Potential Use of Common Administration of Emulsion for Parenteral Nutrition and Vinpocetine: Compatibility Study and Prospect
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials: Vinpocetine Solutions and Parenteral Nutrition Admixtures
2.2. Methods: Compatibility Evaluation
2.2.1. Visual Control
2.2.2. The pH Measurement
2.2.3. Osmolality Measurement
2.2.4. Measurement of Turbidity
2.2.5. Measurement of Droplet Size, PDI, and Zeta Potential
2.2.6. Particle Size Measurements (LO)
2.2.7. Statistical Analysis
3. Results
3.1. Characterization of Total Parenteral Nutrition Admixtures without Drug Solutions
3.2. Compatibility Test Assessment
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mielańczuk-Lubecka, B.; Krzysztoń, K.; Zdrowowicz, A.; Stolarski, J.; Piaścik, R.; Domitrz, I. Dizziness as a first alarming symptom to neurological hospital admission: Reasons and differentiation problem—A pilot study. J. Med. Sci. 2021, 90, e562. [Google Scholar] [CrossRef]
- Petric, Z.; Paixão, P.; Filipe, A.; Guimarães Morais, J. Clinical Pharmacology of Vinpocetine: Properties Revisited and Introduction of a Population Pharmacokinetic Model for Its Metabolite, Apovincaminic Acid (AVA). Pharmaceutics 2023, 15, 2502. [Google Scholar] [CrossRef]
- Bereczki, D.; Fekete, I. Vinpocetine for acute ischaemic stroke. Cochrane Database Syst. Rev. 2008, 2008, CD000480. [Google Scholar] [CrossRef] [PubMed]
- Panda, P.K.; Ramachandran, A.; Panda, P.; Sharawat, I.K. Safety and Efficacy of Vinpocetine as a Neuroprotective Agent in Acute Ischemic Stroke: A Systematic Review and Meta-Analysis. Neurocrit. Care 2022, 37, 314–325. [Google Scholar] [CrossRef] [PubMed]
- August, D.A.; Huhmann, M.B.; American Society for Parenteral and Enteral Nutrition. Clinical Guidelines: Nutrition Support Therapy During Adult Anticancer Treatment and in Hematopoietic Cell Transplantation. J. Parenter. Enter. Nutr. 2009, 33, 472–500. [Google Scholar] [CrossRef]
- Mirtallo, J.; Canada, T.; Johnson, D.; Kumpf, V.; Petersen, C.; Sacks, G.; Seres, D.; Guenter, P. Task Force for the Revision of Safe Practices for Parenteral Nutrition Safe practices for parenteral nutrition. J. Parenter. Enter. Nutr. 2004, 28, S39–S70. [Google Scholar] [CrossRef]
- Braga, M.; Ljungqvist, O.; Soeters, P.; Fearon, K.; Weimann, A.; Bozzetti, F. ESPEN Guidelines on Parenteral Nutrition: Surgery. Clin. Nutr. 2009, 28, 378–386. [Google Scholar] [CrossRef]
- Lourenço, R. Enteral feeding: Drug/nutrient interaction. Clin. Nutr. 2001, 20, 187–193. [Google Scholar] [CrossRef] [PubMed]
- El-Laithy, H.M.; Shoukry, O.; Mahran, L.G. Novel sugar esters proniosomes for transdermal delivery of vinpocetine: Preclinical and clinical studies. Eur. J. Pharm. Biopharm. 2011, 77, 43–55. [Google Scholar] [CrossRef]
- Dhaval, M.; Vaghela, P.; Patel, K.; Sojitra, K.; Patel, M.; Patel, S.; Dudhat, K.; Shah, S.; Manek, R.; Parmar, R. Lipid-based emulsion drug delivery systems—A comprehensive review. Drug Deliv. Transl. Res. 2022, 12, 1616–1639. [Google Scholar] [CrossRef]
- Tomczak, S.; Stawny, M.; Dettlaff, K.; Kieliszek, M.; Słomińska, D.; Jelińska, A. Physicochemical compatibility and stability of linezolid with parenteral nutrition. Molecules 2019, 24, 1242. [Google Scholar] [CrossRef]
- Aeberhard, C.; Steuer, C.; Saxer, C.; Huber, A.; Stanga, Z.; Mühlebach, S. Physicochemical stability and compatibility testing of levetiracetam in all-in-one parenteral nutrition admixtures in daily practice. Eur. J. Pharm. Sci. 2017, 96, 449–455. [Google Scholar] [CrossRef]
- Angare, D.; Giri, T.; Tripathi, D.K.; Ajazuddin, A. Unexplored Areas and New Findings in Lipid Emulsion Serving as a Potential Drug Carrier for Lipophilic Drugs: A Review. Trends Med. Res. 2012, 7, 1–24. [Google Scholar] [CrossRef]
- Cavassin, F.B.; Baú-Carneiro, J.L.; Vilas-Boas, R.R.; Queiroz-Telles, F. Sixty years of Amphotericin B: An Overview of the Main Antifungal Agent Used to Treat Invasive Fungal Infections. Infect. Dis. Ther. 2021, 10, 115–147. [Google Scholar] [CrossRef] [PubMed]
- Trissel, L.A.; Gilbert, D.L.; Martinez, J.F.; Baker, M.B.; Walter, W.V.; Mirtallo, J.M. Compatibility of Medications with 3-in-1 Parenteral Nutrition Admixtures. J. Parenter. Enter. Nutr. 1999, 23, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Robinson, C.A.; Sawyer, J.E. Y-Site Compatibility of Medications with Parenteral Nutrition. J. Pediatr. Pharmacol. Ther. 2009, 14, 48–56. [Google Scholar] [CrossRef]
- Gostyńska, A.; Stawny, M.; Dettlaff, K.; Jelińska, A. The interactions between ciprofloxacin and parenteral nutrition admixtures. Pharmaceutics 2020, 12, 27. [Google Scholar] [CrossRef] [PubMed]
- Tomczak, S.; Gostyńska, A.; Nadolna, M.; Reisner, K.; Orlando, M.; Jelińska, A.; Stawny, M. Stability and compatibility aspects of drugs: The case of selected cephalosporins. Antibiotics 2021, 10, 549. [Google Scholar] [CrossRef] [PubMed]
- Tomczak, S.; Stawny, M.; Jelińska, A. Co-administration of drugs and parenteral nutrition: In vitro compatibility studies of loop diuretics for safer clinical practice. Pharmaceutics 2020, 12, 1092. [Google Scholar] [CrossRef]
- Bouchoud, L.; Fonzo-Christe, C.; Klingmüller, M.; Bonnabry, P. Compatibility of intravenous medications with parenteral nutrition: In vitro evaluation. J. Parenter. Enter. Nutr. 2013, 37, 416–424. [Google Scholar] [CrossRef]
- Lee, T.M.; Villareal, C.L.; Meyer, L.M. Y-Site Compatibility of Intravenous Levetiracetam with Commonly Used Critical Care Medications. Hosp. Pharm. 2019, 56, 282–286. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, T.; Forest, J.M.; Leclair, G. Compatibility of cloxacillin sodium with selected intravenous drugs during simulated Y-site administration. Hosp. Pharm. 2015, 50, 214–220. [Google Scholar] [CrossRef] [PubMed]
- Dettlaff, K.; Anglart, G.; Gruszczyńska, A.; Jelińska, A. Compatibility studies of selected multichamber bag parenteral nutrition with fluconazole. Nutrition 2024, 123, 112417. [Google Scholar] [CrossRef] [PubMed]
- Newton, D.W.; Driscoll, D.F. Calcium and phosphate compatibility: Revisited again. Am. J. Health Syst. Pharm. 2008, 65, 73–80. [Google Scholar] [CrossRef] [PubMed]
- Particulate contamination: Visible particles. In European Pharmacopoeia (Ph. Eur.) | European Directorate for Quality in Medicines and Healthcare, 9th ed.; European Directorate for Quality in Medicines and Healthcare (EDQM): Strasburg, France, 2017; Available online: https://www.edqm.eu/en/european-pharmacopoeia-ph-eur-9th-edition (accessed on 20 May 2024).
- Peng, J.; Dong, W.-J.; Li, L.; Xu, J.-M.; Jin, D.-J.; Xia, X.-J.; Liu, Y.-L. Effect of high-pressure homogenization preparation on mean globule size and large-diameter tail of oil-in-water injectable emulsions. J. Food Drug Anal. 2015, 23, 828–835. [Google Scholar] [CrossRef] [PubMed]
- Staven, V.; Wang, S.; Grønlie, I.; Tho, I. Development and evaluation of a test program for Y-site compatibility testing of total parenteral nutrition and intravenous drugs. Nutr. J. 2016, 15, 29. [Google Scholar] [CrossRef] [PubMed]
- Trissel, L.A.; Bready, B.B. Turbidimetric assessment of the compatibility of taxol with selected other drugs during simulated Y-site injection. Am. J. Hosp. Pharm. 1992, 49, 1716–1719. [Google Scholar] [CrossRef] [PubMed]
- Globule Size Distribution in Lipid Injectable Emulsions <729>. The United States Pharmacopeia 33/National Formulary 28. 2009. Available online: https://www.drugfuture.com/Pharmacopoeia/USP32/pub/data/v32270/usp32nf27s0_c729.html (accessed on 24 May 2024).
- Lin, C.; Chen, F.; Ye, T.; Zhang, L.; Zhang, W.; Liu, D.; Xiong, W.; Yang, X.; Pan, W. A novel oral delivery system consisting in “drug-in cyclodextrin-in nanostructured lipid carriers” for poorly water-soluble drug: Vinpocetine. Int. J. Pharm. 2014, 465, 90–96. [Google Scholar] [CrossRef] [PubMed]
- Song, S.; Tian, B.; Chen, F.; Zhang, W.; Pan, Y.; Zhang, Q.; Yang, X.; Pan, W. Potentials of proniosomes for improving the oral bioavailability of poorly water-soluble drugs. Drug Dev. Ind. Pharm. 2015, 41, 51–62. [Google Scholar] [CrossRef]
- Kováčik, A.; Kopečná, M.; Vávrová, K. Permeation enhancers in transdermal drug delivery: Benefits and limitations. Expert Opin. Drug Deliv. 2020, 17, 145–155. [Google Scholar] [CrossRef]
- Srivastava, P.K.; Thakkar, H.P. Vinpocetine loaded ultradeformable liposomes as fast dissolving microneedle patch: Tackling treatment challenges of dementia. Eur. J. Pharm. Biopharm. 2020, 156, 176–190. [Google Scholar] [CrossRef]
- Hard, S.A.A.A.; Shivakumar, H.N.; Redhwan, M.A.M. Development and optimization of in-situ gel containing chitosan nanoparticles for possible nose-to-brain delivery of vinpocetine. Int. J. Biol. Macromol. 2023, 253, 127217. [Google Scholar] [CrossRef]
- Ahmed, T.A.; El-Say, K.M.; Ahmed, O.A.; Aljaeid, B.M. Superiority of TPGS-loaded micelles in the brain delivery of vinpocetine via administration of thermosensitive intranasal gel. Int. J. Nanomed. 2019, 14, 5555–5567. [Google Scholar] [CrossRef]
- Stawny, M.; Nadolna, M.; Jelińska, A. In vitro compatibility studies of vancomycin with ready-to-use parenteral nutrition admixtures for safer clinical practice. Clin. Nutr. 2019, 39, 2539–2546. [Google Scholar] [CrossRef]
- Piwowarczyk, L.; Tomczak, S.; Antkowiak, P.; Jelińska, A.; Stawny, M. Sodium Valproate Incompatibility with Parenteral Nutrition Admixtures-A Risk to Patient Safety: An In Vitro Evaluation Study. Pharmaceutics 2022, 14, 371. [Google Scholar] [CrossRef]
- Dettlaff, K.; Dominiak, K.; Klimaszewska, M.; Gostyńska, A. Physical compatibility of ibuprofen and selected parenteral drugs during simulated Y-site administration. Acta Pol. Pharm. 2023, 80, 255–267. [Google Scholar] [CrossRef] [PubMed]
- Driscoll, D.F.; Ling, P.-R.; Silvestri, A.P.; Bistrian, B.R. Fine vs. coarse complete all-in-one admixture infusions over 96 hours in rats: Fat globule size and hepatic function. Clin. Nutr. 2008, 27, 889–894. [Google Scholar] [CrossRef] [PubMed]
- Cowl, C.T.; Weinstock, J.V.; Al-Jurf, A.; Ephgrave, K.; Murray, J.A.; Dillon, K. Complications and cost associated with parenteral nutrition delivered to hospitalized patients through either subclavian or peripherally-inserted central catheters. Clin. Nutr. 2000, 19, 237–243. [Google Scholar] [CrossRef] [PubMed]
- Driscoll, D.F.; Ling, P.-R.; Bistrian, B.R. Pathological consequences to reticuloendothelial system organs following infusion of unstable all-in-one mixtures in rats. Clin. Nutr. 2006, 25, 842–850. [Google Scholar] [CrossRef] [PubMed]
- Boullata, J.I.; Mirtallo, J.M.; Sacks, G.S.; Salman, G.; Gura, K.; Canada, T.; Maguire, A.; the ASPEN Parenteral Nutrition Safety Committee. Parenteral nutrition compatibility and stability: A comprehensive review. J. Parenter. Enter. Nutr. 2022, 46, 273–299. [Google Scholar] [CrossRef]
- Staven, V.; Iqbal, H.; Wang, S.; Grønlie, I.; Tho, I. Physical compatibility of total parenteral nutrition and drugs in Y-site administration to children from neonates to adolescents. J. Pharm. Pharmacol. 2017, 69, 448–462. [Google Scholar] [CrossRef] [PubMed]
- Driscoll, D.F.; Bhargava, H.N.; Li, L.; Zaim, R.H.; Babayan, V.K.; Bistrian, B.R. Physicochemical stability of total nutrient admixtures. Am. J. Health-Syst. Pharm. 1995, 52, 623–634. [Google Scholar] [CrossRef] [PubMed]
- WHO. Water Quality and Health: Review of Turbidity. Water, Sanitation, Hygiene and Health (WSH). 2017. Available online: https://www.who.int/publications-detail-redirect/WHO-FWC-WSH-17.01 (accessed on 21 January 2024).
- Washington, C. The electrokinetic properties of phospholipid stabilized fat emulsions VI. Zeta potentials of Intralipid 20% in TPN mixtures. Int. J. Pharm. 1992, 87, 167–174. [Google Scholar] [CrossRef]


| Ingredient | Omegaflex Special | Omegaflex Peri | Lipoflex Special | Lipoflex Peri | SmofKabiven | Kabiven | Kabiven Peripheral | Olimel Peri N4E |
|---|---|---|---|---|---|---|---|---|
| ILE | Lipidem® | Lipidem® | Lipofundin® | Lipofundin® | SMOFlipid® | Intralipid® | Intralipid® | ClinOleic® |
| Lipids [g] | 40 | 40 | 40 | 40 | 38 | 39 | 35 | 30 |
| MCT [g] | 20 | 20 | 20 | 20 | 11 | - | - | - |
| Soybean oil [g] | 16 | 16 | 20 | 20 | 11 | 39 | 35 | 6 |
| Fish oil [g] | - | - | - | - | 5.7 | - | - | - |
| Olive oil [g] | - | - | - | - | 9.6 | - | - | 24 |
| ω-3 fatty acids [g] | 4 | 4 | - | - | - | - | - | - |
| Glucose [g] | 144 | 64 | 144 | 64 | 127 | 97 | 67 | 75 |
| Amino acids [g] | 56 | 32 | 56 | 32 | 50.8 | 33.1 | 23.6 | 25.3 |
| Nitrogen [g] | 8 | 4.6 | 8 | 4.6 | 8.1 | 5.3 | 3.8 | 4 |
| Total energy [kcal] | 1184 | 764 | 1184 | 764 | 1084 | 909 | 695 | 700 |
| Sample | pH ± SD | Osmolality ± SD (mOsm/kgH2O) | Turbidity ± SD (NTU) | ZP ± SD (mV) | PDI ± SD | MDD ± SD (nm) |
|---|---|---|---|---|---|---|
| parenteral fluids | ||||||
| 0.9% NaCl | 6.60 ± 0.01 | 289 ± 1 | 0.093 ± 0.007 | N/A | N/A | N/A |
| 5% glucose | 4.85 ± 0.01 | 291 ± 1 | 0.160 ± 0.004 | N/A | N/A | N/A |
| drug solution | ||||||
| VP+ 0.9% NaCl | 3.48 ± 0.02 | 296 ± 1 | 0.168 ± 0.012 | N/A | N/A | N/A |
| VP + 5% glucose | 3.71 ± 0.04 | 301 ± 2 | 0.174 ± 0.003 | N/A | N/A | N/A |
| parenteral nutrition admixtures | ||||||
| Omegaflex special | 5.57 ± 0.01 | 1932 ± 6 | 0.186 ± 0.004 | −9.5 ± 0.2 | 0.111 ± 0.020 | 251.2 ± 1.8 |
| Omegaflex peri | 5.48 ± 0.00 | 921 ± 4 | 0.133 ± 0.015 | −14.3 ± 0.5 | 0.095 ± 0.014 | 249.9 ± 5.3 |
| Lipoflex special | 5.51 ± 0.01 | 2008 ± 6 | 0.134 ± 0.002 | −8.35 ± 0.6 | 0.102 ± 0.024 | 267.3 ± 3.8 |
| Lipoflex peri | 5.74 ± 0.00 | 909 ± 1 | 0.121 ± 0.004 | −11.2 ± 0.1 | 0.116 ± 0.008 | 256.5 ± 1.7 |
| SmofKabiven | 5.47 ± 0.00 | 1582 ± 14 | 0.220 ± 0.002 | −11.6 ± 0.3 | 0.119 ± 0.013 | 243.4 ± 3.7 |
| Kabiven | 5.51 ± 0.01 | 1153 ± 8 | 0.441 ± 0.014 | −10.8 ± 0.5 | 0.133 ± 0.028 | 281.7 ± 0.3 |
| Kabiven Peripheral | 5.58 ± 0.00 | 795 ± 2 | 0.223 ± 0.002 | −15.0 ± 0.5 | 0.130 ± 0.023 | 267.2 ± 3.0 |
| Olimel Peri N4E | 6.40 ± 0.00 | 850 ± 1 | 0.209 ± 0.021 | −17.2 ± 0.4 | 0.113 ± 0.018 | 256.5 ± 1.5 |
| PNA | Drug Solution | VP:PNA Ratio | pH | Osmolality, mOsm/kg H2O | Turbidity, NTU | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Average ± SD | Δ (t4h − t0h) | Average ± SD | Δ% | Average ± SD | Δ (t4h − t0h) | ||||||
| t0h | t4h | t0h | t4h | t0h | t4h | ||||||
| Omegaflex special | VP + NS | 8:2 | 5.29 ± 0.00 | 5.23 ± 0.01 | −0.06 | 559.5 ± 2.1 | 559.0 ± 4.2 | −0.5 | 0.112 ± 0.002 | 0.112 ± 0.002 | −0.109 |
| 6:4 | 5.46 ± 0.00 | 5.41 ± 0.01 | −0.05 | 857.5 ± 0.7 | 860.5 ± 0.7 | 3.0 | 0.228 ± 0.004 | 0.145 ± 0.040 | −0.083 | ||
| 5:5 | 5.48 ± 0.00 | 5.47 ± 0.01 | −0.01 | 998 ± 0.0 | 998.5 ± 0.7 | 0.5 | 0.198 ± 0.006 | 0.150 ± 0.053 | −0.048 | ||
| VP + 5Gl | 8:2 | 5.41 ± 0.00 | 5.39 ± 0.01 | −0.02 | 610.5 ± 0.7 | 609.0 ± 0.0 | −1.5 | 0.181 ± 0.003 | 0.180 ± 0.012 | −0.001 | |
| 6:4 | 5.49 ± 0.00 | 5.48 ± 0.00 | −0.01 | 850.5 ± 0.7 | 843.0 ± 5.7 | −7.5 | 0.247 ± 0.006 | 0.165 ± 0.006 | −0.081 | ||
| 5:5 | 5.51 ± 0.01 | 5.51 ± 0.00 | 0.00 | 985 ± 2.8 | 980.5 ± 2.1 | −4.5 | 0.207 ± 0.014 | 0.138 ± 0.006 | −0.069 | ||
| Lipoflex special | VP + NS | 8:2 | 5.13 ± 0.01 | 5.20 ± 0.00 | 0.07 | 498.5 ± 3.5 | 509.0 ± 1.4 | 10.5 | 0.330 ± 0.044 | 0.233 ± 0.015 | −0.097 |
| 6:4 | 5.39 ± 0.01 | 5.41 ± 0.01 | 0.02 | 818 ± 2.8 | 817.0 ± 5.7 | −1.0 | 0.268 ± 0.002 | 0.28 ± 0.045 | 0.012 | ||
| 5:5 | 5.44 ± 0.00 | 5.45 ± 0.00 | 0.01 | 936.5 ± 2.1 | 938.5 ± 3.5 | 2.0 | 0.436 ± 0.037 | 0.393 ± 0.002 | −0.043 | ||
| VP + 5Gl | 8:2 | 5.33 ± 0.00 | 5.36 ± 0.01 | 0.03 | 549.5 ± 3.5 | 554.5 ± 3.5 | 5.0 | 0.265 ± 0.019 | 0.231 ± 0.005 | −0.034 | |
| 6:4 | 5.46 ± 0.01 | 5.46 ± 0.01 | 0.01 | 855 ± 1.4 | 856.0 ± 2.8 | 1.0 | 0.372 ± 0.004 | 0.334 ± 0.001 | −0.038 | ||
| 5:5 | 5.48 ± 0.00 | 5.49 ± 0.00 | 0.01 | 1003.5 ± 2.1 | 1018.5 ± 10.6 | 15.0 | 0.310 ± 0.006 | 0.267 ± 0.034 | −0.043 | ||
| Kabiven | VP + NS | 7:3 | 5.35 ± 0.01 | 5.36 ± 0.00 | 0.01 | 510.5 ± 3.5 | 522.5 ± 2.1 | 12.0 | 0.245 ± 0.002 | 0.207 ± 0.004 | −0.038 |
| 6:4 | 5.40 ± 0.01 | 5.41 ± 0.00 | 0.01 | 583.5 ± 3.5 | 594.5 ± 0.7 | 11.0 | 0.249 ± 0.006 | 0.263 ± 0.008 | 0.014 | ||
| 5:5 | 5.46 ± 0.01 | 5.45 ± 0.00 | −0.01 | 666.5 ± 0.7 | 679.0 ± 1.4 | 12.5 | 0.252 ± 0.001 | 0.270 ± 0.001 | 0.018 | ||
| VP + 5Gl | 7:3 | 5.39 ± 0.00 | 5.38 ± 0.00 | −0.01 | 510.5 ± 0.7 | 522.5 ± 0.7 | 12.0 | 0.203 ± 0.007 | 0.194 ± 0.005 | −0.009 | |
| 6:4 | 5.44 ± 0.00 | 5.45 ± 0.00 | 0.01 | 604 ± 0.0 | 614.5 ± 0.7 | 10.5 | 0.241 ± 0.004 | 0.252 ± 0.001 | 0.010 | ||
| 5:5 | 5.48 ± 0.00 | 5.48 ± 0.01 | 0.00 | 699 ± 0.0 | 704.0 ± 1.4 | 5.0 | 0.259 ± 0.002 | 0.276 ± 0.007 | 0.017 | ||
| SmofKabiven | VP + NS | 7:3 | 5.38 ± 0.01 | 5.38 ± 0.01 | 0.01 | 644.5 ± 0.7 | 647 ± 1.4 | 2.5 | 1.723 ± 0.006 | 1.520 ± 0.000 | −0.203 |
| 5:5 | 5.45 ± 0.00 | 5.46 ± 0.02 | 0.01 | 928 ± 2.8 | 928.5 ± 2.1 | 0.5 | 1.347 ± 0.147 | 1.183 ± 0.006 | −0.163 | ||
| VP + 5Gl | 7:3 | 5.41 ± 0.01 | 5.45 ± 0.01 | 0.04 | 642 ± 7.1 | 638.5 ± 2.1 | −3.5 | 0.182 ± 0.002 | 0.169 ± 0.001 | −0.012 | |
| 5:5 | 5.47 ± 0.00 | 5.48 ± 0.00 | 0.01 | 927 ± 0.0 | 926.5 ± 4.9 | −0.5 | 0.247 ± 0.002 | 0.224 ± 0.003 | −0.022 | ||
| Kabiven Peripheral | VP + NS | 7:3 | 5.28 ± 0.00 | 5.30 ± 0.00 | 0.02 | 471.5 ± 71.4 | 478 ± 69.3 | 6.5 | 0.215 ± 0.002 | 0.209 ± 0.019 | −0.006 |
| 5:5 | 5.45 ± 0.00 | 5.47 ± 0.01 | 0.02 | 516.5 ± 3.5 | 522.5 ± 0.7 | 6.0 | 0.225 ± 0.001 | 0.220 ± 0.006 | −0.006 | ||
| 3:7 | 5.54 ± 0.00 | 5.56 ± 0.00 | 0.02 | 620 ± 0.0 | 628.0 ± 0.0 | 8.0 | 0.221 ± 0.001 | 0.213 ± 0.012 | −0.008 | ||
| VP + 5Gl | 7:3 | 5.36 ± 0.00 | 5.35 ± 0.00 | −0.01 | 433.5 ± 0.7 | 439.5 ± 2.1 | 6.0 | 0.154 ± 0.002 | 0.153 ± 0.004 | −0.001 | |
| 5:5 | 5.48 ± 0.00 | 5.48 ± 0.00 | 0.00 | 530.5 ± 0.7 | 537.5 ± 0.7 | 7.0 | 0.178 ± 0.002 | 0.221 ± 0.010 | 0.044 | ||
| 3:7 | 5.55 ± 0.00 | 5.56 ± 0.00 | 0.01 | 635.5 ± 2.1 | 642.5 ± 0.7 | 7.0 | 0.424 ± 0.012 | 0.539 ± 0.005 | 0.115 | ||
| Lipoflex peri | VP + NS | 7:3 | 5.24 ± 0.01 | 5.26 ± 0.00 | 0.02 | 455.5 ± 0.7 | 458.5 ± 0.7 | 3.0 | 1.187 ± 0.012 | 1.083 ± 0.006 | −0.103 |
| 6:4 | 5.32 ± 0.01 | 5.32 ± 0.01 | 0.00 | 508.5 ± 7.8 | 517.0 ± 0.0 | 8.5 | 1.020 ± 0.010 | 1.057 ± 0.021 | 0.037 | ||
| 5:5 | 5.38 ± 0.00 | 5.83 ± 0.01 | 0.00 | 576 ± 2.8 | 577.0 ± 2.8 | 1.0 | 0.860 ± 0.001 | 0.835 ± 0.001 | −0.025 | ||
| VP + 5Gl | 7:3 | 5.31 ± 0.00 | 5.31 ± 0.01 | 0.00 | 467.5 ± 2.1 | 472.5 ± 0.7 | 5.0 | 0.486 ± 0.048 | 0.354 ± 0.001 | −0.132 | |
| 6:4 | 5.35 ± 0.00 | 5.34 ± 0.00 | −0.01 | 528.5 ± 0.7 | 530.0 ± 1.4 | 1.5 | 0.415 ± 0.025 | 0.52 ± 0.018 | 0.105 | ||
| 5:5 | 5.39 ± 0.00 | 5.38 ± 0.00 | −0.01 | 582 ± 4.2 | 587.0 ± 1.4 | 5.0 | 0.330 ± 0.002 | 0.362 ± 0.006 | 0.032 | ||
| Omega flex peri | VP + NS | 7:3 | 5.28 ± 0.00 | 5.30 ± 0.00 | 0.02 | 472 ± 2.8 | 473.0 ± 2.8 | 1.0 | 1.797 ± 0.064 | 1.543 ± 0.006 | −0.253 |
| 6:4 | 5.34 ± 0.01 | 5.36 ± 0.00 | 0.02 | 529.5 ± 0.7 | 529.5 ± 3.5 | 0.0 | 2.227 ± 0.012 | 2.063 ± 0.050 | −0.163 | ||
| 5:5 | 5.38 ± 0.00 | 5.40 ± 0.00 | 0.02 | 589.5 ± 0.7 | 582.5 ± 4.9 | −7.0 | 2.670 ± 0.010 | 2.39 ± 0.010 | −0.280 | ||
| VP + 5Gl | 7:3 | 5.22 ± 0.00 | 5.21 ± 0.01 | −0.01 | 461 ± 0.0 | 455.5 ± 0.7 | −5.5 | 1.887 ± 0.035 | 1.553 ± 0.006 | −0.333 | |
| 6:4 | 5.25 ± 0.00 | 5.31 ± 0.00 | 0.06 | 520 ± 0.0 | 520.5 ± 2.1 | 0.5 | 2.367 ± 0.035 | 2.117 ± 0.006 | −0.250 | ||
| 5:5 | 5.31 ± 0.00 | 5.37 ± 0.00 | 0.06 | 572.5 ± 3.5 | 580.0 ± 4.2 | 7.5 | 2.770 ± 0.010 | 2.440 ± 0.000 | −0.330 | ||
| Olimel Peri | VP + NS | 7:3 | 6.01 ± 0.02 | 6.07 ± 0.01 | 0.06 | 445 ± 0.0 | 445.5 ± 0.7 | 0.5 | 0.275 ± 0.012 | 0.200 ± 0.002 | −0.075 |
| 6:4 | 6.13 ± 0.01 | 6.16 ± 0.00 | 0.03 | 493 ± 1.4 | 494.5 ± 0.7 | 1.5 | 0.428 ± 0.020 | 0.289 ± 0.003 | −0.139 | ||
| 5:5 | 6.23 ± 0.00 | 6.24 ± 0.00 | 0.01 | 553 ± 0.0 | 551.5 ± 2.1 | −1.5 | 0.457 ± 0.046 | 0.227 ± 0.002 | −0.230 | ||
| VP + 5Gl | 7:3 | 6.05 ± 0.01 | 6.07 ± 0.01 | 0.02 | 440.5 ± 2.1 | 440.0 ± 0.0 | −0.5 | 0.321 ± 0.003 | 0.220 ± 0.021 | −0.101 | |
| 6:4 | 6.20 ± 0.00 | 6.21 ± 0.00 | 0.01 | 502.5 ± 0.7 | 503.0 ± 1.4 | 0.5 | 0.285 ± 0.004 | 0.246 ± 0.014 | −0.039 | ||
| 5:5 | 6.26 ± 0.00 | 6.26 ± 0.01 | 0.00 | 549 ± 1.4 | 550.5 ± 0.7 | 1.5 | 0.215 ± 0.005 | 0.186 ± 0.014 | −0.030 | ||
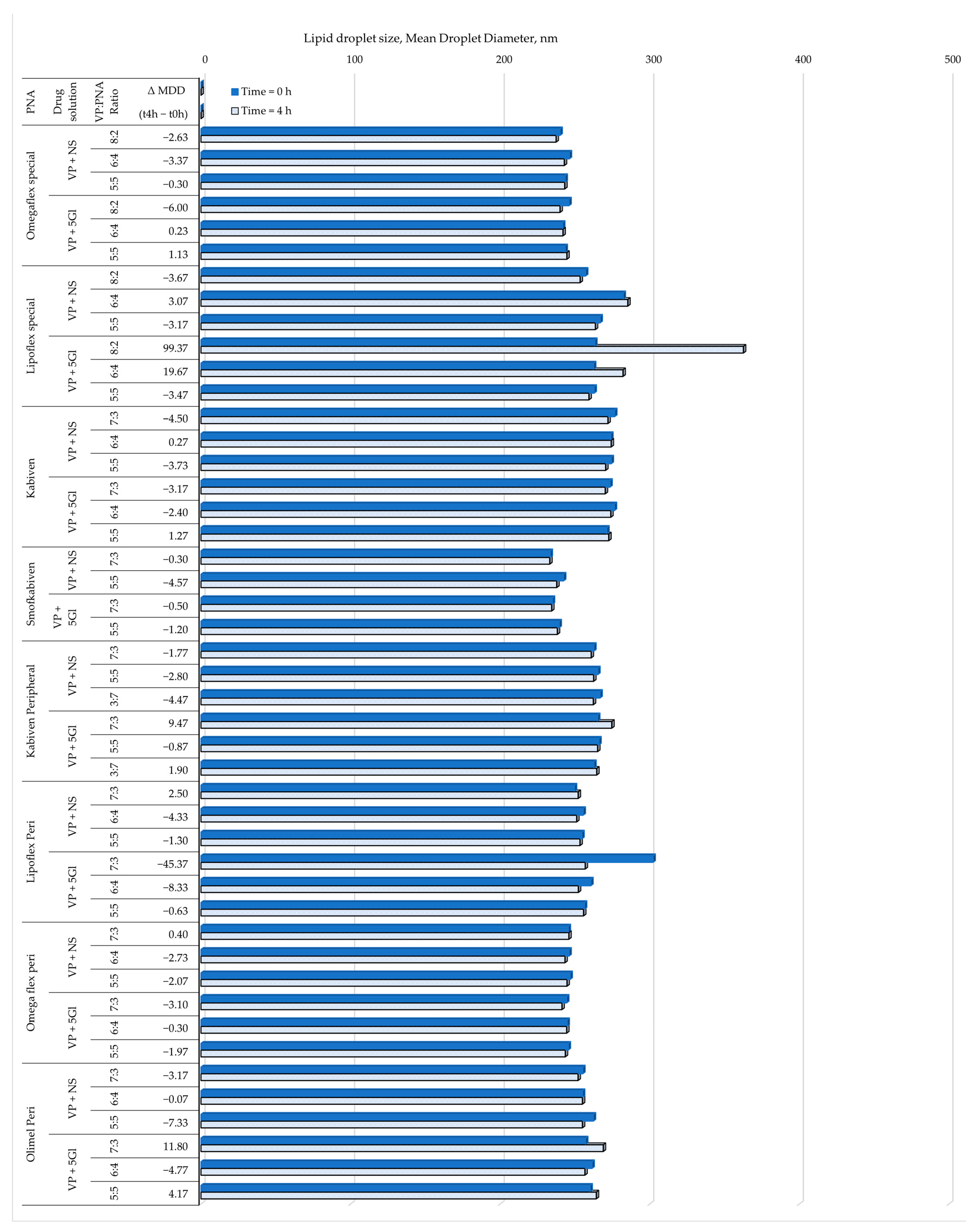
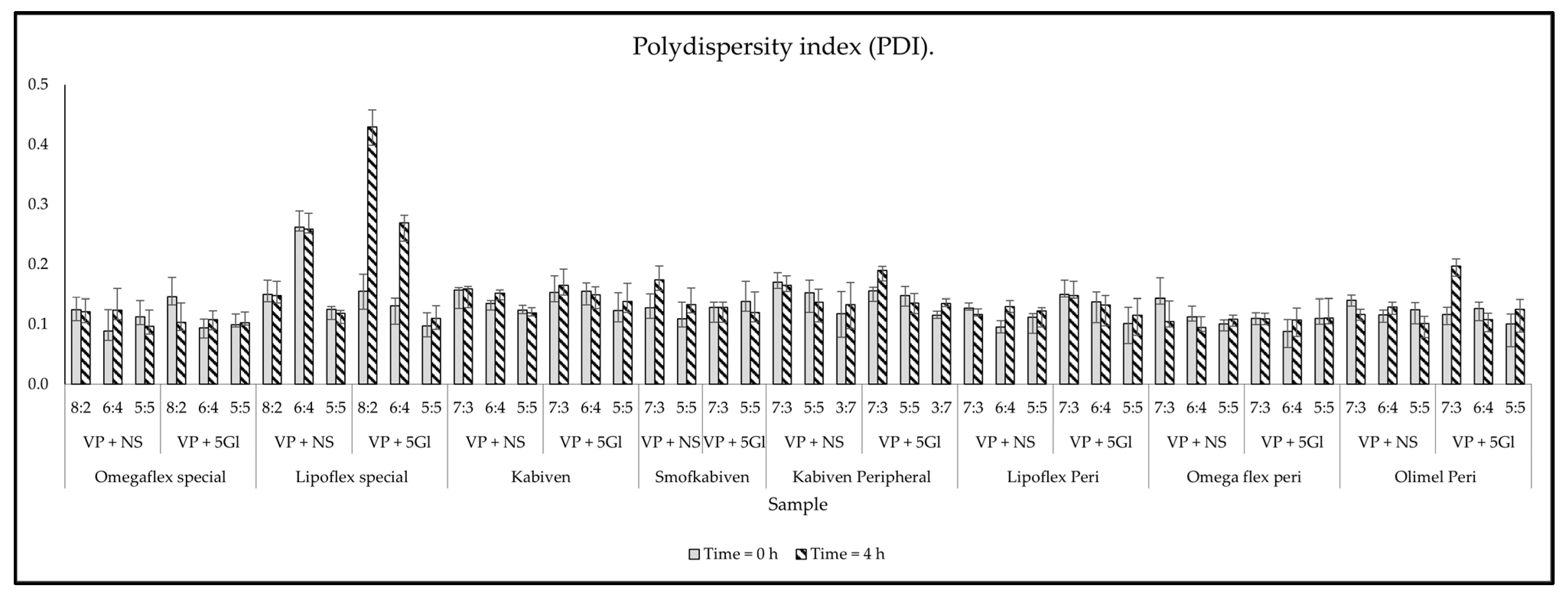
| PNA | Drug Solution | VP:PNA Ratio | Measurements | ||||||
|---|---|---|---|---|---|---|---|---|---|
| pH | Osmolality | Turbidity | ZP | PDI | MDD | PFAT5 | |||
| Omegaflex special | VP + NS | 8:2 | C | C | C | C | C | C | I |
| 6:4 | C | C | C | C | C | C | C | ||
| 5:5 | C | C | C | C | C | C | C | ||
| VP + 5Gl | 8:2 | C | C | C | C | C | I | I | |
| 6:4 | C | C | C | C | C | C | C | ||
| 5:5 | C | C | C | C | C | C | C | ||
| Lipoflex special | VP + NS | 8:2 | C | C | C | C | C | I | I |
| 6:4 | C | C | C | C | C | I | I | ||
| 5:5 | C | C | C | C | C | I | I | ||
| VP + 5Gl | 8:2 | C | C | C | C | C | I | I | |
| 6:4 | C | C | C | C | C | I | I | ||
| 5:5 | C | C | C | C | C | C | I | ||
| Kabiven | VP + NS | 7:3 | C | C | C | C | C | I | I |
| 6:4 | C | C | C | C | C | C | C | ||
| 5:5 | C | C | C | C | C | C | C | ||
| VP + 5Gl | 7:3 | C | C | C | C | C | I | I | |
| 6:4 | C | C | C | C | C | I | I | ||
| 5:5 | C | C | C | C | C | I | I | ||
| SmofKabiven | VP + NS | 7:3 | C | C | C | C | C | I | I |
| 5:5 | C | C | C | C | C | C | C | ||
| VP + 5Gl | 7:3 | C | C | C | C | C | C | C | |
| 5:5 | C | C | C | C | C | I | I | ||
| Kabiven Peripheral | VP + NS | 7:3 | C | C | C | C | C | I | I |
| 5:5 | C | C | C | C | C | I | I | ||
| 3:7 | C | C | C | C | C | C | C | ||
| VP + 5Gl | 7:3 | C | C | C | C | C | I | I | |
| 5:5 | C | C | C | C | C | I | I | ||
| 3:7 | C | C | C | C | C | C | C | ||
| Lipoflex peri | VP + NS | 7:3 | C | C | C | C | C | C | I |
| 6:4 | C | C | C | C | C | C | C | ||
| 5:5 | C | C | C | C | C | C | C | ||
| VP + 5Gl | 7:3 | C | C | C | C | C | I | I | |
| 6:4 | C | C | C | C | C | I | I | ||
| 5:5 | C | C | C | C | C | C | C | ||
| Omegaflex peri | VP + NS | 7:3 | C | C | C | C | C | I | I |
| 6:4 | C | C | C | C | C | C | I | ||
| 5:5 | C | C | C | C | C | C | I | ||
| VP + 5Gl | 7:3 | C | C | C | C | C | I | I | |
| 6:4 | C | C | C | C | C | C | C | ||
| 5:5 | C | C | C | C | C | C | C | ||
| Olimel Peri | VP + NS | 7:3 | C | C | C | C | C | C | I |
| 6:4 | C | C | C | C | C | C | I | ||
| 5:5 | C | C | C | C | C | C | C | ||
| VP + 5Gl | 7:3 | C | C | C | C | C | I | I | |
| 6:4 | C | C | C | C | C | C | I | ||
| 5:5 | C | C | C | C | C | C | C | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tomczak, S.; Kaszuba, K.; Szkudlarek, J.; Piwowarczyk, L.; Jelińska, A. Potential Use of Common Administration of Emulsion for Parenteral Nutrition and Vinpocetine: Compatibility Study and Prospect. Metabolites 2024, 14, 439. https://doi.org/10.3390/metabo14080439
Tomczak S, Kaszuba K, Szkudlarek J, Piwowarczyk L, Jelińska A. Potential Use of Common Administration of Emulsion for Parenteral Nutrition and Vinpocetine: Compatibility Study and Prospect. Metabolites. 2024; 14(8):439. https://doi.org/10.3390/metabo14080439
Chicago/Turabian StyleTomczak, Szymon, Kornelia Kaszuba, Jagoda Szkudlarek, Ludwika Piwowarczyk, and Anna Jelińska. 2024. "Potential Use of Common Administration of Emulsion for Parenteral Nutrition and Vinpocetine: Compatibility Study and Prospect" Metabolites 14, no. 8: 439. https://doi.org/10.3390/metabo14080439
APA StyleTomczak, S., Kaszuba, K., Szkudlarek, J., Piwowarczyk, L., & Jelińska, A. (2024). Potential Use of Common Administration of Emulsion for Parenteral Nutrition and Vinpocetine: Compatibility Study and Prospect. Metabolites, 14(8), 439. https://doi.org/10.3390/metabo14080439








