Cardiometabolic and Cellular Adaptations to Multiple vs. Single Daily HIIT Sessions in Wistar Rats: Impact of Short-Term Detraining
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals and Study Design
2.2. Test to Determine VO2max
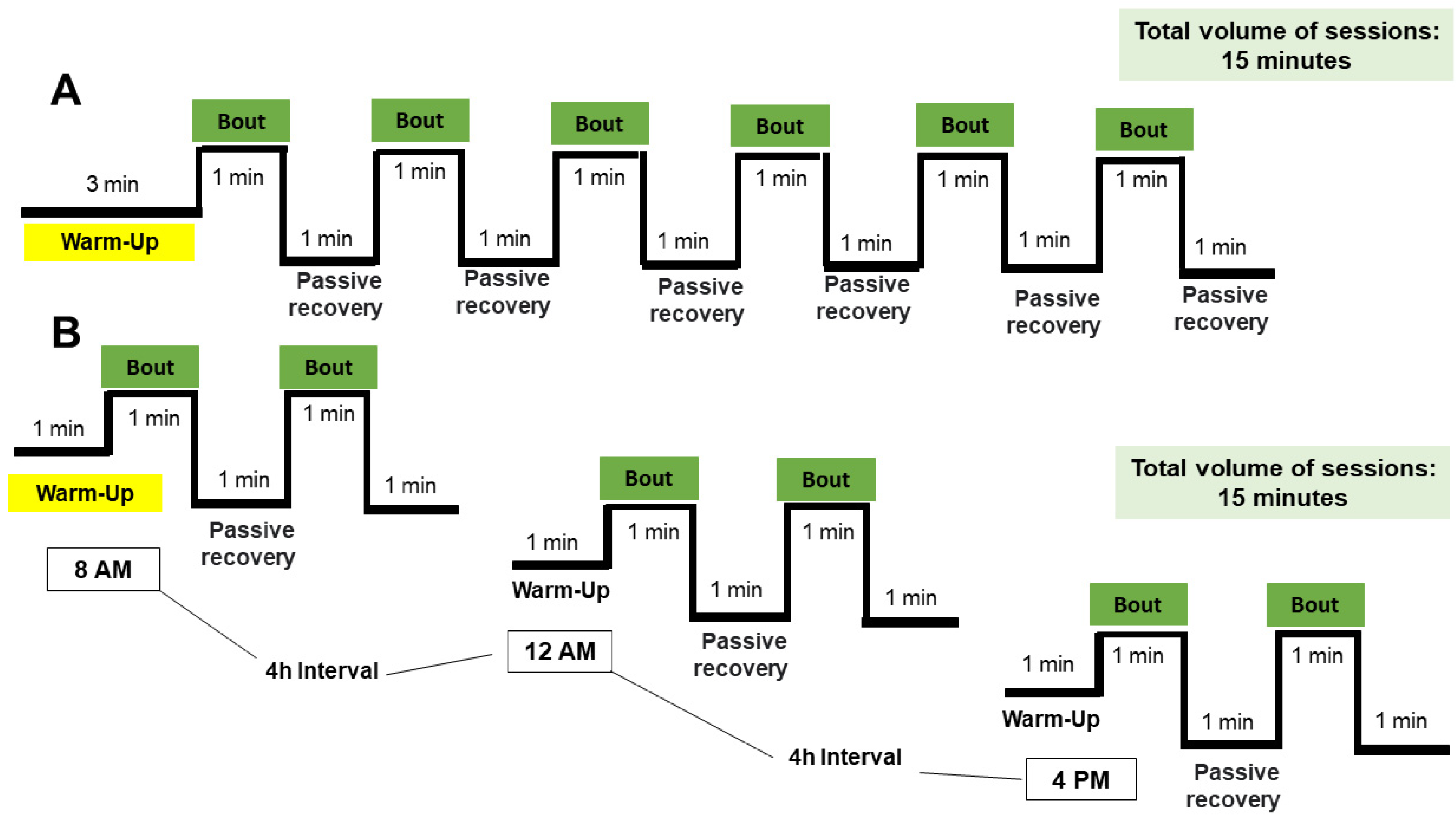
2.3. Training and Detraining Protocols
2.4. Body Composition Analysis
2.5. Heart Rate and Systolic Blood Pressure Records
2.6. Resting Oxygen Consumption Records
2.7. Oral Glucose Tolerance Test (OGTT)
2.8. Intraperitoneal Insulin Tolerance Test (IpITT)
2.9. Euthanasia
2.10. Heart Function Records
2.11. Histological Analyses
2.12. Mitochondrial Density Analyses
2.13. Statistical Analysis
3. Results
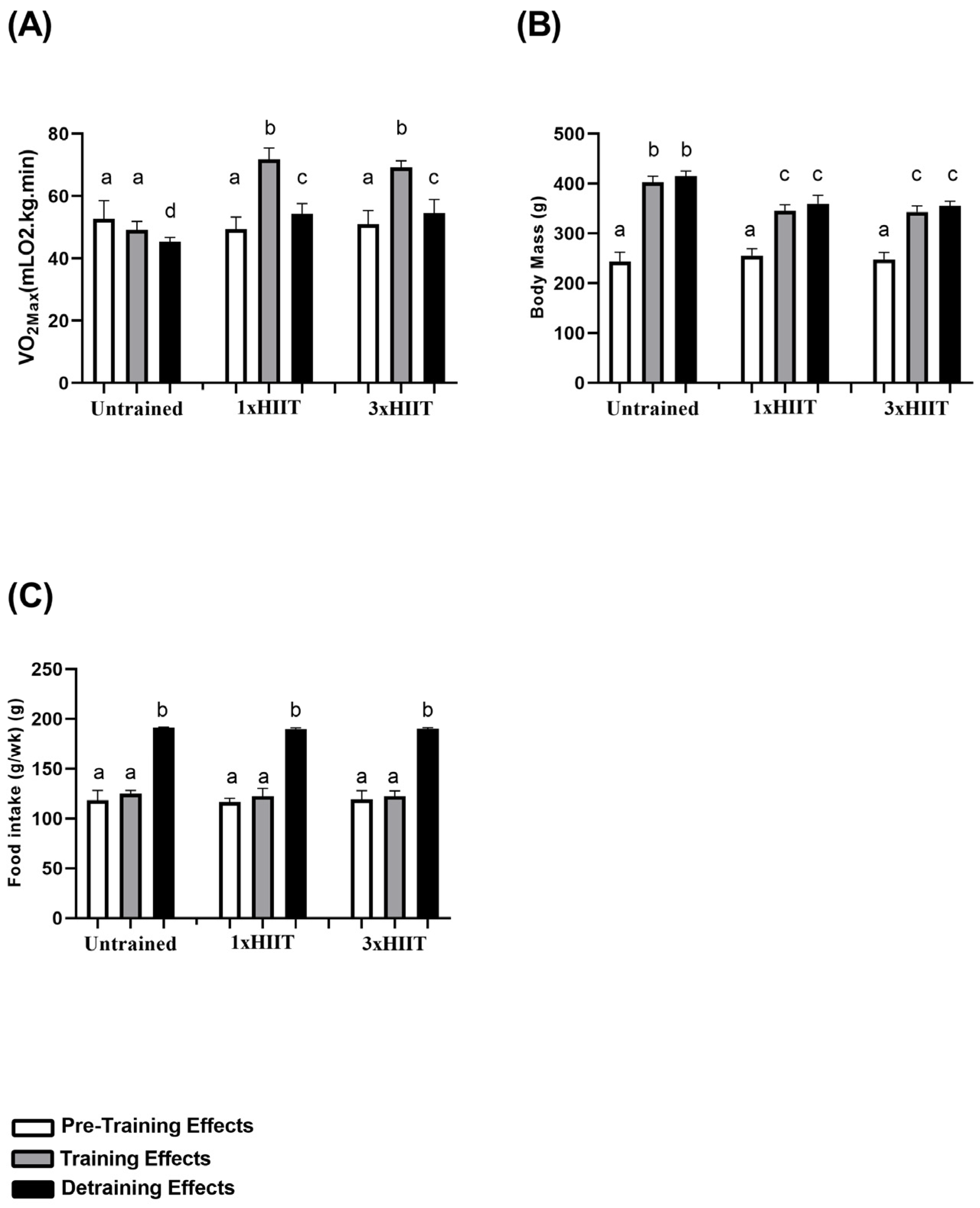
3.1. Training and Detraining Effects of 1xHIIT and 3xHIIT on VO2max, Body Mass and Food Intake
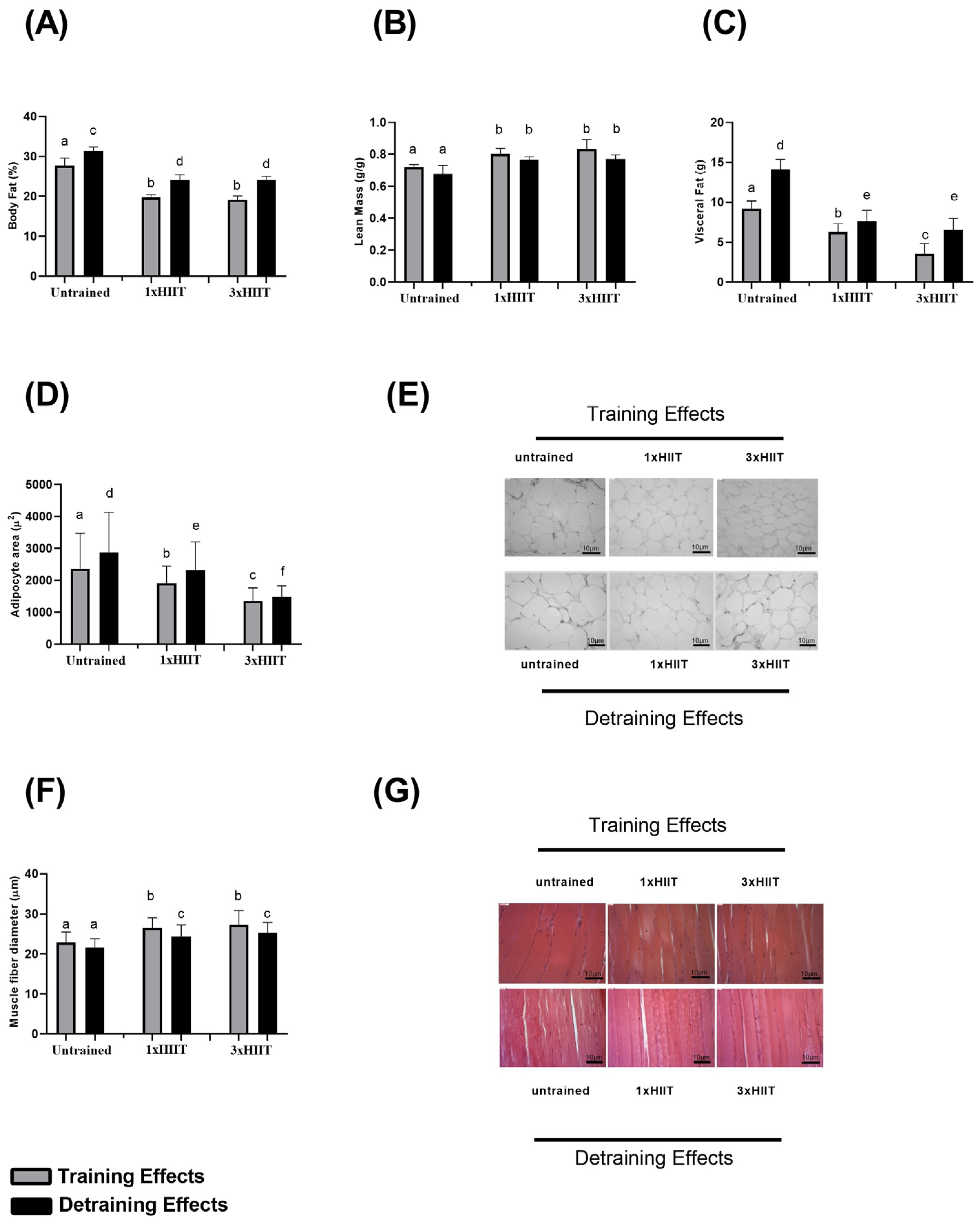
3.2. Training and Detraining Effects of 1xHIIT and 3xHIIT on Body Composition
3.3. Training and Detraining Effects of 1xHIIT and 3xHIIT on Resting Oxygen Consumption and Blood Glucose Homeostasis
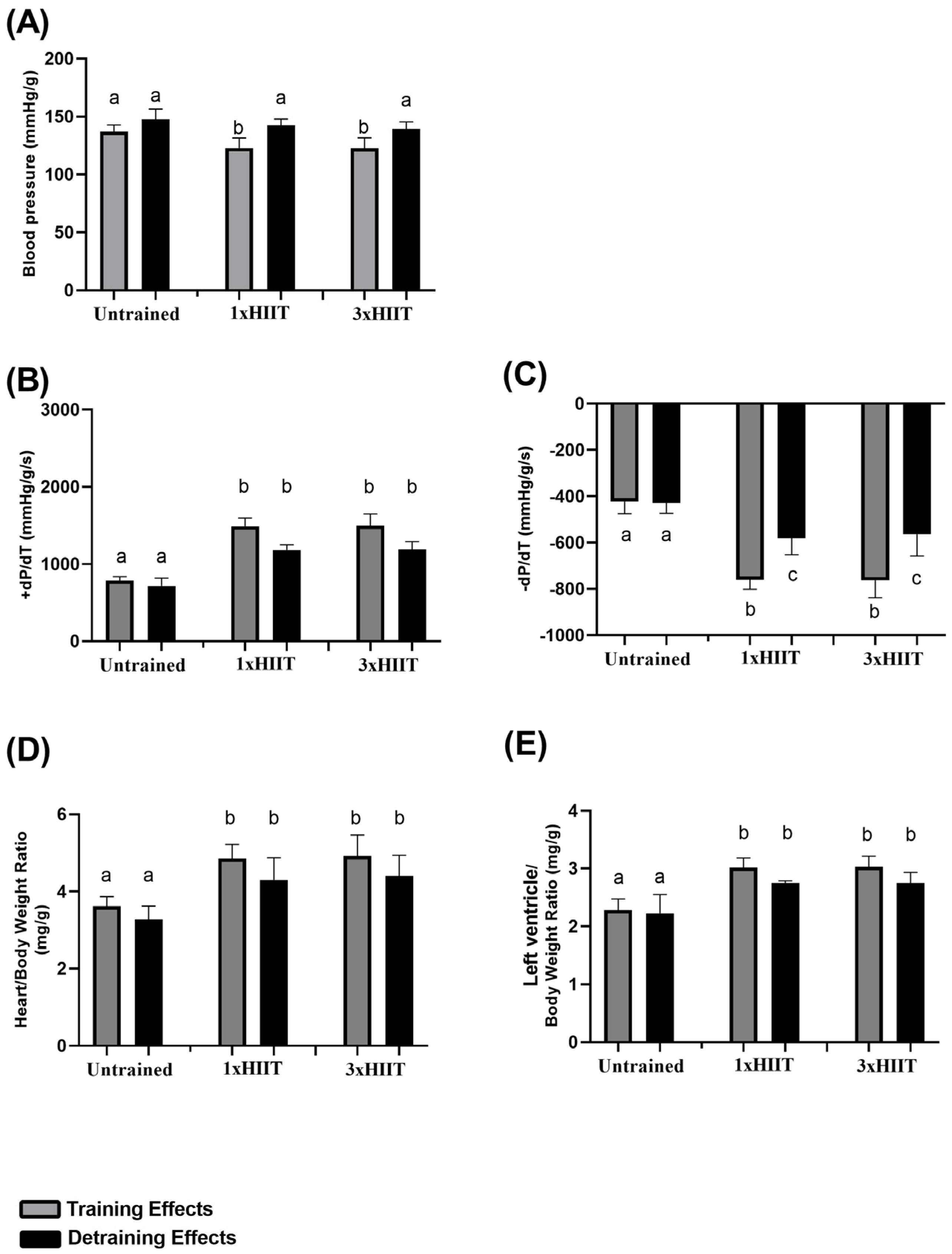
3.4. Training and Detraining Effects of 1xHIIT and 3xHIIT on Cardiovascular Parameters
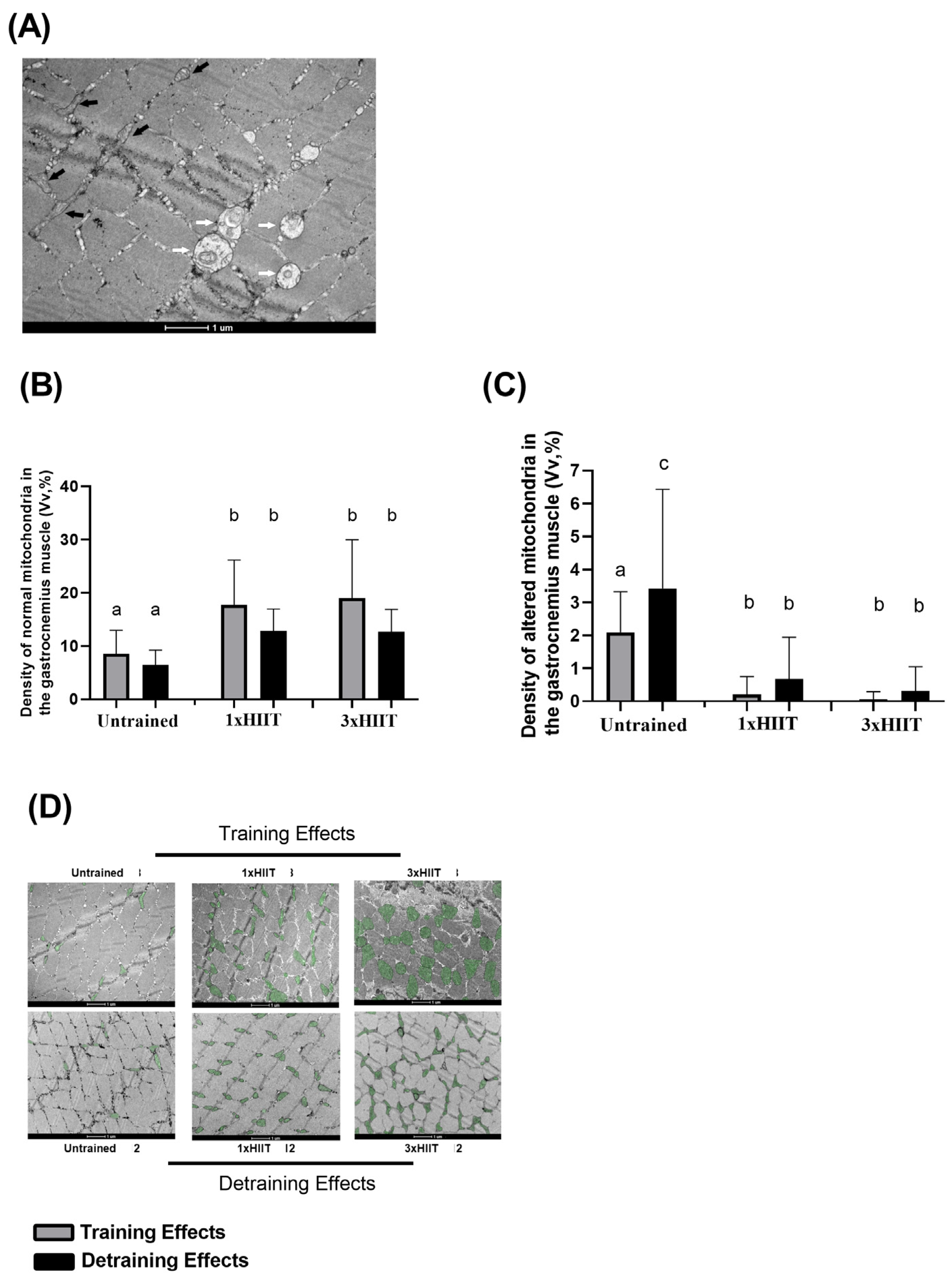
3.5. Training and Detraining Effects of 1xHIIT and 3xHIIT on Mitochondrial Densities of Cardiac and Skeletal Muscles
4. Discussion
4.1. Cardiovascular System
4.2. Metabolic System
4.3. Body Composition
4.4. Study Limitations and Future Directions
4.5. Practical Implications
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Girardi, M.; Casolo, A.; Nuccio, S.; Gattoni, C.; Capelli, C. Detraining Effects Prevention: A New Rising Challenge for Athletes. Front. Physiol. 2020, 11, 588784. [Google Scholar] [CrossRef]
- Mujika, I.; Padilla, S. Detraining: Loss of Training-Induced Physiological and Performance Adaptations. Part I. Sports Med. 2000, 30, 79–87. [Google Scholar] [CrossRef] [PubMed]
- Blocquiaux, S.; Gorski, T.; Van Roie, E.; Ramaekers, M.; Van Thienen, R.; Nielens, H.; Delecluse, C.; De Bock, K.; Thomis, M. The effect of resistance training, detraining and retraining on muscle strength and power, myofibre size, satellite cells and myonuclei in older men. Exp. Gerontol. 2020, 133, 110860. [Google Scholar] [CrossRef]
- Kite, C.; Lagojda, L.; Clark, C.C.T.; Uthman, O.; Denton, F.; McGregor, G.; Harwood, A.E.; Atkinson, L.; Broom, D.R.; Kyrou, I.; et al. Changes in Physical Activity and Sedentary Behaviour Due to Enforced COVID-19-Related Lockdown and Movement Restrictions: A Protocol for a Systematic Review and Meta-Analysis. Int. J. Environ. Res. Public Health 2021, 18, 5251. [Google Scholar] [CrossRef] [PubMed]
- Murphy, M.H.; Lahart, I.; Carlin, A.; Murtagh, E. The Effects of Continuous Compared to Accumulated Exercise on Health: A Meta-Analytic Review. Sports Med. 2019, 49, 1585–1607. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Tong, T.K.; Qiu, W.; Zhang, X.; Zhou, S.; Liu, Y.; He, Y. Comparable Effects of High-Intensity Interval Training and Prolonged Continuous Exercise Training on Abdominal Visceral Fat Reduction in Obese Young Women. J. Diabetes Res. 2017, 2017, 5071740. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, D.G.; Devin, J.L.; Weston, K.L.; Jenkins, J.G.; Skinner, T.L. Benefits beyond cardiometabolic health: The potential of frequent high intensity ‘exercise snacks’ to improve outcomes for those living with and beyond cancer. J. Physiol. 2023, 601, 4691–4697. [Google Scholar] [CrossRef] [PubMed]
- Costa-Pereira, L.V.; Melo, D.S.; Santos, C.S.; Mendes, B.F.; Esteves, E.A.; Lacerda, A.C.R.; de Miranda, J.L.; Rocha-Vieira, E.; Gripp, F.; Amorim, F.T.; et al. Distinct beneficial effects of continuous vs accumulated exercise training on cardiovascular risk factors in Wistar rats. Scand. J. Med. Sci. Sports 2017, 27, 1384–1394. [Google Scholar] [CrossRef] [PubMed]
- De Sousa, R.A.L.; Mendes, B.F.; Costa-Pereira, L.; Pereira, R.R.d.S.; de Andrade, J.A.; e Magalhães, C.O.D.; Gripp, F.; Magalhães, F.d.C.; Andrade, E.F.; Cassilhas, R.C.; et al. Accumulated High-intensity Interval Training Protocol: A New Approach to Study Health Markers in Wistar Rats. J. Vis. Exp. 2022, e63328. [Google Scholar] [CrossRef]
- Mendes, B.F.; Costa-Pereira, L.V.; de Andrade, J.A.; Magalhães, C.O.D.; de Pereira, R.R.S.; Esteves, E.A.; Cassilhas, R.C.; Andrade, E.F.; Gripp, F.; de Magalhães, F.C.; et al. Superior cardiometabolic and cellular adaptive responses to multiple versus single daily sessions of high-intensity interval training in Wistar rats. Sci. Rep. 2022, 12, 21187. [Google Scholar] [CrossRef]
- Høydal, M.A.; Wisløff, U.; Kemi, O.J.; Ellingsen, O. Running speed and maximal oxygen uptake in rats and mice: Practical implications for exercise training. Eur. J. Prev. Cardiol. 2007, 14, 753–760. [Google Scholar] [CrossRef] [PubMed]
- Stevenson, K.T.; van Tets, I.G. Dual-Energy X-Ray Absorptiometry (DXA) Can Accurately and Nondestructively Measure the Body Composition of Small, Free-Living Rodents. Physiol. Biochem. Zool. 2008, 81, 373–382. [Google Scholar] [CrossRef]
- Melo, D.d.S.; Santos, C.S.; Pereira, L.C.; Mendes, B.F.; Jesus, L.S.; Pelaez, J.M.N.; Aguilar, E.C.; Nascimento, D.R.; Martins, A.d.S.; Magalhães, F.d.C.; et al. Refeeding abolishes beneficial effects of severe calorie restriction from birth on adipose tissue and glucose homeostasis of adult rats. Nutrition 2019, 66, 87–93. [Google Scholar] [CrossRef] [PubMed]
- Melo, D.S.; Costa-Pereira, L.V.; Santos, C.S.; Mendes, B.F.; Costa, K.B.; Santos, C.F.F.; Rocha-Vieira, E.; Magalhães, F.C.; Esteves, E.A.; Ferreira, A.J.; et al. Severe calorie restriction reduces cardiometabolic risk factors and protects rat hearts from ischemia/reperfusion injury. Front. Physiol. 2016, 7, 106. [Google Scholar] [CrossRef] [PubMed]
- de Queiroz, K.B.; Honorato-Sampaio, K.; Júnior, J.V.R.; Leal, D.A.; Pinto, A.B.G.; Kappes-Becker, L.; Evangelista, E.A.; Guerra-Sá, R. Physical activity prevents alterations in mitochondrial ultrastructure and glucometabolic parameters in a high-sugar diet model. PLoS ONE 2017, 12, e0172103. [Google Scholar] [CrossRef]
- Oláh, A.; Kellermayer, D.; Mátyás, C.; Németh, B.T.; Lux, Á.; Szabó, L.; Török, M.; Ruppert, M.; Meltzer, A.; Sayour, A.A.; et al. Complete Reversion of Cardiac Functional Adaptation Induced by Exercise Training. Med. Sci. Sports Exerc. 2017, 49, 420–429. [Google Scholar] [CrossRef]
- Bonsu, B.; Terblanche, E. The training and detraining effect of high-intensity interval training on post-exercise hypotension in young overweight/obese women. Eur. J. Appl. Physiol. 2016, 116, 77–84. [Google Scholar] [CrossRef] [PubMed]
- Oláh, A.; Kovács, A.; Lux, Á.; Tokodi, M.; Braun, S.; Lakatos, B.K.; Mátyás, C.; Kellermayer, D.; Ruppert, M.; Sayour, A.A.; et al. Characterization of the dynamic changes in left ventricular morphology and function induced by exercise training and detraining. Int. J. Cardiol. 2019, 277, 178–185. [Google Scholar] [CrossRef]
- Kilic-Toprak, E.; Kilic-Erkek, O.; Abban-Mete, G.; Caner, V.; Baris, I.C.; Turhan, G.; Kucukatay, V.; Senol, H.; Kuru, O.; Bor-Kucukatay, M. Contribution of Heme Oxygenase 2 to Blood Pressure Regulation in Response to Swimming Exercise and Detraining in Spontaneously Hypertensive Rats. Med. Sci. Monit. 2018, 24, 51–59. [Google Scholar] [CrossRef]
- Kilic-Erkek, O.; Kilic-Toprak, E.; Caliskan, S.; Ekbic, Y.; Akbudak, I.H.; Kucukatay, V.; Bor-Kucukatay, M. Detraining reverses exercise-induced improvement in blood pressure associated with decrements of oxidative stress in various tissues in spontaneously hypertensive rats. Mol. Cell. Biochem. 2016, 412, 209–219. [Google Scholar] [CrossRef]
- Lehnen, A.M.; Leguisamo, N.M.; Pinto, G.H.; Markoski, M.M.; De Angelis, K.; Machado, U.F.; Schaan, B. The beneficial effects of exercise in rodents are preserved after detraining: A phenomenon unrelated to GLUT4 expression. Cardiovasc. Diabetol. 2010, 9, 67. [Google Scholar] [CrossRef] [PubMed]
- Coyle, E.F.; Martin, W.H.; Sinacore, D.R.; Joyner, M.J.; Hagberg, J.M.; Holloszy, J.O. Time course of loss of adaptations after stopping prolonged intense endurance training. J. Appl. Physiol. 1984, 57, 1857–1864. [Google Scholar] [CrossRef]
- Lennon, S.L.; Quindry, J.; Hamilton, K.L.; French, J.; Staib, J.; Mehta, J.L.; Powers, S.K. Loss of exercise-induced cardioprotection after cessation of exercise. J. Appl. Physiol. 2004, 96, 1299–1305. [Google Scholar] [CrossRef] [PubMed]
- Gripp, F.; Nava, R.C.; Cassilhas, R.C.; Esteves, E.A.; Magalhães, C.O.D.; Dias-Peixoto, M.F.; Magalhães, F.d.C.; Amorim, F.T. HIIT is superior than MICT on cardiometabolic health during training and detraining. Eur. J. Appl. Physiol. 2021, 121, 159–172. [Google Scholar] [CrossRef] [PubMed]
- Bosquet, L.; Berryman, N.; Dupuy, O.; Mekary, S.; Arvisais, D.; Bherer, L.; Mujika, I. Effect of training cessation on muscular performance: A meta-analysis. Scand. J. Med. Sci. Sports 2013, 23, e140–e149. [Google Scholar] [CrossRef]
- Waring, C.D.; Henning, B.J.; Smith, A.J.; Nadal-Ginard, B.; Torella, D.; Ellison, G.M. Cardiac adaptations from four weeks of intensity-controlled vigorous exercise are lost after a similar period of detraining. Physiol. Rep. 2015, 3, e12302. [Google Scholar] [CrossRef] [PubMed]
- Francois, M.E.; Baldi, J.C.; Manning, P.J.; Lucas, S.J.E.; Hawley, J.A.; Williams, M.J.A.; Cotter, J.D. ‘Exercise snacks’ before meals: A novel strategy to improve glycaemic control in individuals with insulin resistance. Diabetologia 2014, 57, 1437–1445. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Costa-Pereira, L.V.; Mendes, B.F.; Magalhães, C.O.D.; Rodrigues, C.M.; de Andrade, J.A.; de Pereira, R.R.S.; Esteves, E.A.; Cassilhas, R.C.; Andrade, E.F.; Gripp, F.; et al. Cardiometabolic and Cellular Adaptations to Multiple vs. Single Daily HIIT Sessions in Wistar Rats: Impact of Short-Term Detraining. Metabolites 2024, 14, 447. https://doi.org/10.3390/metabo14080447
Costa-Pereira LV, Mendes BF, Magalhães COD, Rodrigues CM, de Andrade JA, de Pereira RRS, Esteves EA, Cassilhas RC, Andrade EF, Gripp F, et al. Cardiometabolic and Cellular Adaptations to Multiple vs. Single Daily HIIT Sessions in Wistar Rats: Impact of Short-Term Detraining. Metabolites. 2024; 14(8):447. https://doi.org/10.3390/metabo14080447
Chicago/Turabian StyleCosta-Pereira, Liliane Vanessa, Bruno Ferreira Mendes, Caíque Olegário Diniz Magalhães, Cíntia Maria Rodrigues, Júllia Alves de Andrade, Ramona Ramalho Souza de Pereira, Elizabethe Adriana Esteves, Ricardo Cardoso Cassilhas, Eric Francelino Andrade, Fernando Gripp, and et al. 2024. "Cardiometabolic and Cellular Adaptations to Multiple vs. Single Daily HIIT Sessions in Wistar Rats: Impact of Short-Term Detraining" Metabolites 14, no. 8: 447. https://doi.org/10.3390/metabo14080447
APA StyleCosta-Pereira, L. V., Mendes, B. F., Magalhães, C. O. D., Rodrigues, C. M., de Andrade, J. A., de Pereira, R. R. S., Esteves, E. A., Cassilhas, R. C., Andrade, E. F., Gripp, F., de Magalhães, F. C., Sampaio, K. H., Improta-Caria, A. C., Amorim, F. T., & Dias-Peixoto, M. F. (2024). Cardiometabolic and Cellular Adaptations to Multiple vs. Single Daily HIIT Sessions in Wistar Rats: Impact of Short-Term Detraining. Metabolites, 14(8), 447. https://doi.org/10.3390/metabo14080447







