Hyperpolarized Magnetic Resonance Imaging, Nuclear Magnetic Resonance Metabolomics, and Artificial Intelligence to Interrogate the Metabolic Evolution of Glioblastoma
Abstract
1. Introduction
2. Material and Methods
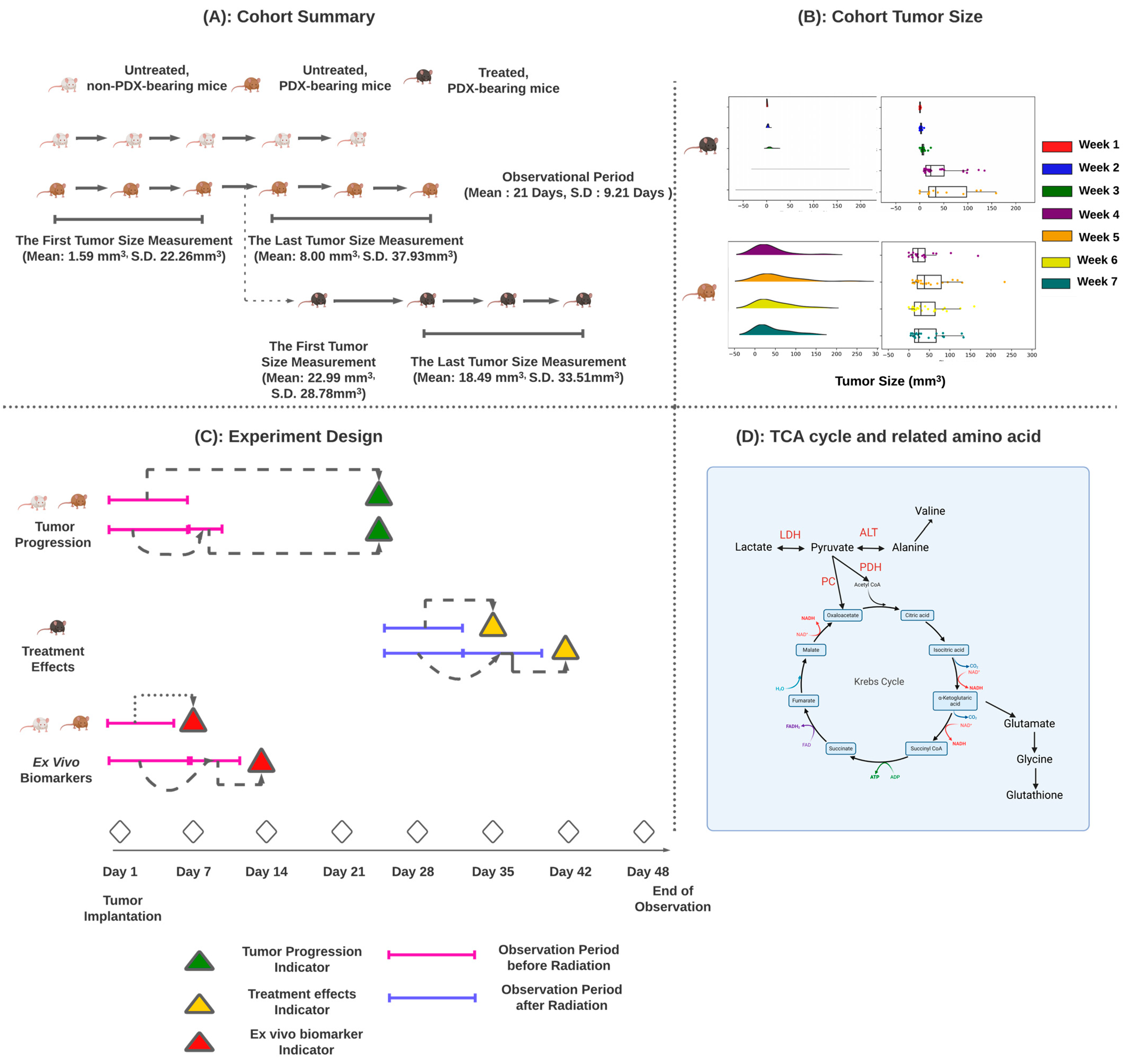
2.1. Xenograft Mice
2.2. Mouse Cohorts
- Untreated, non-PDX-bearing control mice (n = 27)
- Untreated PDX-bearing mice (n = 67)
- Treated PDX-bearing mice (n = 46) with 2 × 5 Gy of radiation daily on days 25 and 27.
2.3. Experimental Design
2.4. Tumor Progression
2.5. Treatment Effects
- Effective radiation therapy should result in a reduction in tumor size over the tumor regression period. This reduction is indicated by a negative correlation between day and tumor volume. We classified mice with a negative correlation as having a successful treatment response and vice versa.
- If a mouse only had one tumor measurement within the tumor regression period, we established a reference line from untreated PDX-bearing mice. This line provides an estimate of tumor size in the event of treatment failure. We believe that effective treatment should result in a tumor size smaller than the lower-bound reference line. We used a generalized linear model to construct this line, which represents the estimated average tumor volume minus one standard deviation of the estimated tumor volume. If a mouse’s tumor volume falls below this line, radiation therapy is considered effective.
2.6. Ex Vivo Metabolomic Prediction
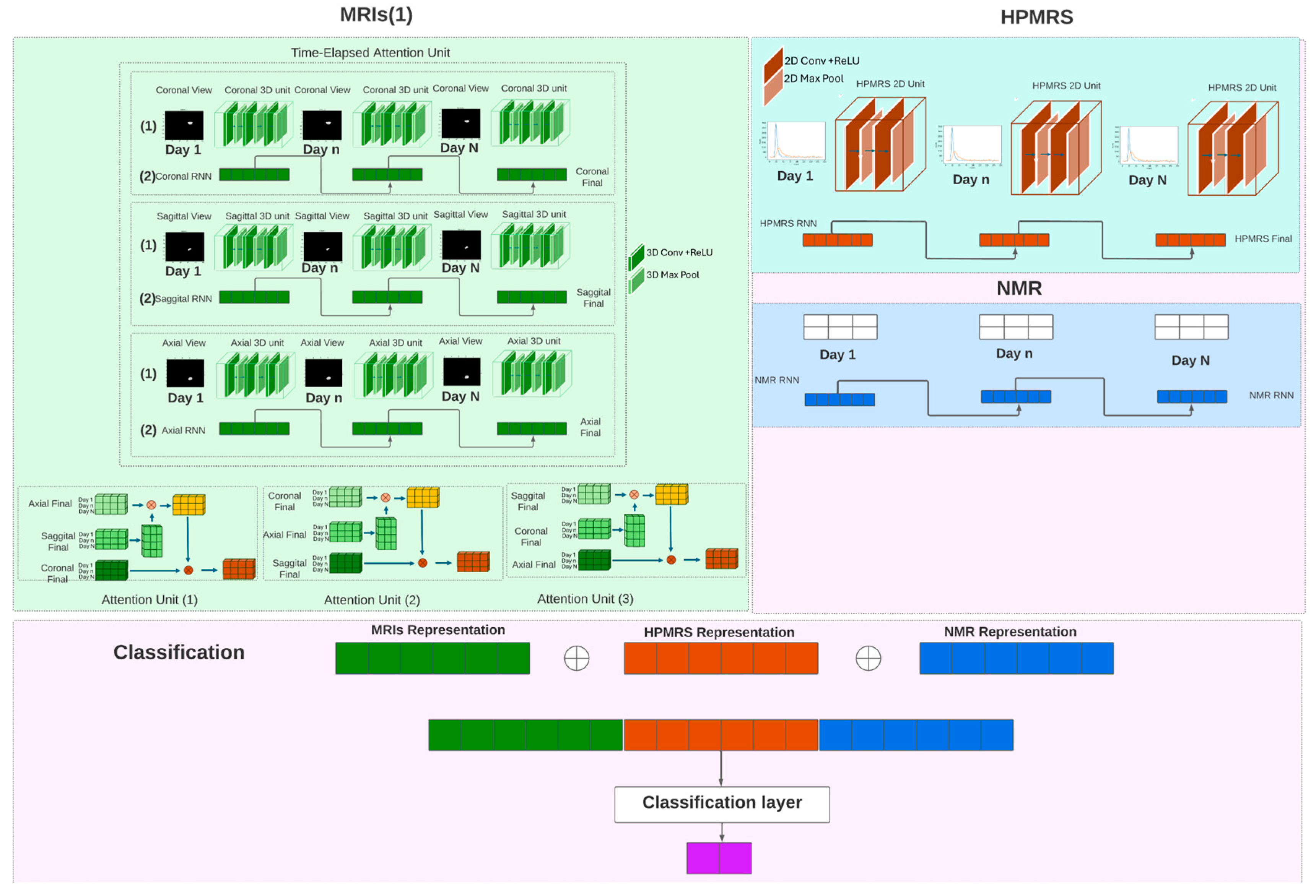
2.7. Model Design
2.8. Model Components
2.9. MRIs Processing Unit
2.9.1. MRIs Encoder
2.9.2. Time-Elapsed Attention Module
2.10. HPMRS Processing Unit
2.11. Classifier
2.12. Training and Evaluation
2.13. Missing Value Handling
3. Results
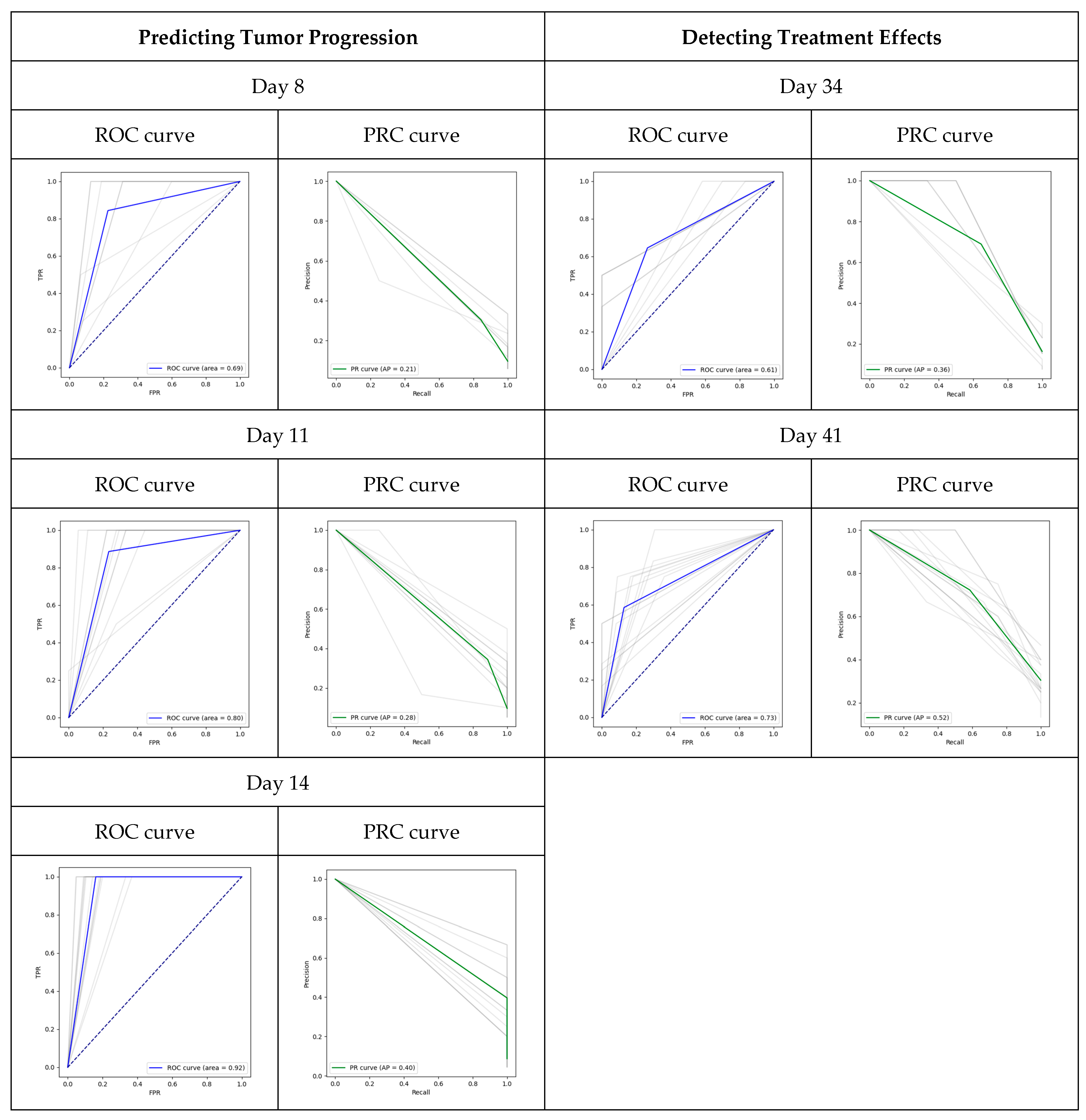
3.1. Prediction of Tumor Progression
3.2. Detection of Treatment Efficacy
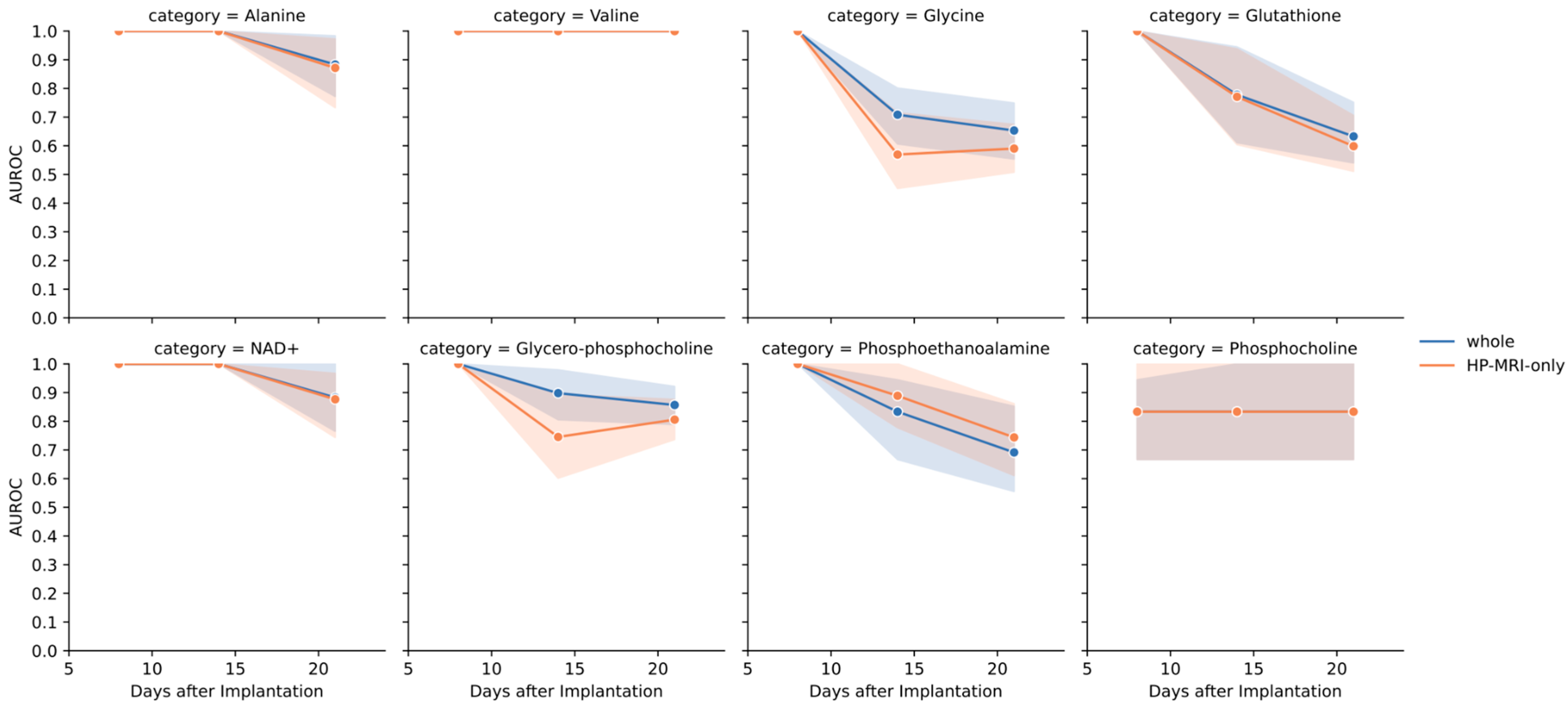
3.3. Prediction of Biomarkers Ex Vivo
4. Discussion
4.1. Temporal Patterns Improve Model Performance
4.2. Deep Learning Can Be Used to Predict Treatment Effects in Preclinical Models of GBM
4.3. Deep Learning Can Be Combined with HPMRS to Predict Metabolomic Patterns
5. Limitations
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ostrom, Q.T.; Price, M.; Ryan, K.; Edelson, J.; Neff, C.; Cioffi, G.; Waite, K.A.; Kruchko, C.; Barnholtz-Sloan, J.S. CBTRUS Statistical Report: Pediatric Brain Tumor Foundation Childhood and Adolescent Primary Brain and Other Central Nervous System Tumors Diagnosed in the United States in 2014–2018. Neuro-Oncology 2022, 24, iii1–iii38. [Google Scholar] [CrossRef] [PubMed]
- Tan, A.C.; Ashley, D.M.; López, G.Y.; Malinzak, M.; Friedman, H.S.; Khasraw, M. Management of Glioblastoma: State of the Art and Future Directions. CA Cancer J. Clin. 2020, 70, 299–312. [Google Scholar] [CrossRef] [PubMed]
- Warburg, O. On the Origin of Cancer Cells. Science 1956, 123, 309–314. [Google Scholar] [CrossRef] [PubMed]
- Vander Heiden, M.G.; Cantley, L.C.; Thompson, C.B. Understanding the Warburg Effect: The Metabolic Requirements of Cell Proliferation. Science 2009, 324, 1029–1033. [Google Scholar] [CrossRef]
- Ardenkjaer-Larsen, J.H.; Fridlund, B.; Gram, A.; Hansson, G.; Hansson, L.; Lerche, M.H.; Servin, R.; Thaning, M.; Golman, K. Increase in Signal-to-Noise Ratio of >10,000 Times in Liquid-State NMR. Proc. Natl. Acad. Sci. USA 2003, 100, 10158–10163. [Google Scholar] [CrossRef] [PubMed]
- Salzillo, T.C.; Mawoneke, V.; Weygand, J.; Shetty, A.; Gumin, J.; Zacharias, N.M.; Gammon, S.T.; Piwnica-Worms, D.; Fuller, G.N.; Logothetis, C.J.; et al. Measuring the Metabolic Evolution of Glioblastoma throughout Tumor Development, Regression, and Recurrence with Hyperpolarized Magnetic Resonance. Cells 2021, 10, 2621. [Google Scholar] [CrossRef] [PubMed]
- Mansouri, S.; Singh, S.; Alamsahebpour, A.; Burrell, K.; Li, M.; Karabork, M.; Ekinci, C.; Koch, E.; Solaroglu, I.; Chang, J.T.; et al. DICER Governs Characteristics of Glioma Stem Cells and the Resulting Tumors in Xenograft Mouse Models of Glioblastoma. Oncotarget 2016, 7, 56431–56446. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Wei, J.; Barr, J.; Kong, L.Y.; Wang, Y.; Wu, A.; Sharma, A.K.; Gumin, J.; Henry, V.; Colman, H.; Priebe, W.; et al. Glioblastoma Cancer-Initiating Cells Inhibit T-Cell Proliferation and Effector Responses by the Signal Transducers and Activators of Transcription 3 Pathway. Mol. Cancer Ther. 2010, 9, 67–78. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.K.; Hawkins, C.; Clarke, I.D.; Squire, J.A.; Bayani, J.; Hide, T.; Henkelman, R.M.; Cusimano; Dirks, P.B. Identification of Human Brain Tumour Initiating Cells. Nature 2004, 432, 396–401. [Google Scholar] [CrossRef] [PubMed]
- McMillan, K.M.; Ehtesham, M.; Stevenson, C.B.; Edgeworth, M.L.; Thompson, R.C.; Price, R.R. T2 Detection of Tumor Invasion within Segmented Components of Glioblastoma Multiforme. J. Magn. Reson. Imaging 2009, 29, 251–257. [Google Scholar] [CrossRef] [PubMed]
- Tran, D.; Bourdev, L.; Fergus, R.; Torresani, L.; Paluri, M. Learning Spatiotemporal Features with 3D Convolutional Networks. In Proceedings of the 2015 IEEE International Conference on Computer Vision (ICCV), Santiago, Chile, 7–13 December 2015. [Google Scholar]
- Vaswani, A.; Shazeer, N.; Parmar, N.; Uszkoreit, J.; Jones, L.; Kaiser, A.G.; Polosukhin, I. Attention is all you need. Adv. Neural Inf. Process. Syst. 2017, 5998–6008. [Google Scholar]
- Cordonnier, J.; Loukas, A.; Jaggi, M. Multi-Head Attention: Collaborate Instead of Concatenate. arXiv 2020, arXiv:2006.16362. [Google Scholar]
- Dwibedi, D.; Aytar, Y.; Tompson, J.; Sermanet, P.; Zisserman, A. Temporal Cycle-Consistency Learning. In Proceedings of the 2019 IEEE/CVF Conference on Computer Vision and Pattern Recognition (CVPR), Long Beach, CA, USA, 15–20 June 2019. [Google Scholar]
- Kaggie, J.D.; Khan, A.S.; Matys, T.; Schulte, R.F.; Locke, M.J.; Grimmer, A.; Frary, A.; Menih, I.H.; Latimer, E.; Graves, M.J.; et al. Deuterium Metabolic Imaging and Hyperpolarized 13C-MRI of the Normal Human Brain at Clinical Field Strength Reveals Differential Cerebral Metabolism. NeuroImage 2022, 257, 119284. [Google Scholar] [CrossRef] [PubMed]
- Caniglia, J.L.; Jalasutram, A.; Asuthkar, S.; Sahagun, J.; Park, S.; Ravindra, A.; Tsung, A.J.; Guda, M.R.; Velpula, K.K. Beyond Glucose: Alternative Sources of Energy in Glioblastoma. Theranostics 2021, 11, 2048–2057. [Google Scholar] [CrossRef] [PubMed]
- Bankson, J.A.; Walker, C.M.; Ramirez, M.S.; Stefan, W.; Fuentes, D.; Merritt, M.E.; Lee, J.; Sandulache, V.C.; Chen, Y.; Phan, L.; et al. Kinetic Modeling and Constrained Reconstruction of Hyperpolarized [1-13C]-Pyruvate Offers Improved Metabolic Imaging of Tumors. Cancer Res. 2015, 75, 4708–4717. [Google Scholar] [CrossRef]
| No. of Days after Index Date or Treatment | Cohort | No. of Mice |
|---|---|---|
| Predicting Tumor Progression | ||
| 8 | Control | 10 |
| Untreated | 42 | |
| 11 | Control | 13 |
| Untreated | 47 | |
| 14 | Control | 17 |
| Untreated | 51 | |
| Predicting Treatment Effects | ||
| 7 | Treated | 39 |
| 14 | Treated | 46 |
| Predicting in vivo biomarkers | ||
| 8 | Control | 3 |
| Untreated | 7 | |
| 14 | Control | 9 |
| Untreated | 17 | |
| 21 | Control | 12 |
| Untreated | 22 | |
| Modula_Name | Kernel Size | Output Size | Number of Parameters |
|---|---|---|---|
| Fianl Model | [4, 2] | 42,304,277 | |
| Sagittal_3D_Encoder-1 | [4, 64, 3, 35, 48] | 1216 | |
| Conv3d: 2 | [2, 3, 3] | [4, 64, 6, 105, 145] | 1216 |
| ReLU: 2 | [4, 64, 6, 105, 145] | 0 | |
| MaxPool3d: 2 | [2, 3, 3] | [4, 64, 3, 35, 48] | 0 |
| Sagittal_3D_Encoder-2 | [4, 128, 3, 11, 15] | 73,856 | |
| Conv3d: 2 | [1, 3, 3] | [4, 128, 3, 33, 46] | 73,856 |
| ReLU: 2 | [4, 128, 3, 33, 46] | 0 | |
| MaxPool3d: 2 | [1, 3, 3] | [4, 128, 3, 11, 15] | 0 |
| Sagittal_3D_Encoder-3 | [4, 256, 3, 3, 4] | 295,168 | |
| Conv3d: 2 | [1, 3, 3] | [4, 256, 3, 9, 13] | 295,168 |
| ReLU: 2 | [4, 256, 3, 9, 13] | 0 | |
| MaxPool3d: 2 | [1, 3, 3] | [4, 256, 3, 3, 4] | 0 |
| Axial_3D_Encoder-1 | [4, 64, 2, 35, 48] | 1216 | |
| Conv3d: 2 | [2, 3, 3] | [4, 64, 5, 105, 145] | 1216 |
| ReLU: 2 | [4, 64, 5, 105, 145] | 0 | |
| MaxPool3d: 2 | [2, 3, 3] | [4, 64, 2, 35, 48] | 0 |
| Axial_3D_Encoder-2 | [4, 128, 2, 11, 15] | 73,856 | |
| Conv3d: 2 | [1, 3, 3] | [4, 128, 2, 33, 46] | 73,856 |
| ReLU: 2 | [4, 128, 2, 33, 46] | 0 | |
| MaxPool3d: 2 | [1, 3, 3] | [4, 128, 2, 11, 15] | 0 |
| Axial_3D_Encoder-3 | [4, 256, 2, 3, 4] | 295,168 | |
| Conv3d: 2 | [1, 3, 3] | [4, 256, 2, 9, 13] | 295,168 |
| ReLU: 2 | [4, 256, 2, 9, 13] | 0 | |
| MaxPool3d: 2 | [1, 3, 3] | [4, 256, 2, 3, 4] | 0 |
| Coronal_3D_Encoder-1 | [4, 64, 4, 35, 48] | 1216 | |
| Conv3d: 2 | [2, 3, 3] | [4, 64, 8, 105, 145] | 1216 |
| ReLU: 2 | [4, 64, 8, 105, 145] | 0 | |
| MaxPool3d: 2 | [2, 3, 3] | [4, 64, 4, 35, 48] | 0 |
| Coronal_3D_Encoder-2 | [4, 128, 4, 11, 15] | 73,856 | |
| Conv3d: 2 | [1, 3, 3] | [4, 128, 4, 33, 46] | 73,856 |
| ReLU: 2 | [4, 128, 4, 33, 46] | 0 | |
| MaxPool3d: 2 | [1, 3, 3] | [4, 128, 4, 11, 15] | 0 |
| Coronal_3D_Encoder-3 | [4, 256, 4, 3, 4] | 295,168 | |
| Conv3d: 2 | [1, 3, 3] | [4, 256, 4, 9, 13] | 295,168 |
| ReLU: 2 | [4, 256, 4, 9, 13] | 0 | |
| MaxPool3d: 2 | [1, 3, 3] | [4, 256, 4, 3, 4] | 0 |
| HPMRS_2D_Encoder-1 | [4, 8, 5, 843] | 80 | |
| Conv2d: 2 | [3, 3] | [4, 8, 16, 2530] | 80 |
| ReLU: 2 | [4, 8, 16, 2530] | 0 | |
| MaxPool2d: 2 | [3, 3] | [4, 8, 5, 843] | 0 |
| HPMRS_2D_Encoder-2 | [4, 16, 1, 280] | 1168 | |
| Conv2d: 2 | [3, 3] | [4, 16, 3, 841] | 1168 |
| ReLU: 2 | [4, 16, 3, 841] | 0 | |
| MaxPool2d: 2 | [3, 3] | [4, 16, 1, 280] | 0 |
| Time-Elapsed Attention:1 | [4, 768] | 786,432 | |
| Axial Attention: 1 | [1, 1, 256] | 262,144 | |
| ReLU: 2 | [1, 4, 256] | 0 | |
| Coronal Attention: 1 | [1, 1, 256] | 262,144 | |
| Sagittal Attention: 1 | [1, 1, 256] | 262,144 | |
| ReLU: 2 | [4, 768] | 0 | |
| Time-Elapsed Attention:2 | [4, 768] | 786,432 | |
| Coronal Attention: 2 | [1, 1, 256] | 262,144 | |
| ReLU: 2 | [1, 4, 256] | 0 | |
| Axial Attention: 2 | [1, 1, 256] | 262,144 | |
| Sagittal Attention: 2 | [1, 1, 256] | 262,144 | |
| ReLU: 2 | [4, 768] | 0 | |
| Time-Elapsed Attention:3 | [4, 768] | 786,432 | |
| Sagittal Attention: 3 | [1, 1, 256] | 262,144 | |
| ReLU: 2 | [1, 4, 256] | 0 | |
| Coronal Attention: 3 | [1, 1, 256] | 262,144 | |
| Axial Attention: 3 | [1, 1, 256] | 262,144 | |
| ReLU: 2 | [4, 768] | 0 | |
| Classification: 1 | [4, 768] | 1,770,240 | |
| ReLU: 1 | [4, 768] | 0 | |
| NMR_RNN: 1 | [4, 1, 9] | 180 | |
| HP_RNN: 1 | [4, 1, 128] | 52,480 | |
| Classification: 2 | [4, 2] | 1818 | |
| Overall (Mean/S.D.) | |||||
| Day | AUC | AUPRC | |||
| 7 | 0.690/0.184 | 0.211/0.086 | |||
| 11 | 0.799/0.145 | 0.279/0.094 | |||
| 14 | 0.919/0.049 | 0.396/0.174 | |||
| By Cohort (Mean/S.D.) | |||||
| Day | Cohort | TPR | FNR | FPR | TNR |
| 7 | non-PDX-bearing mice | 0/0 | 0/0 | 0.052/0.184 | 0.948/0.184 |
| 7 | untreated PDX-bearing mice | 0.519/0.483 | 0.480/0.483 | 0.159/0.201 | 0.840/0.201 |
| 11 | non-PDX-bearing mice | 0/0 | 0/0 | 0.059 ± 0.150 | 0.941/0.150 |
| 11 | untreated PDX-bearing mice | 0.812/0.355 | 0.187/0.355 | 0.261/0.175 | 0.738/0.175 |
| 14 | non-PDX-bearing mice | 0/0 | 0/0 | 0.059/0.069 | 0.941/0.150 |
| 14 | untreated PDX-bearing mice | 1/0 | 0/0 | 0.206/0.105 | 0.793/0.105 |
| Overall (Mean/S.D.) | |||||
| Day | AUC | AUPRC | |||
| 7 | 0.608/0.607 | 0.356/0.166 | |||
| 14 | 0.728/0.081 | 0.520/0.107 | |||
| By Cohort (Mean/S.D.) | |||||
| Day | Cohort | TPR | FNR | FPR | TNR |
| 7 | Treated | 0.369/0.398 | 0.630/0.398 | 0.151/0.304 | 0.848/0.304 |
| 14 | Treated | 0.585/0.244 | 0.414/0.244 | 0.129/0.126 | 0.870/0.126 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hsieh, K.L.; Chen, Q.; Salzillo, T.C.; Zhang, J.; Jiang, X.; Bhattacharya, P.K.; Shams, S. Hyperpolarized Magnetic Resonance Imaging, Nuclear Magnetic Resonance Metabolomics, and Artificial Intelligence to Interrogate the Metabolic Evolution of Glioblastoma. Metabolites 2024, 14, 448. https://doi.org/10.3390/metabo14080448
Hsieh KL, Chen Q, Salzillo TC, Zhang J, Jiang X, Bhattacharya PK, Shams S. Hyperpolarized Magnetic Resonance Imaging, Nuclear Magnetic Resonance Metabolomics, and Artificial Intelligence to Interrogate the Metabolic Evolution of Glioblastoma. Metabolites. 2024; 14(8):448. https://doi.org/10.3390/metabo14080448
Chicago/Turabian StyleHsieh, Kang Lin, Qing Chen, Travis C. Salzillo, Jian Zhang, Xiaoqian Jiang, Pratip K. Bhattacharya, and Shyan Shams. 2024. "Hyperpolarized Magnetic Resonance Imaging, Nuclear Magnetic Resonance Metabolomics, and Artificial Intelligence to Interrogate the Metabolic Evolution of Glioblastoma" Metabolites 14, no. 8: 448. https://doi.org/10.3390/metabo14080448
APA StyleHsieh, K. L., Chen, Q., Salzillo, T. C., Zhang, J., Jiang, X., Bhattacharya, P. K., & Shams, S. (2024). Hyperpolarized Magnetic Resonance Imaging, Nuclear Magnetic Resonance Metabolomics, and Artificial Intelligence to Interrogate the Metabolic Evolution of Glioblastoma. Metabolites, 14(8), 448. https://doi.org/10.3390/metabo14080448






