A GC-MS Metabolic Study on Lipophilic Compounds in the Leaves of Common Wheat Triticum aestivum L.
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Reagents and Solvents, Synthesis of Pure Substances
Synthesis of Fatty Acid Amides
2.3. Extraction of Metabolites
2.4. Chromatographic Separation and Mass Spectrometric Detection
2.4.1. GC-MS Detection
2.4.2. GCxGC-MS Detection
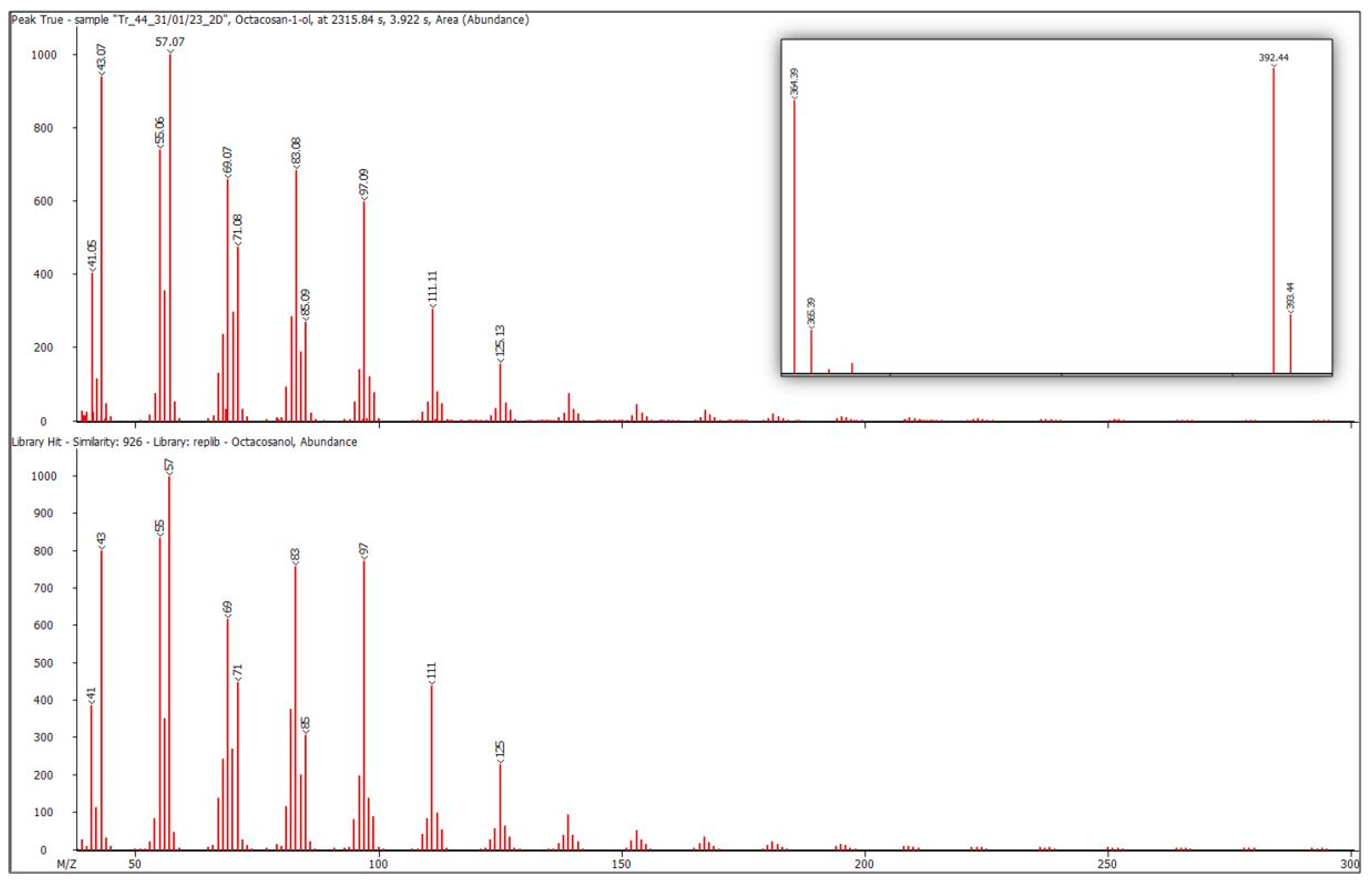
2.5. Data Processing and Annotation
2.6. Statistical Analysis
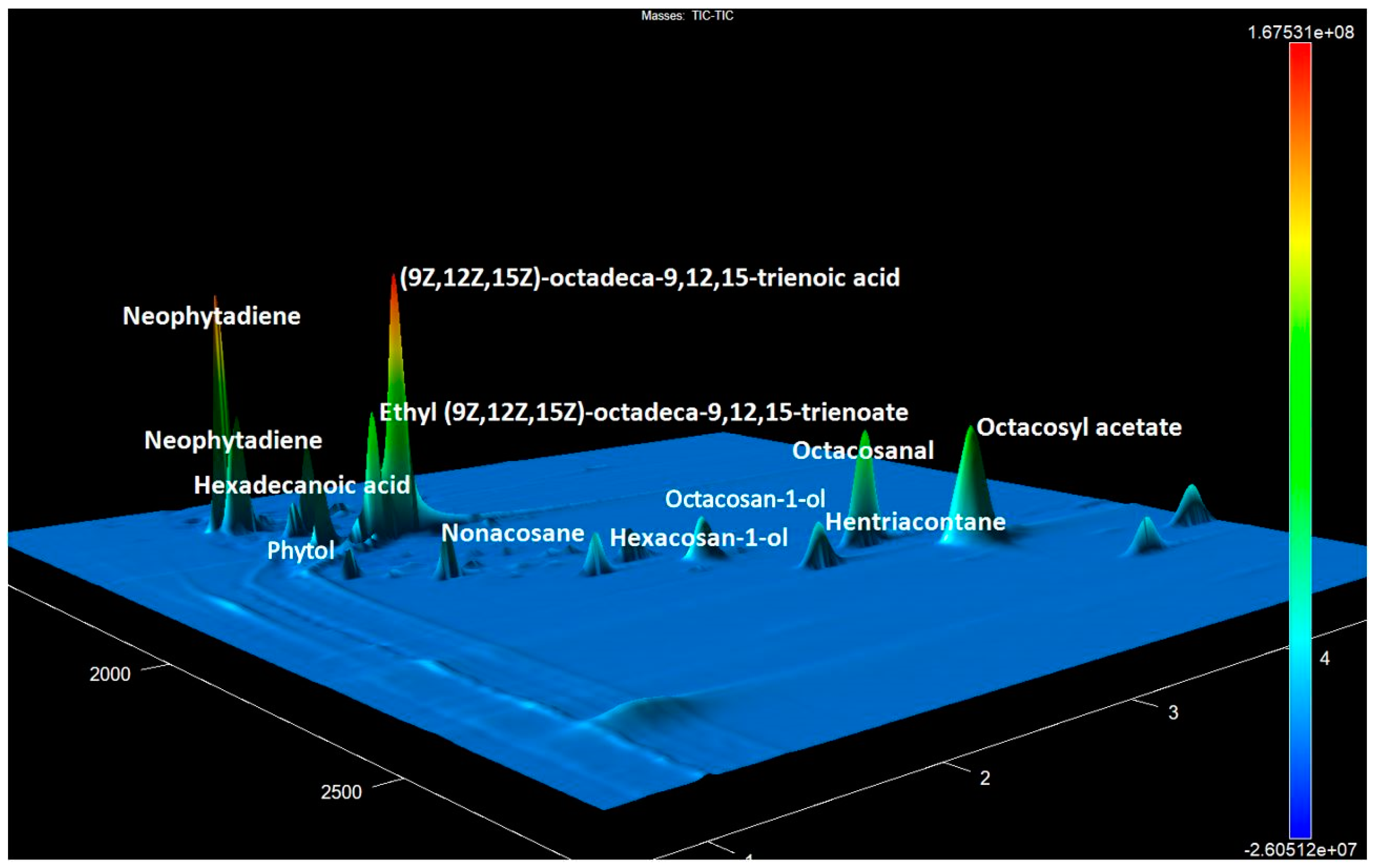
3. Results
4. Discussion
4.1. Aliphatic Alcohols
4.2. Esters of Aliphatic Alcohols
4.3. Alkanes
4.4. Fatty Acids
4.5. β-Diketones and Their Hydroxy Derivatives
4.6. Other Compounds of Signaling Systems Functioning in Leaves
4.7. Fatty Acid Amides
4.8. Aliphatic Aldehydes
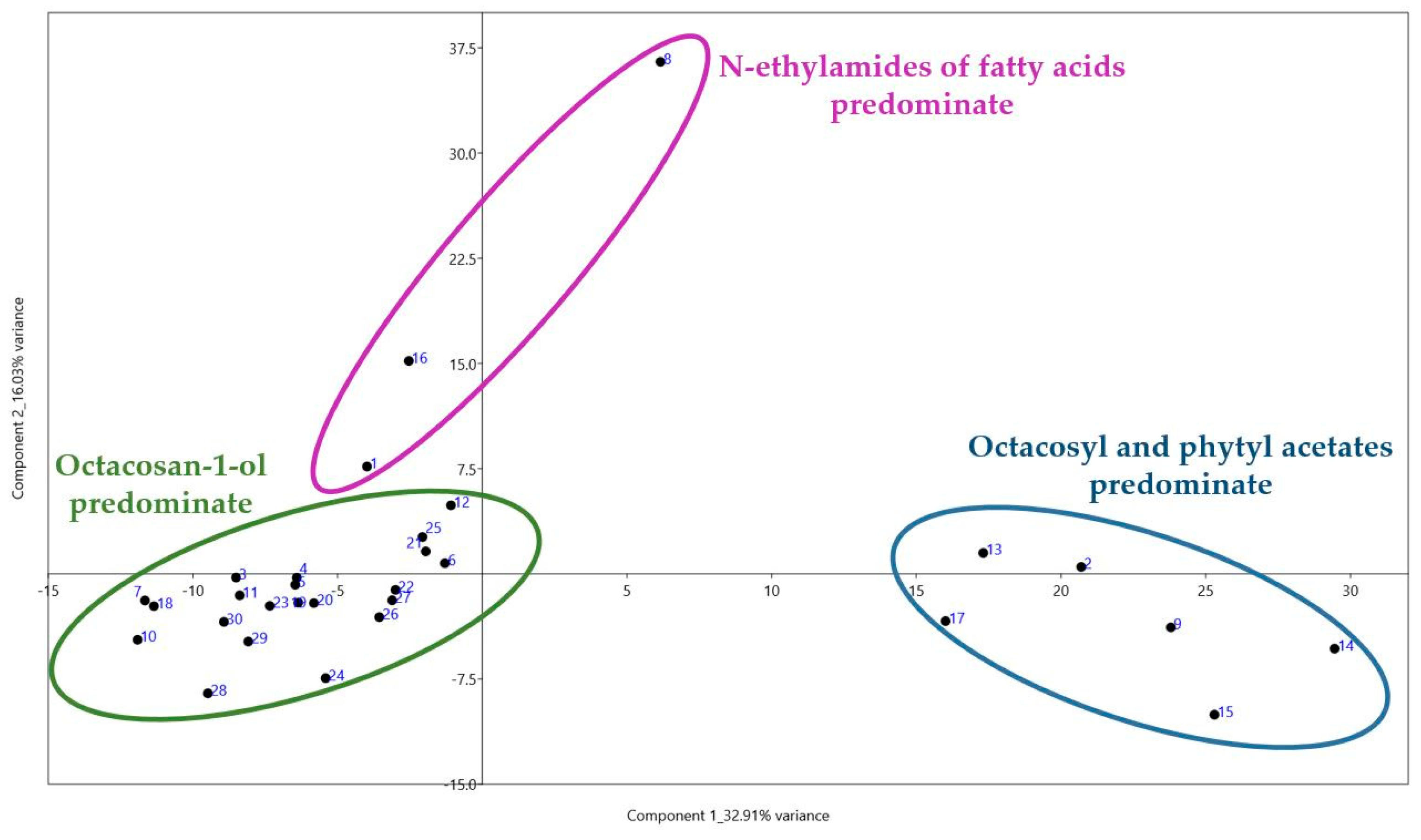
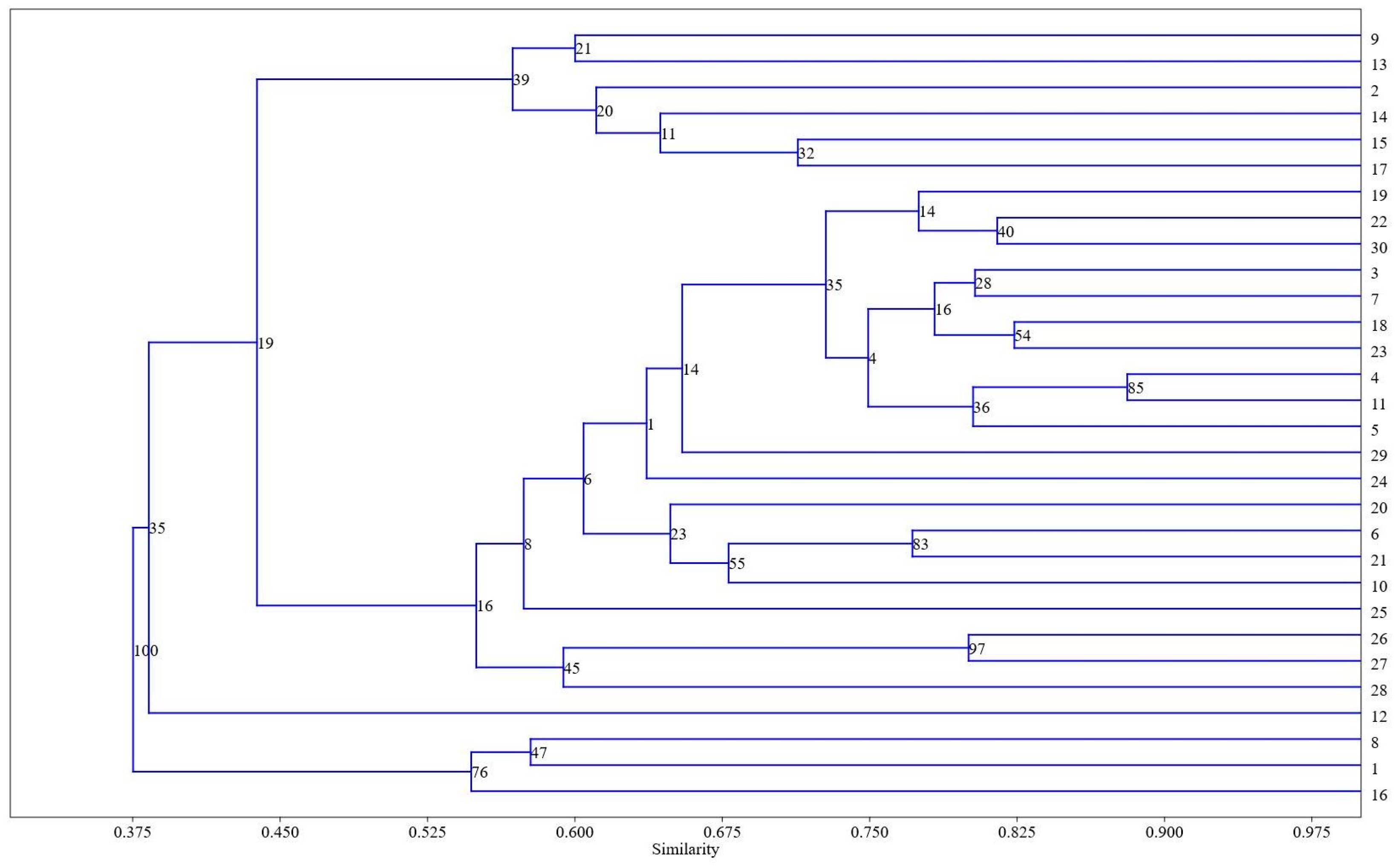
5. Statistical Processing Findings
6. Conclusions
- The differences observed in the composition of the extracts provide evidence for the existence of metabolic polymorphism in the studied T. aestivum varieties, which will allow breeding for these metabolites.
- The predominant component identified in the leaf extracts of most of the studied varieties was found to be octacosan-1-ol. Other markers of this polymorphism include N-ethylamides of fatty acids, acetates of octacosan-1-ol and phytol, and β-diketones.
- For the first time, N-ethylamides of fatty acids that may play an important role in plant adaptation to stress have been detected in plant extracts.
- No correlations were found between cuticle composition and the place of origin of wheat accessions.
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Facts & Figures on Food and Biodiversity. Available online: https://idrc-crdi.ca/en/research-in-action/facts-figures-food-and-biodiversity (accessed on 14 June 2024).
- Feldman, M.; Levy, A.A. Evolution of wheat under cultivation. In Wheat Evolution and Domestication; Springer Nature: Cham, Switzerland, 2023; pp. 605–663. [Google Scholar] [CrossRef]
- Thynne, E.; Mead, O.L.; Chooi, Y.H.; Mcdonald, M.C.; Solomon, P.S.; Katz, L.A. Acquisition and loss of secondary metabolites shaped the evolutionary path of three emerging phytopathogens of wheat. Genome Biol Evol. 2019, 11, 890–905. [Google Scholar] [CrossRef] [PubMed]
- Fotev, Y.V.; Pivovarov, V.F.; Artemyeva, A.M.; Kulikov, I.M.; Goncharova, Y.K.; Syso, A.I.; Goncharov, N.P. Concept of producing of the Russian national system of functional food. Vavilovskii Zhournal Genet. I Sel. 2018, 22, 776–783. [Google Scholar] [CrossRef]
- Khaneghah, A.M.; Martins, L.M.; von Hertwig, A.M.; Bertoldo, R.; Sant’Ana, A.S. Deoxynivalenol and its masked forms: Characteristics, incidence, control and fate during wheat and wheat based products processing—A review. Trends Food Sci. Technol. 2018, 71, 13–24. [Google Scholar] [CrossRef]
- Saied, D.B.; Ramadan, N.S.; El-Sayed, M.M.; Farag, M.A. Effect of Maturity Stage on Cereal and Leguminous Seeds’ Metabolome as Analyzed Using Gas Chromatography Mass-Spectrometry (GC-MS) and Chemometric Tools. Metabolites 2023, 13, 163. [Google Scholar] [CrossRef] [PubMed]
- Shi, T.; Zhu, A.; Jia, J.; Hu, X.; Chen, J.; Liu, W.; Chen, W. Metabolomics analysis and metabolite-agronomic trait associations using kernels of wheat (Triticum aestivum) recombinant inbred lines. Plant J. 2020, 103, 279–292. [Google Scholar] [CrossRef] [PubMed]
- Zidorn, C. Plant chemophenetics—A new term for plant chemosystematics/plant chemotaxonomy in the macro-molecular era. Phytochemistry 2019, 163, 147–148. [Google Scholar] [CrossRef] [PubMed]
- Litvinov, D.Y.; Karlov, G.I.; Divashuk, M.G. Metabolomics for Crop Breeding: General Considerations. Genes 2021, 12, 1602. [Google Scholar] [CrossRef] [PubMed]
- Laskoś, K.; Czyczyło-Mysza, I. Wosk epikutykularny liści jako cecha warunkująca odporność na suszę u zbóż. Postępy Biochem. 2023, 69, 104–112. [Google Scholar] [CrossRef]
- Pastor, K.; Ačanski, M.; Vujić, Đ.; Jovanović, Đ.; Wiekoop, S. Authentication of Cereal Flours by Multivariate Analysis of GC–MS Data. Chromatographia 2016, 79, 1387–1393. [Google Scholar] [CrossRef]
- Meng, X.; Liu, Y.; Xu, S.; Yang, L.; Yin, R. Review on analytical technologies and applications in metabolomics. Biocell 2024, 48, 65–78. [Google Scholar] [CrossRef]
- Hajjar, G.; Santos, M.C.B.; Bertrand-Michel, J.; Canlet, C.; Castelli, F.; Creusot, N.; Dechaumet, S.; Diémé, B.; Giacomoni, F.; Giraudeau, P.; et al. Scaling-up metabolomics: Current state and perspectives. TrAC-Trends Anal. Chem. 2023, 167, 117225. [Google Scholar] [CrossRef]
- Ebbels, T.M.D.; van der Hooft, J.J.J.; Chatelaine, H.; Broeckling, C.; Zamboni, N.; Hassoun, S.; Mathé, E.A. Recent advances in mass spectrometry-based computational metabolomics. Curr. Opin. Chem. Biol. 2023, 74, 102288. [Google Scholar] [CrossRef] [PubMed]
- Loscher, C.; Mccarthy, C.; Guiry, P.; Murphy, C.; Maingot, C.; Dublin City University; University College Dublin; Dublin National University of Ireland. A Method for Treating Peripheral Inflammatory Disease. U.S. Patent Application filed by Dublin City University, University College Dublin, National University of Ireland, Dublin 2015. WO2016185025A1, 20 May 2016. [Google Scholar]
- Staudinger, H.; Rößler, K. Über hochpolymere Verbindungen, 124. Mitteil.: Viscositäts-Messungen an Fettsäureamiden und -aniliden. Chem. Ber. 1936, 69, 61–73. [Google Scholar] [CrossRef]
- Braun, J.V.; Jostes, F.; Münch, W. Zur Kenntnis der Imidchloride und Imidbromide aliphatischer Säuren. Liebigs Ann. 1927, 453, 113–147. [Google Scholar] [CrossRef]
- Vasilieva, A.R.; Slynko, N.M.; Tatarova, L.E.; Efimov, V.M.; Kuibida, L.V.; Asbaganov, S.V.; Peltek, S.E. A GC-MS Chemotaxonomic Study on Lipophilic Compounds in the Bark of S. aucuparia subsp. sibirica Trees from the Population Growing in Akademgorodok, Novosibirsk (Russia). Metabolites 2023, 13, 768. [Google Scholar] [CrossRef]
- Dool, V.D.; Kratz, P.D. A Generalization of the Retention Index System Including Linear Temperature Programmed Gas-Liquid Partition Chromatography. J. Chromatogr. A. 1963, 11, 463–471. [Google Scholar] [CrossRef]
- Gas Chromatographic Retention Data. Available online: https://webbook.nist.gov/chemistry/gc-ri/ (accessed on 26 July 2024).
- Hammer, Ø.H.; Harper, D.A.T.; Ryan, P.D. Past: Paleontological Statistics Software Package for Education and Data Analysis. Palaeontol. Electron. 2001, 4, 1–9. Available online: http://palaeo-electronica.org/2001_1/past/issue1_01.htm (accessed on 14 June 2024).
- Lebedev, A.T. Mass Spectrometry in Organic Chemistry, 2nd ed.; Tekhnosphere: Moscow, Russia, 2015; p. 704. ISBN 978-5-94836. [Google Scholar]
- Silverstein, R.M.; Webster, F.X.; Kiemle, D.; Bryce, D.L. Spectrometric Identification of Organic Compounds, 8th ed.; Wiley: Hoboken, NJ, USA, 2014; p. 502. [Google Scholar]
- Tulloch, A.P.; Weenink, R.O. Composition of the leaf wax of Little Club wheat. Can. J. Chem. 1969, 47, 3119–3126. [Google Scholar] [CrossRef]
- Paparella, P.; Shaltiel-Harpaza, L.; Ibdah, M. β-Ionone: Its Occurrence and Biological Function and Metabolic Engineering. Plants 2021, 10, 754. [Google Scholar] [CrossRef]
- Schuster, A.; Burghardt, M.; Alfarhan, A.; Bueno, A.; Hedrich, R.; Leide, J.; Thomas, J.; Riederer, M. Effectiveness of cuticular transpiration barriers in a desert plant at controlling water loss at high temperatures. AoB Plants 2016, 8, plw027. [Google Scholar] [CrossRef]
- Domínguez, E.; Cuartero, J.; Heredia, A. An overview on plant cuticle biomechanics. Plant Sci. 2011, 181, 77–84. [Google Scholar] [CrossRef]
- Ensikat, H.J.; Boese, M.; Mader, W.; Barthlott, W.; Koch, K. Crystallinity of plant epicuticular waxes: Electron and X-ray diffraction studies. Chem. Phys. Lipids. 2006, 144, 45–59. [Google Scholar] [CrossRef] [PubMed]
- Barthlott, W.; Neinhuis, C.; Cutler, D.; Ditsch, F.; Meusel, I.; Theisen, I.; Wilhelmi, H. Classification and terminology of plant epicuticular waxes. Bot. J. Linn. Soc. 1998, 126, 237–260. [Google Scholar] [CrossRef]
- Koch, K.; Barthlott, W.; Koch, S.; Hommes, A.; Wandelt, K.; Mamdouh, W.; De-Feyter, S.; Broekmann, P. Structural Analysis of Wheat Wax (Triticum aestivum, c.v. “Naturastar” L.): From the Molecular Level to Three Dimensional Crystals. Planta 2006, 223, 258–270. [Google Scholar] [CrossRef] [PubMed]
- Munné-Bosch, S.; Alegre, L. Vitamin E in plants: Biosynthesis, transport, and function. Trends Plant Sci. 2002, 7, 41–47. [Google Scholar] [CrossRef]
- Tulloch, A.P.; Hoffman, L.L. Leaf Wax of Triticum aestivum. Phytochemistry 1973, 12, 2217–2223. [Google Scholar] [CrossRef]
- Tulloch, A.P.; Baum, B.R.; Hoffman, L.L. A survey of epicuticular waxes among genera of Triticeae. 2. Chemistry. Can. J. Bot. 1980, 58, 2602–2615. [Google Scholar] [CrossRef]
- Su, R.; Chen, L.; Wang, Z.; Hu, Y. Differential response of cuticular wax and photosynthetic capacity by glaucous and non-glaucous wheat cultivars under mild and severe droughts. Plant Physiol. Biochem. 2020, 147, 303–312. [Google Scholar] [CrossRef] [PubMed]
- Florent, D.L.; Corey, D.B.; Darren, M.C.; Scott, D.H.; Frank, B.P.; Courtney, E.J.; Heuberger, A.L. GC-MS Metabolomics to Evaluate the Composition of Plant Cuticular Waxes for Four Triticum aestivum Cultivars. Int. J. Mol. Sci. 2018, 19, 249. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, J.; Chai, G.; Li, C.; Hu, Y.; Chen, X.; Wang, Z. Developmental Changes in Composition and Morphology of Cuticular Waxes on Leaves and Spikes of Glossy and Glaucous Wheat (Triticum aestivum L.). PLoS ONE 2015, 10, e0141239. [Google Scholar] [CrossRef]
- Wang, J.; Fan, J.; Yang, B.; Chen, X.; Zhang, F.; Cheng, Z.; Zhang, J. Analysis of composition and morphology of cuticular wax of wheat at different development stages. Int. J. Agric. Biol. 2021, 25, 813–819. [Google Scholar] [CrossRef]
- Samuels, L.; Kunst, L.; Jetter, R. Sealing Plant Surfaces: Cuticular Wax Formation by Epidermal Cells. Annu. Rev. Plant Biol. 2008, 59, 683–707. [Google Scholar] [CrossRef] [PubMed]
- Allebone, J.E.; Hamilton, R.J.; Knights, B.A.; Middleditch, B.S.; Power, T.D. Cuticular leaf waxes. Part II. Chenopodium album L. and Lolium perenne L. Chem. Phys. Lipids 1970, 4, 37–46. [Google Scholar] [CrossRef]
- Maffei, M.E. Chemotaxonomic Significance of Leaf Wax Alkanes in the Gramineae. Biochem. Syst. Ecol. 1996, 24, 53–64. [Google Scholar] [CrossRef]
- Tulloch, P. Composition of epicuticular waxes from 28 genera of Gramineae: Differences between subfamilies. Can. J. Bot. 1981, 59, 1213–1221. [Google Scholar] [CrossRef]
- Busta, L.; Jetter, R. Structure and Biosynthesis of Branched Wax Compounds on Wild Type and Wax Biosynthesis Mutants of Arabidopsis thaliana. Plant Cell Physiol. 2017, 58, 1059–1074. [Google Scholar] [CrossRef]
- Leide, J.; Nierop, K.G.J.; Deininger, A.-C.; Staiger, S.; Riederer, M.; de Leeuw, J.W. Leaf Cuticle Analyses: Implications for the Existence of Cutan/non-ester Cutin and Its Biosynthetic Origin. Annals Bot. 2020, 126, 141–162. [Google Scholar] [CrossRef]
- Richards, R.A.; Rawson, H.M.; Johnson, D.A. Glaucousness in wheat: Its development and effect on water use efficiency, gas exchange and photosynthetic tissue temperatures. Aust. J. Plant Physiol. 1986, 13, 465–473. [Google Scholar] [CrossRef]
- Johnson, D.A.; Richards, R.A.; Turner, N.C. Yield, water relations, gas exchange, and surface reflectances of near-isogenic wheat lines differing in glaucousness. Crop Sci. 1983, 23, 318–325. [Google Scholar] [CrossRef]
- Barber, H.N.; Netting, A.G. Chemical Genetics of β-diketone Formation in Wheat. Phytochemistry 1968, 7, 2089–2093. [Google Scholar] [CrossRef]
- Tulloch, A.P. Composition of Leaf Surface Waxes of Triticum Species: Variation with Age and Tissue. Phytochemistry 1973, 12, 2225–2232. [Google Scholar] [CrossRef]
- El-khwaga, A.A.; Fattouh, H.M.; Draz, I.S.; Abdelmaksoud, H.M.; Mostafa, F.A.; Abdelghany, R.E. Biological Induction of Metabolic Defense Mechanisms against Wheat Yellow Rust Using Bacterial and Organic Biocontrol Agents. Egypt. J. Phytopathol. 2023, 51, 98–113. [Google Scholar] [CrossRef]
- Morris, B.D.; Foster, S.P.; Harris, M.O. Identification of 1-octacosanal and 6-methoxy-2-benzoxazolinone from wheat as ovipositional stimulants for the Hessian fly, Mayetiola destructor. J. Chem. Ecol. 2000, 26, 859–874. [Google Scholar] [CrossRef]
- Greger, H. Alkamides: A critical reconsideration of a multifunctional class of unsaturated fatty acid amides. Phytochem. Rev. 2015, 15, 729–770. [Google Scholar] [CrossRef]
- Zhao, J.P.; Muhammad, I.; Dunbar, D.C.; Mustafa, J.; Khan, I.A. New alkamides from Maca (Lepidium meyenii). J Agric. Food Chem. 2005, 53, 690–693. [Google Scholar] [CrossRef] [PubMed]
- Wansi, J.D.; Nwozo, S.O.; Mbaze, L.M.; Devkota, K.P.; Moladje, S.M.D.; Fomum, Z.T.; Sewald, N. Amides from the stem bark of Fagara macrophylla. Planta Med. 2009, 75, 517–521. [Google Scholar] [CrossRef] [PubMed]
- Ndom, J.C.; Mbafor, J.T.; Meva´a, L.M.; Kakam, Z.; Phanuel, A.S.; Ndongo, E.; Harwood, L.M.; Mpondo, T.N. New alkamide and ent-kaurane diterpenoid derivatives from Senecio erechtitoides (Asteraceae). Phytochem. Lett. 2010, 3, 201–206. [Google Scholar] [CrossRef]
- Muhammad, I.; Dunbar, D.C.; Khan, I.A. Constituents of Lepidium meyenii “maca”. Phytochemistry 2002, 59, 105–110. [Google Scholar] [CrossRef] [PubMed]
- Bisht, K.; Merkler, D. The Biosynthesis and Metabolism of the N-Acylated Aromatic Amino Acids: N-Acylphenylalanine, N-Acyltyrosine, N-Acyltryptophan, and N-Acylhistidine. Front. Mol. Biosci. 2021, 8, 801749. [Google Scholar] [CrossRef]
- Buschhaus, C.; Jetter, R. Composition and Physiological Function of the Wax Layers Coating Arabidopsis Leaves: β-amyrin Negatively Affects the Intracuticular Water Barrier. Plant Physiol. 2012, 160, 1120–1129. [Google Scholar] [CrossRef]


| Injection | 1 mkl, inlet split ratio 1:10, front inlet temp. 280 °C |
| Carrier gas | Helium, constant flow 1.4 mL/min |
| Column one | Rxi-5MS, 30 m × 0.25 mm i.d. × 0.25 µm coating (Restek) |
| Column two | Rxi-17Sil MS, 2 m × 0.25 mm i.d. × 0.25 µm coating (Restek) |
| Temp. program | 50 °C (1 min); 5 °C/min to 150 °C: 10 °C/min to 250 °C: 20 °C/min to 280 °C (60 min): primary oven maintained +5 °C relative to secondary oven |
| Modulator timing | 8 s; temp. maintained + 15 °C relative to secondary oven |
| Transfer line temp. | 280 °C |
| Electron energy | 70 eV |
| Ion source temp. | 280 °C |
| Mass range (m/z) | 40–650 |
| Acquisition delay | 300 s |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vasilieva, A.R.; Slynko, N.M.; Goncharov, N.P.; Tatarova, L.E.; Kuibida, L.V.; Peltek, S.E. A GC-MS Metabolic Study on Lipophilic Compounds in the Leaves of Common Wheat Triticum aestivum L. Metabolites 2024, 14, 426. https://doi.org/10.3390/metabo14080426
Vasilieva AR, Slynko NM, Goncharov NP, Tatarova LE, Kuibida LV, Peltek SE. A GC-MS Metabolic Study on Lipophilic Compounds in the Leaves of Common Wheat Triticum aestivum L. Metabolites. 2024; 14(8):426. https://doi.org/10.3390/metabo14080426
Chicago/Turabian StyleVasilieva, Asya R., Nikolay M. Slynko, Nikolay P. Goncharov, Ljudmila E. Tatarova, Leonid V. Kuibida, and Sergey E. Peltek. 2024. "A GC-MS Metabolic Study on Lipophilic Compounds in the Leaves of Common Wheat Triticum aestivum L." Metabolites 14, no. 8: 426. https://doi.org/10.3390/metabo14080426
APA StyleVasilieva, A. R., Slynko, N. M., Goncharov, N. P., Tatarova, L. E., Kuibida, L. V., & Peltek, S. E. (2024). A GC-MS Metabolic Study on Lipophilic Compounds in the Leaves of Common Wheat Triticum aestivum L. Metabolites, 14(8), 426. https://doi.org/10.3390/metabo14080426







