An Exploratory Study on the Rapid Detection of Volatile Organic Compounds in Gardenia Fruit Using the Heracles NEO Ultra-Fast Gas Phase Electronic Nose
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Sample Preparation
2.3. Heracles NEO Ultra-Fast Gas Phase Electronic Nose Analysis Conditions
2.4. Statistical Analysis
3. Results
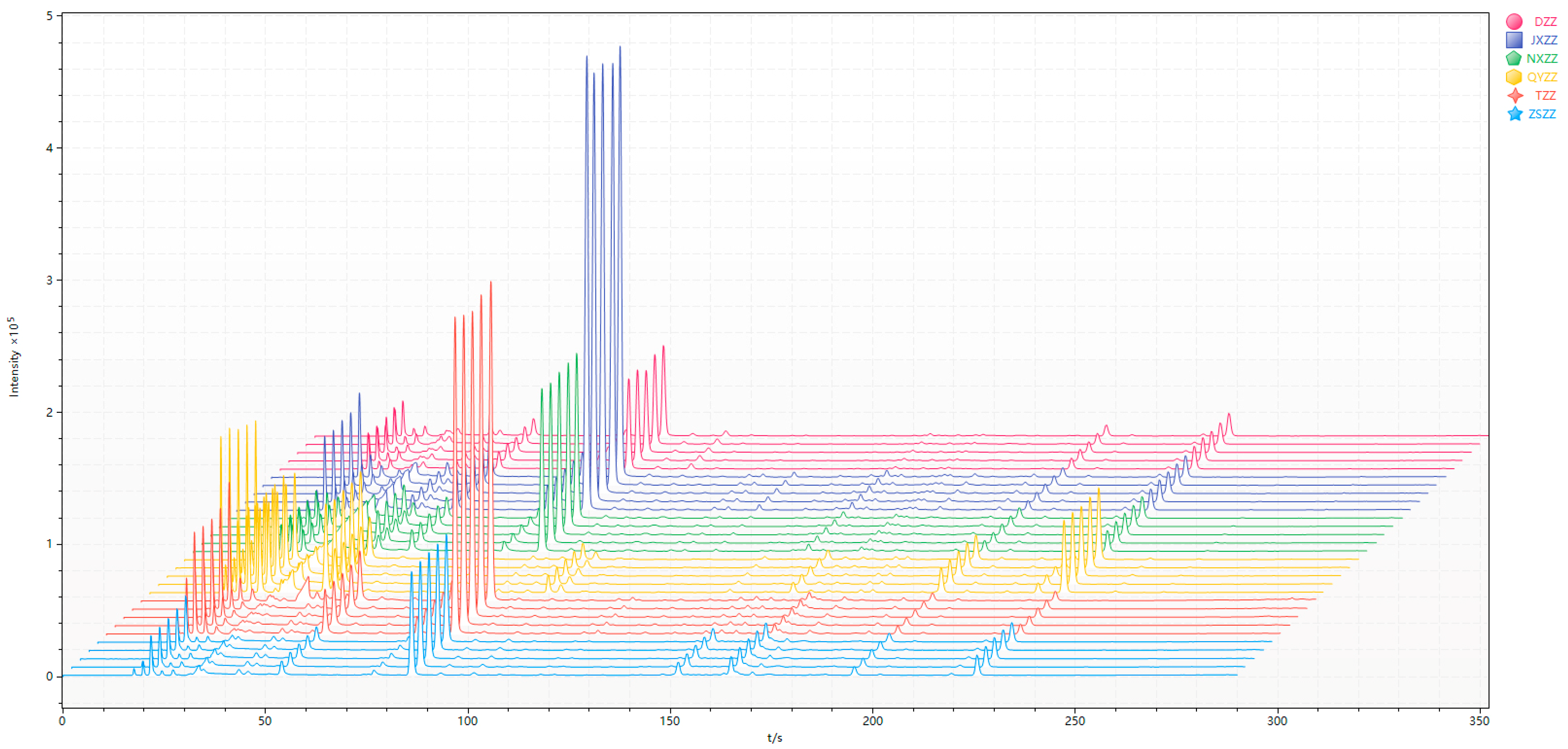
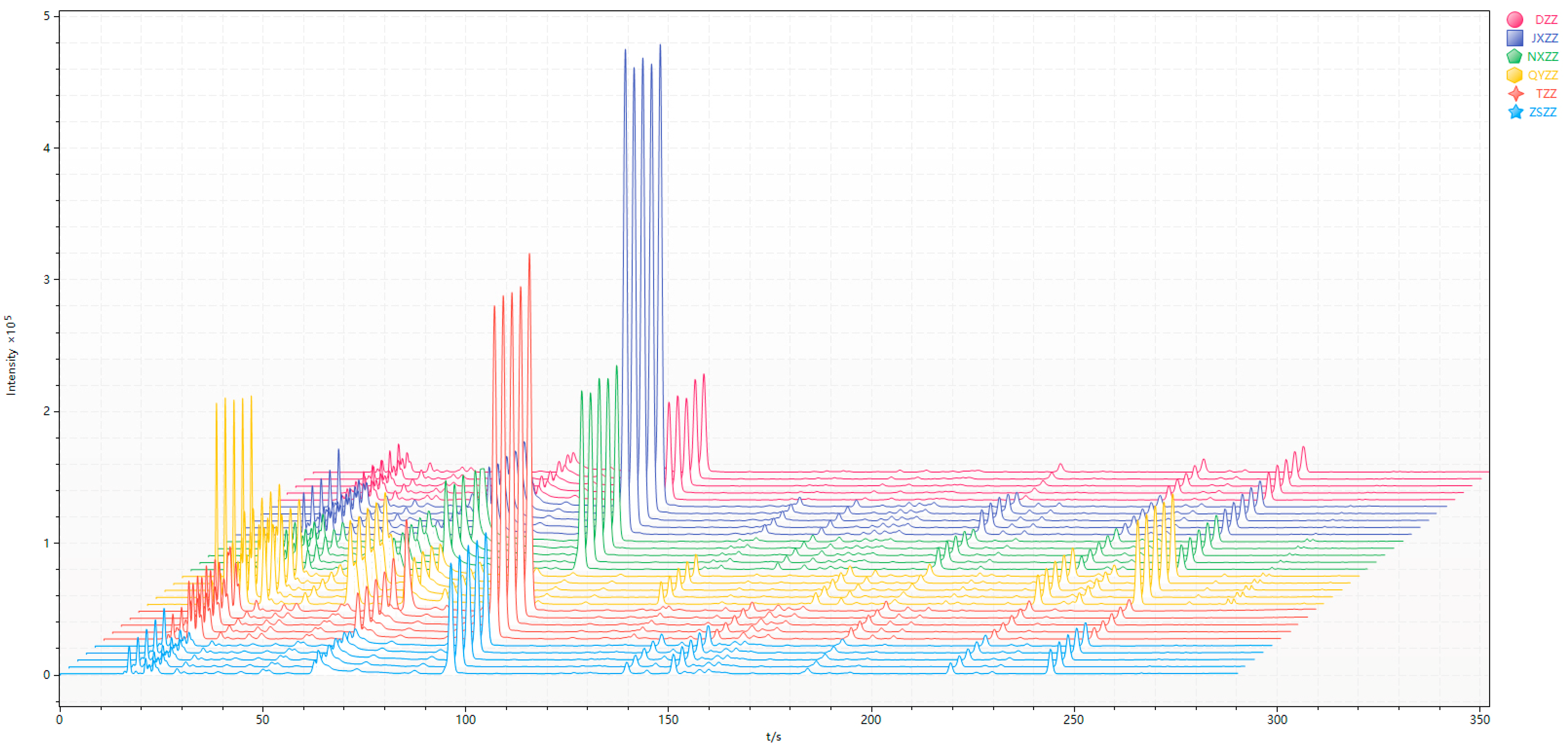
3.1. Gas Chromatogram Analysis
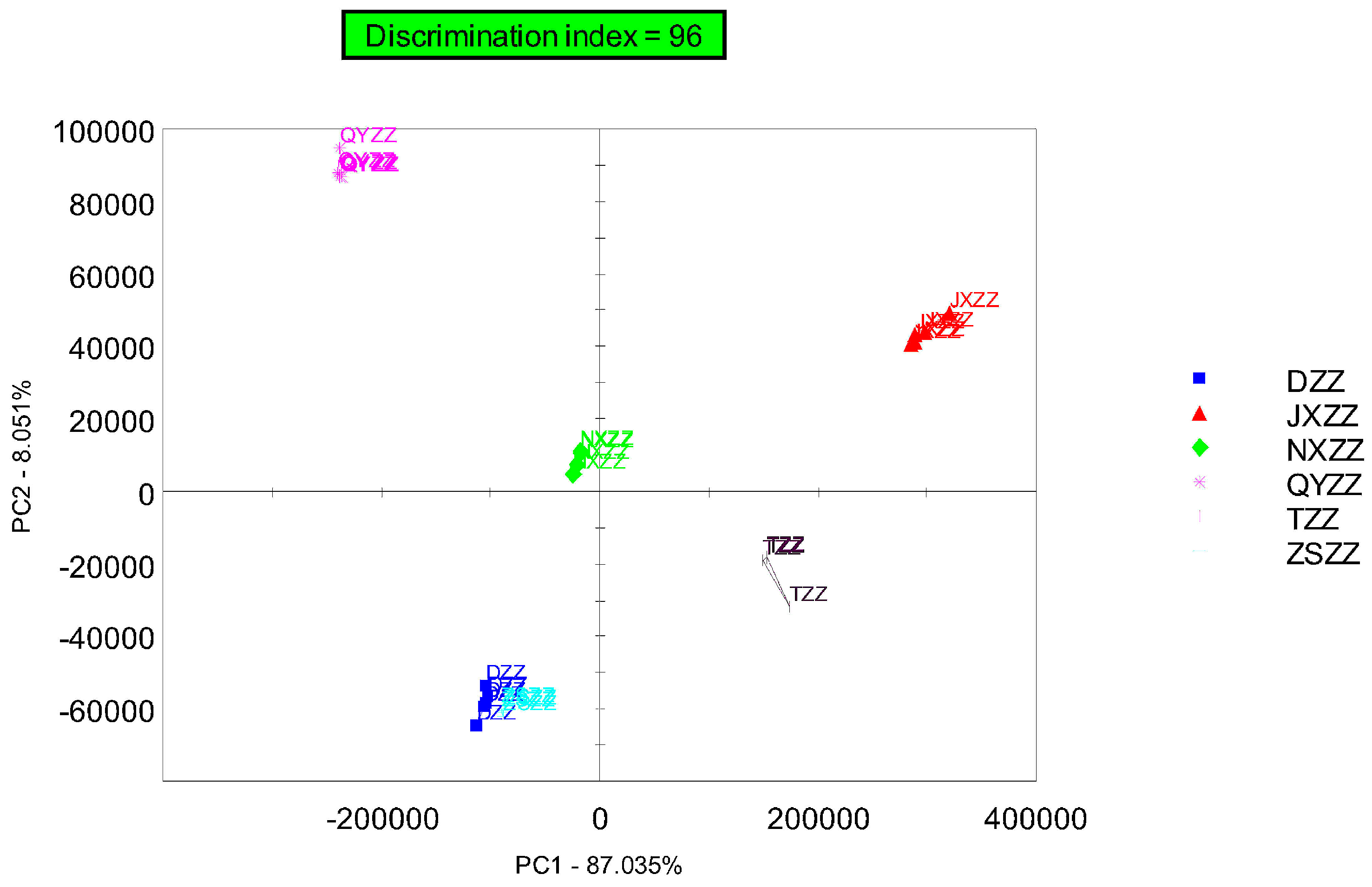
3.2. Principal Component Analysis (PCA)
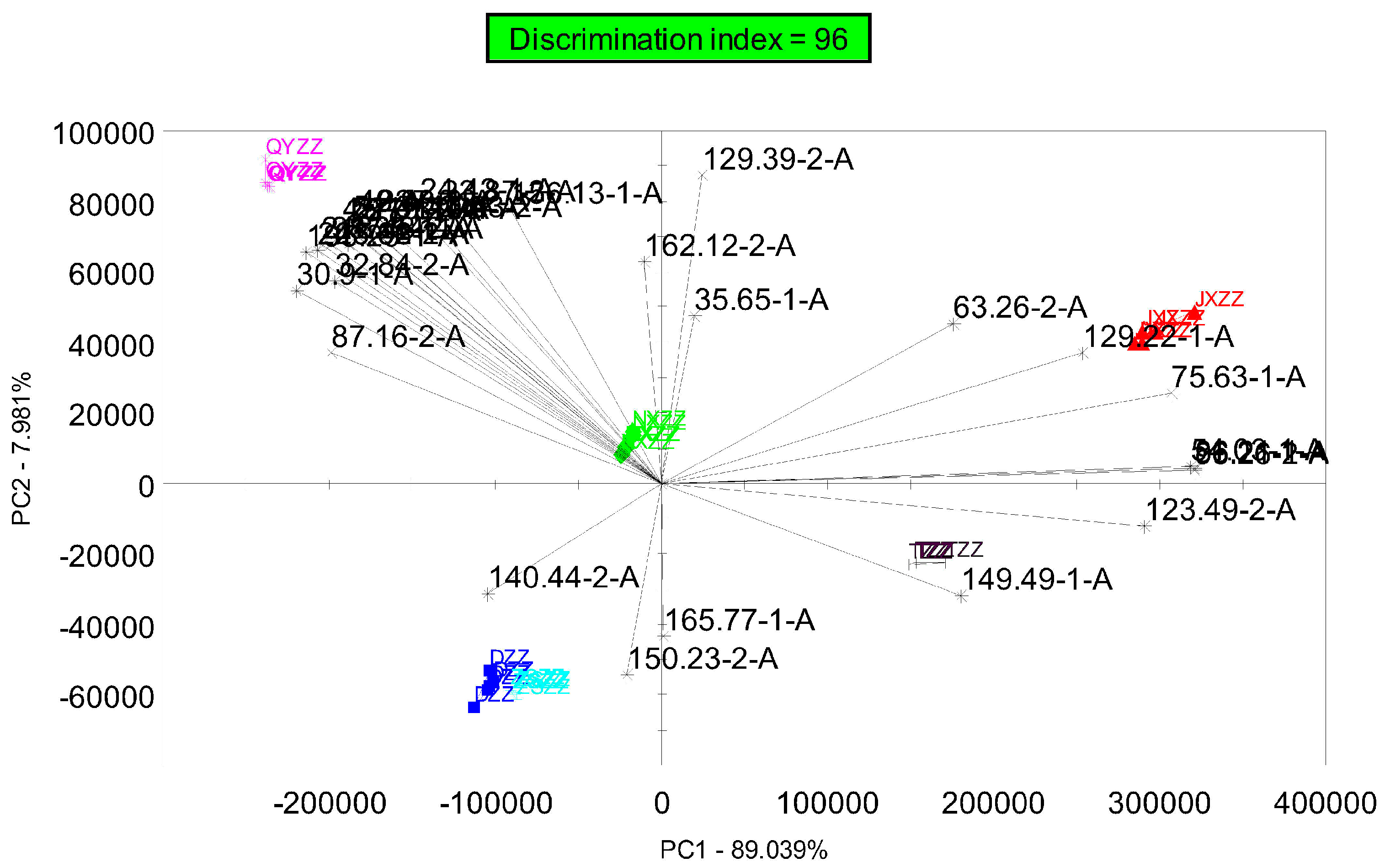
3.3. Compound Identification
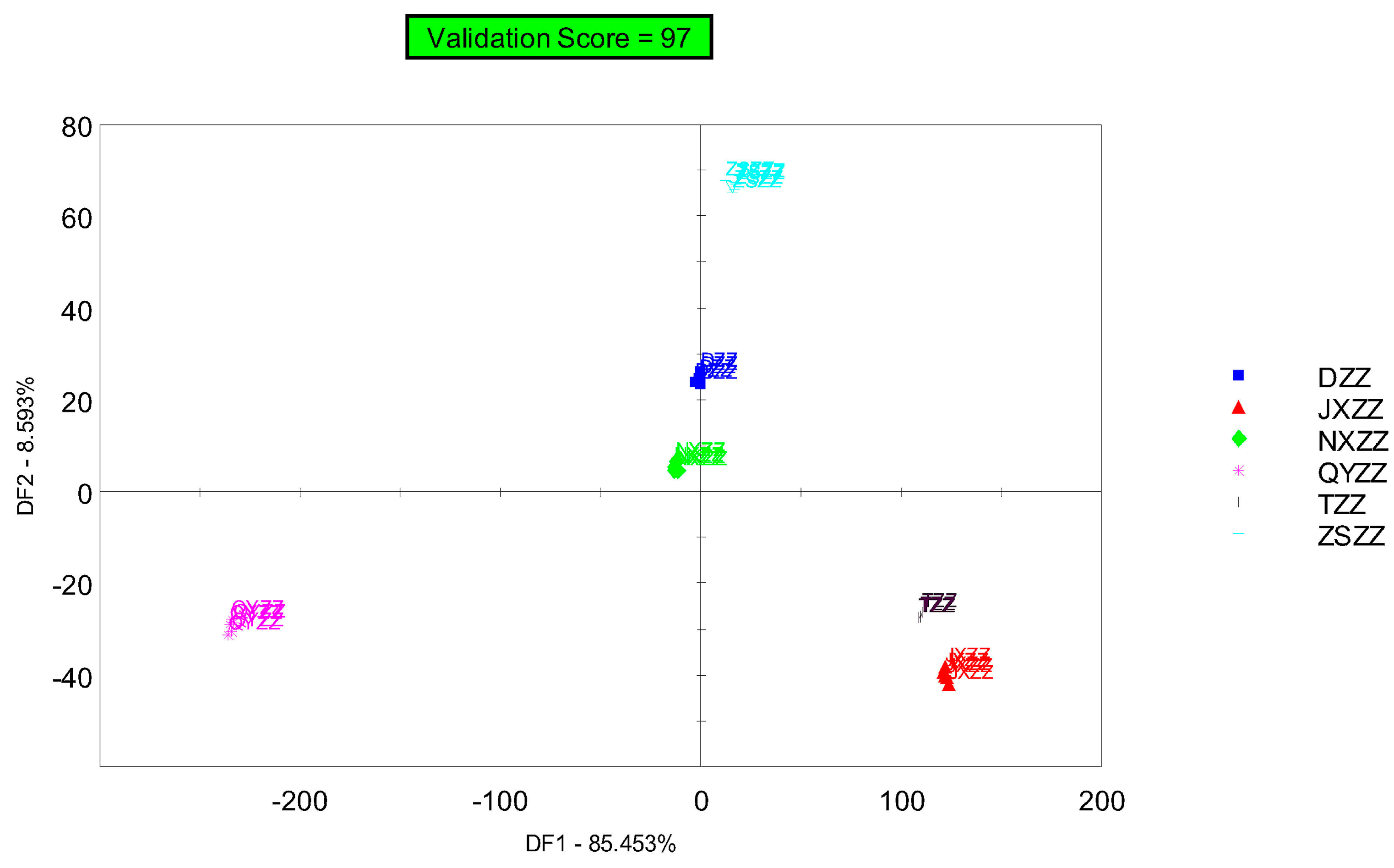
3.4. Discriminant Factor Analysis (DFA)
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Committee for the National Pharmacopoeia of P.R. China. Pharmacopoeia of P.R. China, Part I; Beijing Chemical Industry Press: Beijing, China, 2020. [Google Scholar]
- Chen, L.; Li, M.; Yang, Z.; Tao, W.; Wang, P.; Tian, X.; Li, X.; Wang, W. Gardenia jasminoides Ellis: Ethnopharmacology, phytochemistry, and pharmacological and industrial applications of an important traditional Chinese medicine. J. Ethnopharmacol. 2020, 257, 112829. [Google Scholar] [CrossRef] [PubMed]
- Tian, J.; Qin, S.; Han, J.; Meng, J.; Liang, A. A review of the ethnopharmacology, phytochemistry, pharmacology and toxicology of Fructus Gardeniae (Zhi-zi). J. Ethnopharmacol. 2022, 289, 114984. [Google Scholar] [CrossRef] [PubMed]
- Umigai, N.; Takeda, R.; Mori, A. Effect of crocetin on quality of sleep: A randomized, double-blind, placebo-controlled, crossover study. Complement. Ther. Med. 2018, 41, 47–51. [Google Scholar] [CrossRef] [PubMed]
- Kuratsune, H.; Umigai, N.; Takeno, R.; Kajimoto, Y.; Nakano, T. Effect of crocetin from Gardenia jasminoides Ellis on sleep: A pilot study. Phytomedicine 2010, 11, 840–843. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.; He, Z.X.; Yan, Y.; Zheng, B.H.; Huang, X.H.; Lin, Z.Y. Research progress on pharmacological and toxic effects of Gardeniae Fructus and its main components. Chin. Tradit. Herb. Drugs 2023, 54, 6092–6105. [Google Scholar] [CrossRef]
- Lomax, J.; Ford, R.; Bar, I. Multi-omic applications for understanding and enhancing tropical fruit flavour. Plant Mol. Biol. 2024, 4, 83. [Google Scholar] [CrossRef]
- Kaldeli, A.; Zakidou, P.; Paraskevopoulou, A. Volatilomics as a tool to ascertain food adulteration, authenticity, and origin. Compr. Rev. Food Sci. Food Saf. 2024, 4, e13387. [Google Scholar] [CrossRef]
- Ding, Y.; Dai, Y.D.; Ding, M.; Chen, P.; Wang, Q. Variation Analysis of Flavors of Stropharia rugosoannulata Cultivated with Different Substrates Basing on Electronic Nose, Electronic Tongue and HS-SPME-GC-MS. J. Fungal Res. 2024, 1–12. [Google Scholar] [CrossRef]
- Gehlken, J.; Pour Nikfardjam, M.; Zörb, C. Prediction of sensory attributes in winemaking grapes by on-line near-infrared spectroscopy based on selected volatile aroma compounds. Anal. Bioanal. Chem. 2023, 415, 1515–1527. [Google Scholar] [CrossRef]
- Dai, Y.; Liu, S.; Yang, L.; He, Y.; Guo, X.; Ma, Y.; Li, S.; Huang, D. Explorative study for the rapid detection of Fritillaria using gas chromatography-ion mobility spectrometry. Front. Nutr. 2024, 11, 361668. [Google Scholar] [CrossRef]
- Srinivasan, A.; Maruthavanan, T.; Mayildurai, R.; Ramasubbu, A. GC-MS investigations of VOCs in South Indian honey samples as environmental biomarkers. Environ. Monit. Assess. 2021, 8, 539. [Google Scholar] [CrossRef] [PubMed]
- Aghili, N.S.; Rasekh, M.; Karami, H.; Edriss, O.; Wilson, A.D.; Ramos, J. Aromatic Fingerprints: VOC Analysis with E-Nose and GC-MS for Rapid Detection of Adulteration in Sesame Oil. Sensors 2023, 23, 6294. [Google Scholar] [CrossRef] [PubMed]
- Fois, M.; Cox, T.; Ratcliffe, N.; de Lacy Costello, B. Rare earth doped metal oxide sensor for the multimodal detection of volatile organic compounds (VOCs). Sens. Actuators B Chem. 2021, 330, 129264. [Google Scholar] [CrossRef]
- Zhang, B.; Rahman, M.A.; Liu, J.; Huang, J.; Yang, Q. Real-time detection and analysis of foodborne pathogens via machine learning based fiber-optic Raman sensor. Measurement 2023, 217, 113121. [Google Scholar] [CrossRef]
- Shuba, A.; Umarkhanov, R.; Bogdanova, E.; Anokhina, E.; Burakova, I. Possibilities of an Electronic Nose on Piezoelectric Sensors with Polycomposite Coatings to Investigate the Microbiological Indicators of Milk. Sensors 2024, 11, 3634. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cai, W.T.; Feng, T.; Song, S.Q.; Yao, L.Y.; Sun, M.; Wang, H.T.; Yu, C.; Liu, Q. Research progress of machine learning in predicting food flavor. Food Sci. 2024, 12, 11–21. [Google Scholar] [CrossRef]
- Zha, S.H.; Wang, J.L.; Zhou, S.Y.; Jiang, S.H.; Zhang, H. Rapid Identification of American Ginseng Originated from Varied Places Based on Heracles Ultra-fast Gas Phase Electronic Nose. Sci. Technol. Food Ind. 2023, 44, 284–291. [Google Scholar] [CrossRef]
- Nai, G.Y.; Zhang, H.L.; Yue, Y.Y.; Chen, K.Y.; Zhang, X.Y. Influences of different aging containers on the wine quality analyzed by electronic nose and HS-SPME-GC-MS. J. Anhui Agric. Univ. 2024, 51, 152–158. [Google Scholar] [CrossRef]
- Sipos, L.; Végh, R.; Bodor, Z.; Zaukuu, J.Z.; Hitka, G.; Bázár, G.; Kovacs, Z. Classification of Bee Pollen and Prediction of Sensory and Colorimetric Attributes-A Sensometric Fusion Approach by e-Nose, e-Tongue and NIR. Sensors 2020, 20, 6768. [Google Scholar] [CrossRef]
- Shen, C.; Cai, Y.; Wu, X.; Gai, S.; Wang, B.; Liu, D. Characterization of Selected Commercially Available Grilled Lamb Shashliks Based on Flavor Profiles Using GC-MS, GC × GC-TOF-MS, GC-IMS, E-Nose and E-Tongue Combined with Chemometrics. Food Chem. 2023, 423, 136257. [Google Scholar] [CrossRef]
- Wang, Q.; Chen, X.; Zhang, C.; Li, X.; Yue, N.; Shao, H.; Wang, J.; Jin, F. Discrimination and Characterization of Volatile Flavor Compounds in Fresh Oriental Melon after Forchlorfenuron Application Using Electronic Nose (E-Nose) and Headspace-Gas Chromatography-Ion Mobility Spectrometry (HS-GC-IMS). Foods 2023, 12, 1272. [Google Scholar] [CrossRef]
- Wei, G.; Dan, M.; Zhao, G.; Wang, D. Recent Advances in Chromatography-Mass Spectrometry and Electronic Nose Technology in Food Flavor Analysis and Detection. Food Chem. 2023, 405, 134814. [Google Scholar] [CrossRef] [PubMed]
- Zhu, G.F.; Wang, J.; Qian, Y.J.; Zhang, K.W.; Lu, T.L.; Mao, C.Q. Rapid identification of Pogostemonis Herba and its odor fingerprint based on Heracles Neo ultra-fast gas phase electronic nose. Chin. Tradit. Herb. Drugs 2022, 53, 1320–1327. [Google Scholar] [CrossRef]
- Zhang, J.B.; Fei, C.H.; Zhang, W.; Qin, Y.W.; Li, Y.; Wang, B.; Jin, S.J.; Su, L.L.; Mao, C.Q.; Ji, D. Rapid Identification for the Species Discrimination of Curcumae Rhizoma Using Spectrophotometry and Flash Gas Chromatography E-Nose Combined with Chemometrics. Chin. J. Anal. Chem. 2022, 50, 100167. [Google Scholar] [CrossRef]
- Mohammad-Razdari, A.; Ghasemi-Varnamkhasti, M.; Yoosefian, S.H.; Izadi, Z.; Siadat, M. Potential Application of Electronic Nose Coupled with Chemometric Tools for Authentication Assessment in Tomato Paste. J. Food Process Eng. 2019, 42, e13119. [Google Scholar] [CrossRef]
- Emkani, M.; Gourrat, K.; Oliete, B.; Saurel, R. Identification of volatile and odor-active compounds in pea protein fractions obtained by a modified extraction method using fermentation. J. Food Sci. 2024, 7, 4229–4249. [Google Scholar] [CrossRef]
- Liu, X.P.; Tang, M.; Yun, Y.; Zhu, H.K.; Zhou, H.Y.; Ma, L.; Zhang, Y.H. Mining of flavor composition and main flavor substances in boiled fish. Food Ferment. Ind. 2024, 1–13. [Google Scholar] [CrossRef]
- Maoz, I.; Kaplunov, T.; Raban, E.; Dynkin, I.; Degani, O.; Lewinsohn, E.; Lichter, A. Insights into the Chemosensory Basis of Flavor in Table Grapes. J. Sci. Food Agric. 2020, 100, 1405–1417. [Google Scholar] [CrossRef] [PubMed]
- King, B.M.; Arents, P.; Bouter, N.; Duineveld, C.A.A.; Meyners, M.; Schroff, S.I.; Soekhai, S.T. Sweetener/Sweetness-Induced Changes in Flavor Perception and Flavor Release of Fruity and Green Character in Beverages. J. Agric. Food Chem. 2006, 54, 2671–2677. [Google Scholar] [CrossRef]
- Mayuoni-Kirshinbaum, L.; Porat, R. The Flavor of Pomegranate Fruit: A Review. J. Sci. Food Agric. 2014, 94, 21–27. [Google Scholar] [CrossRef] [PubMed]
- Peng, Q.; Li, S.; Zheng, H.; Meng, K.; Jiang, X.; Shen, R.; Xue, J.; Xie, G. Characterization of Different Grades of Jiuqu Hongmei Tea Based on Flavor Profiles Using HS-SPME-GC-MS Combined with E-Nose and E-Tongue. Food Res. Int. 2023, 172, 113198. [Google Scholar] [CrossRef] [PubMed]
- Americo da Silva, T.; Acuña Caldeira Juncá, M.; Braunger, M.L.; Riul, A., Jr.; Fernandes Barbin, D. Application of a microfluidic electronic tongue based on impedance spectroscopy for coconut water analysis. Food Res. Int. 2024, 187, 114353. [Google Scholar] [CrossRef] [PubMed]
- Tibaduiza, D.; Anaya, M.; Gómez, J.; Sarmiento, J.; Perez, M.; Lara, C.; Ruiz, J.; Osorio, N.; Rodriguez, K.; Hernandez, I.; et al. Electronic Tongues and Noses: A General Overview. Biosensors 2024, 4, 190. [Google Scholar] [CrossRef] [PubMed]
- Douglas, S.L.; Bernardez-Morales, G.M.; Nichols, B.W.; Johnson, G.F.; Barahona-Dominguez, L.S.; Jessup, A.P.; Belk, A.D.; Ball, J.J.; Cho, S.; Sawyer, J.T. Inclusion of Beef Heart in Ground Beef Patties Alters Quality Characteristics and Consumer Acceptability as Assessed by the Application of Electronic Nose and Tongue Technology. Foods 2024, 5, 811. [Google Scholar] [CrossRef]
- Cascos, G.; Montero-Fernández, I.; Marcía-Fuentes, J.A.; Aleman, R.S.; Ruiz-Canales, A.; Martín-Vertedor, D. Electronic Prediction of Chemical Contaminants in Aroma of Brewed Roasted Coffee and Quantification of Acrylamide Levels. Foods 2024, 5, 768. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Usman, I.; Sana, S.; Afzaal, M.; Imran, A.; Saeed, F.; Ahmed, A.; Shah, Y.A.; Munir, M.; Ateeq, H.; Afzal Av Azam, I.; et al. Advances and challenges in conventional and modern techniques for halal food authentication: A review. Food Sci. Nutr. 2023, 3, 1430–1443. [Google Scholar] [CrossRef]
- Mei, X.; Xu, J.G.; Su, L.L.; Mao, C.Q.; Lu, T.L.; Fang, F. Rapid identification of odor difference markers before and after bran-fried Aurantii Fructus based on Heracles NEO ultra-fast gas phase electronic nose. Chin. Tradit. Herb. Drugs 2023, 54, 5165–5171. [Google Scholar]

| No | Compounds | CAS | RI (RT-5) | RI (RT-1701) | Odor Threshold (mg/m3) |
|---|---|---|---|---|---|
| 1 | Methyl formate | 107-31-3 | 372 | 462 | 2.66 × 10³ (air) |
| 2 | 2-propanol | 67-63-0 | 478 | 603 | 90.00 (air) |
| 3 | Acrylonitrile | 107-13-1 | 521 | 633 | 11.20 (air) |
| 4 | vinyl acetate | 108-05-4 | 579 | 658 | 0.40 (air) |
| 5 | Acetic acid | 64-19-7 | 619 | - | 0.60 (air) |
| 6 | 3-methylbutanal | 590-86-3 | 650 | 738 | 2.0 × 10−³ (air) |
| 7 | 2-methylbutanal | 96-17-3 | 661 | 743 | 3.3 × 10−³ (water) |
| 8 | 2,3-Pentanedione | 600-14-6 | 698 | 786 | 0.02 (air) |
| 9 | Ethane, 1,1,2-trichloro- | 79-00-5 | 767 | 864 | - |
| 10 | Hexanal | 66-25-1 | 801 | 892 | 0.04 (air) |
| 11 | 2-Cyclopenten-1-one | 930-30-3 | 835 | 983 | - |
| 12 | Methyl hexanoate | 106-70-7 | 926 | 995 | 0.08 (water) |
| 13 | Myrcene | 123-35-3 | 993 | 1023 | 0.04 (air) |
| 14 | 2-Ethyl-5-methylpyrazine | 13360-64-0 | 1014 | 1102 | 0.04 (air) |
| 15 | Beta-phellandrene | 555-10-2 | 1033 | 1060 | 0.04 (water) |
| 16 | Phenol, 2,6-dimethyl- | 576-26-1 | 1130 | 1292 | 2.0 × 10−4 (air) |
| 17 | Methyl salicylate | 119-36-8 | 1210 | 1329 | 0.33 (air) |
| 18 | 2-Cyclohexen-1-one, 2-methyl-5-(1-methylethenyl)- | 99-49-0 | 1232 | 1383 | 0.50 (air) |
| No | Compounds | CAS | Average Peak Area | |||||
|---|---|---|---|---|---|---|---|---|
| DZZ | JXZZ | NXZZ | QYZZ | TZZ | ZSZZ | |||
| 1 | Methyl formate | 107-31-3 | 3089 | 3197 | 3959 | 45,152 | 4168 | 2125 |
| 2 | 2-propanol | 67-63-0 | 3327 | 8752 | 10,499 | 34,975 | 7142 | 2041 |
| 3 | Acrylonitrile | 107-13-1 | 6066 | 7430 | 12,022 | 44,024 | 4161 | 3000 |
| 4 | vinyl acetate | 108-05-4 | 955 | 670 | 426 | 2077 | 332 | 488 |
| 5 | Acetic acid | 64-19-7 | 6565 | 26,403 | 51,691 | 21,307 | 3516 | 5502 |
| 6 | 3-methylbutanal | 590-86-3 | 6117 | 10,042 | 24,642 | 60,832 | 4079 | 3491 |
| 7 | 2-methylbutanal | 96-17-3 | 3804 | 4438 | 12,837 | 32,102 | 2570 | 2256 |
| 8 | 2,3-Pentanedione | 600-14-6 | 11,932 | 44,573 | 14,823 | 2115 | 31,796 | 10,405 |
| 9 | Ethane, 1,1,2-trichloro- | 79-00-5 | 6828 | 21,929 | 10,013 | 6634 | 15,761 | 5973 |
| 10 | Hexanal | 66-25-1 | 61,859 | 297,560 | 111,381 | 2550 | 215,125 | 72,907 |
| 11 | 2-Cyclopenten-1-one | 930-30-3 | 594 | 440 | 1145 | 12,047 | 296 | 480 |
| 12 | Methyl hexanoate | 106-70-7 | 363 | 3567 | 1284 | 367 | 613 | 233 |
| 13 | Myrcene | 123-35-3 | 2461 | 9565 | 7882 | 816 | 6959 | 11,591 |
| 14 | 2-Ethyl-5-methylpyrazine | 13360-64-0 | 1627 | 3755 | 2304 | 8534 | 3792 | 1896 |
| 15 | Beta-phellandrene | 555-10-2 | 2354 | 5797 | 8098 | 5104 | 12,887 | 23245 |
| 16 | Phenol, 2,6-dimethyl- | 576-26-1 | 8954 | 7733 | 8669 | 20,162 | 6015 | 7454 |
| 17 | Methyl salicylate | 119-36-8 | 1598 | 2342 | 1953 | 7365 | 703 | 1528 |
| 18 | 2-Cyclohexen-1-one, 2-methyl-5-(1-methylethenyl)- | 99-49-0 | 17,808 | 17,036 | 16,982 | 54,251 | 7442 | 16,010 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cai, W.; Zhou, W.; Liu, J.; Wang, J.; Kuang, D.; Wang, J.; Long, Q.; Huang, D. An Exploratory Study on the Rapid Detection of Volatile Organic Compounds in Gardenia Fruit Using the Heracles NEO Ultra-Fast Gas Phase Electronic Nose. Metabolites 2024, 14, 445. https://doi.org/10.3390/metabo14080445
Cai W, Zhou W, Liu J, Wang J, Kuang D, Wang J, Long Q, Huang D. An Exploratory Study on the Rapid Detection of Volatile Organic Compounds in Gardenia Fruit Using the Heracles NEO Ultra-Fast Gas Phase Electronic Nose. Metabolites. 2024; 14(8):445. https://doi.org/10.3390/metabo14080445
Chicago/Turabian StyleCai, Wenjing, Wei Zhou, Jiayao Liu, Jing Wang, Ding Kuang, Jian Wang, Qing Long, and Dan Huang. 2024. "An Exploratory Study on the Rapid Detection of Volatile Organic Compounds in Gardenia Fruit Using the Heracles NEO Ultra-Fast Gas Phase Electronic Nose" Metabolites 14, no. 8: 445. https://doi.org/10.3390/metabo14080445
APA StyleCai, W., Zhou, W., Liu, J., Wang, J., Kuang, D., Wang, J., Long, Q., & Huang, D. (2024). An Exploratory Study on the Rapid Detection of Volatile Organic Compounds in Gardenia Fruit Using the Heracles NEO Ultra-Fast Gas Phase Electronic Nose. Metabolites, 14(8), 445. https://doi.org/10.3390/metabo14080445





