Genes related to insulin signaling and gluconeogenesis | |||
| Irs1 | 100 ± 9 | 97.6 ± 16.6 | 86.9 ± 26.3 |
| Irs2 | 100 ± 7 | 101 ± 26 | 92.1 ± 17.9 |
| Pik3ca | 100 ± 8 | 103 ± 10 | 114 ± 18 |
| Akt2 | 100 ± 7 | 192 ± 54 | 139 ± 31 |
| Foxo1 | 100 ± 11 | 156 ± 30 | 196 ± 25 |
| Pck1 | 100 ± 14 | 76.1 ± 10.4 | 156 ± 29 † |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Higuchi, M.; Okumura, M.; Mitsuta, S.; Shirouchi, B. Dietary Cholest-4-en-3-one, a Cholesterol Metabolite of Gut Microbiota, Alleviates Hyperlipidemia, Hepatic Cholesterol Accumulation, and Hyperinsulinemia in Obese, Diabetic db/db Mice. Metabolites 2024, 14, 321. https://doi.org/10.3390/metabo14060321
Higuchi M, Okumura M, Mitsuta S, Shirouchi B. Dietary Cholest-4-en-3-one, a Cholesterol Metabolite of Gut Microbiota, Alleviates Hyperlipidemia, Hepatic Cholesterol Accumulation, and Hyperinsulinemia in Obese, Diabetic db/db Mice. Metabolites. 2024; 14(6):321. https://doi.org/10.3390/metabo14060321
Chicago/Turabian StyleHiguchi, Mina, Mai Okumura, Sarasa Mitsuta, and Bungo Shirouchi. 2024. "Dietary Cholest-4-en-3-one, a Cholesterol Metabolite of Gut Microbiota, Alleviates Hyperlipidemia, Hepatic Cholesterol Accumulation, and Hyperinsulinemia in Obese, Diabetic db/db Mice" Metabolites 14, no. 6: 321. https://doi.org/10.3390/metabo14060321
APA StyleHiguchi, M., Okumura, M., Mitsuta, S., & Shirouchi, B. (2024). Dietary Cholest-4-en-3-one, a Cholesterol Metabolite of Gut Microbiota, Alleviates Hyperlipidemia, Hepatic Cholesterol Accumulation, and Hyperinsulinemia in Obese, Diabetic db/db Mice. Metabolites, 14(6), 321. https://doi.org/10.3390/metabo14060321








