Bile Acid Metabolism Analysis Provides Insights into Vascular Endothelial Injury in Salt-Sensitive Hypertensive Rats
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals and Diet
2.2. Sample Collection
2.3. Vascular and Renal Histopathological Examination
2.4. Bile Acid Metabolism Analysis
2.4.1. Metabolite Extraction
2.4.2. Standard Solution Preparation
2.4.3. UHPLC-PRM-MS Analysis
2.4.4. Calibration Curves
2.4.5. Limit of Detection and Limit of Quantitation
2.4.6. Precision and Accuracy
2.4.7. The Detection Result of Bile Acids
2.5. Correlation Analysis
2.6. Statistical Analysis
2.7. Ethical Approval
3. Results
3.1. Blood Pressure Measurement
3.2. Vascular Endothelial Injury Factor Determination
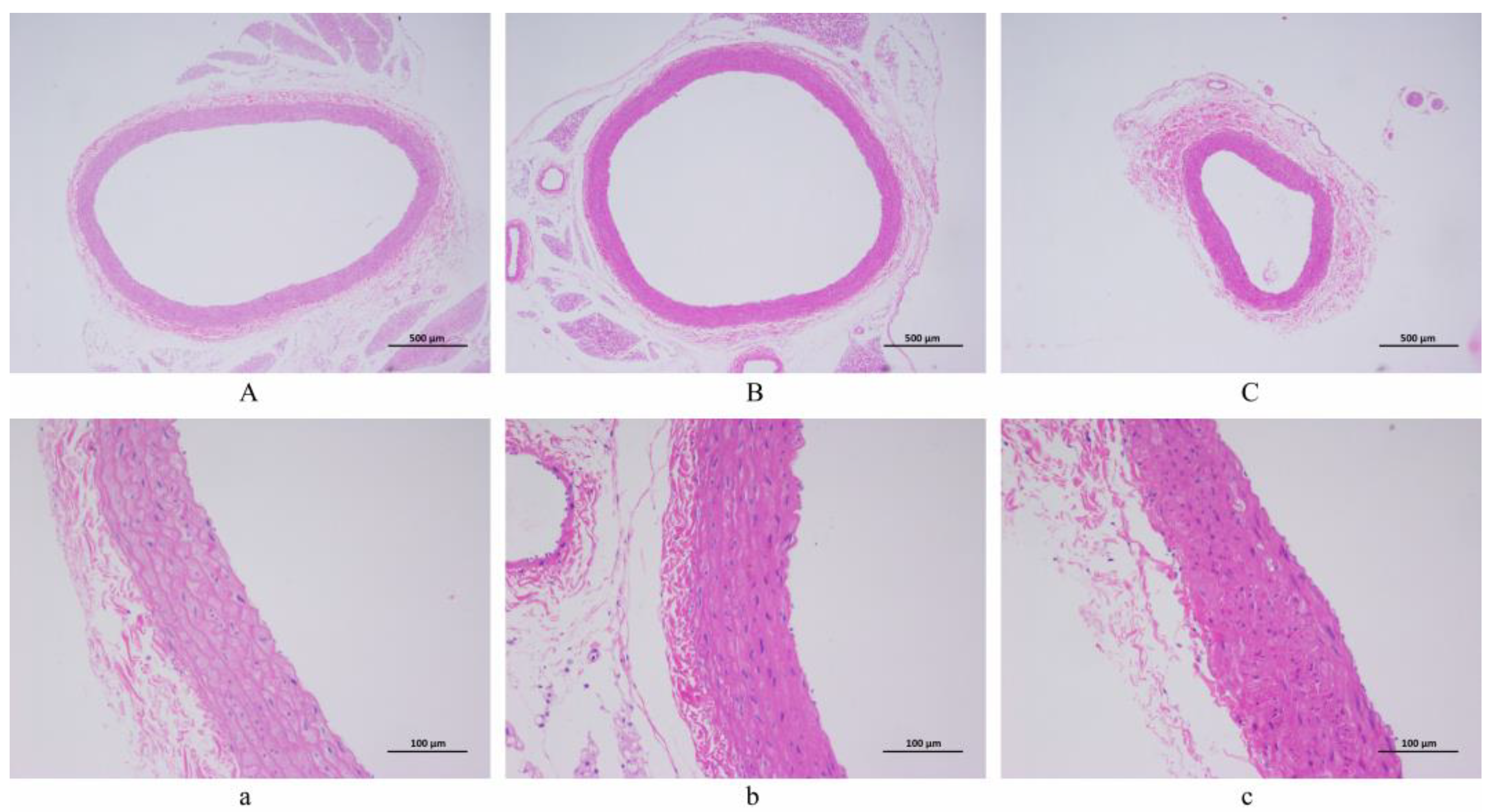
3.3. Artery Histopathological Observation
3.4. Renal Histopathological Observation
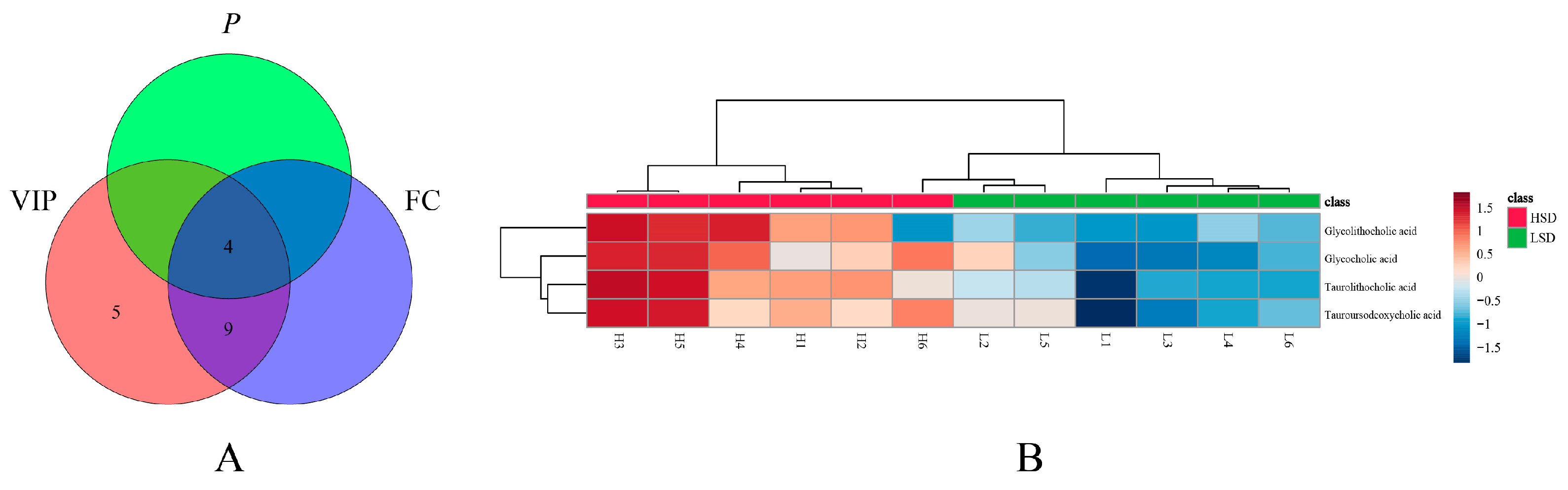
3.5. Bile Acid Metabolomics Analysis
3.6. Correlation Analysis
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Quader, Z.S.; Zhao, L.; Gillespie, C.; Cogswell, M.E.; Terry, A.L.; Moshfegh, A.; Rhodes, D. Sodium Intake among Persons Aged ≥2 Years—United States, 2013–2014. MMWR Morb. Mortal. Wkly. Rep. 2017, 66, 324–328. [Google Scholar] [CrossRef]
- Bigiani, A. Salt Taste, Nutrition, and Health. Nutrients 2020, 12, 1537. [Google Scholar] [CrossRef] [PubMed]
- Borrelli, S.; Provenzano, M.; Gagliardi, I.; Michael, A.; Liberti, M.E.; De Nicola, L.; Conte, G.; Garofalo, C.; Andreucci, M. Sodium Intake and Chronic Kidney Disease. Int. J. Mol. Sci. 2020, 21, 4744. [Google Scholar] [CrossRef]
- Wu, X.; Chen, L.; Cheng, J.; Qian, J.; Fang, Z.; Wu, J. Effect of Dietary Salt Intake on Risk of Gastric Cancer: A Systematic Review and Meta-Analysis of Case-Control Studies. Nutrients 2022, 14, 4260. [Google Scholar] [CrossRef] [PubMed]
- Jiang, K.; He, T.; Ji, Y.; Zhu, T.; Jiang, E. The perspective of hypertension and salt intake in Chinese population. Front. Public Health 2023, 11, 1125608. [Google Scholar] [CrossRef] [PubMed]
- Han, B.; Li, C.; Zhou, Y.; Zhang, M.; Zhao, Y.; Zhao, T.; Hu, D.; Sun, L. Association of Salt-Reduction Knowledge and Behaviors and Salt Intake in Chinese Population. Front. Public Health 2022, 10, 872299. [Google Scholar] [CrossRef] [PubMed]
- Viggiano, J.; Coutinho, D.; Clark-Cutaia, M.N.; Martinez, D. Effects of a high salt diet on blood pressure dipping and the implications on hypertension. Front. Neurosci. 2023, 17, 1212208. [Google Scholar] [CrossRef] [PubMed]
- Vinaiphat, A.; Pazhanchamy, K.; JebaMercy, G.; Ngan, S.C.; Leow, M.K.; Ho, H.H.; Gao, Y.G.; Lim, K.L.; Richards, A.M.; de Kleijn, D.P.V.; et al. Endothelial Damage Arising from High Salt Hypertension Is Elucidated by Vascular Bed Systematic Profiling. Arter. Thromb. Vasc. Biol. 2023, 43, 427–442. [Google Scholar] [CrossRef]
- Li, Y.; Deng, X.; Zhou, H.; Zheng, X.; Zhang, G.; Xiong, Q. Bile acid predicts congenital portosystemic venous shunt in patients with pulmonary arterial hypertension. Eur. J. Med. Res. 2023, 28, 74. [Google Scholar] [CrossRef]
- Fiorillo, B.; Marchiano, S.; Moraca, F.; Sepe, V.; Carino, A.; Rapacciuolo, P.; Biagioli, M.; Limongelli, V.; Zampella, A.; Catalanotti, B.; et al. Discovery of Bile Acid Derivatives as Potent ACE2 Activators by Virtual Screening and Essential Dynamics. J. Chem. Inf. Model. 2022, 62, 196–209. [Google Scholar] [CrossRef]
- Wang, C.; Ma, Q.; Yu, X. Bile Acid Network and Vascular Calcification-Associated Diseases: Unraveling the Intricate Connections and Therapeutic Potential. Clin. Interv. Aging 2023, 18, 1749–1767. [Google Scholar] [CrossRef] [PubMed]
- Geiger, M.; Oppi, S.; Nusser-Stein, S.; Costantino, S.; Mohammed, S.A.; Gorica, E.; Hoogerland, J.A.; Matter, C.M.; Guillaumon, A.T.; Ruschitzka, F.; et al. Genetic deletion of hepatic NCOR1 protects from atherosclerosis by promoting alternative bile acid-metabolism and sterol excretion. Cardiovasc. Diabetol. 2023, 22, 144. [Google Scholar] [CrossRef] [PubMed]
- Ishimwe, J.A.; Dola, T.; Ertuglu, L.A.; Kirabo, A. Bile acids and salt-sensitive hypertension: A role of the gut-liver axis. Am. J. Physiol. Heart Circ. Physiol. 2022, 322, H636–H646. [Google Scholar] [CrossRef] [PubMed]
- Vogt, L.; Marques, F.Z.; Fujita, T.; Hoorn, E.J.; Danser, A.H.J. Novel mechanisms of salt-sensitive hypertension. Kidney Int. 2023, 104, 690–697. [Google Scholar] [CrossRef] [PubMed]
- Ertuglu, L.A.; Mutchler, A.P.; Yu, J.; Kirabo, A. Inflammation and oxidative stress in salt sensitive hypertension; The role of the NLRP3 inflammasome. Front. Physiol. 2022, 13, 1096296. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Qing, T.; Shen, Y.; Huang, J.; Liu, Y.; Li, J.; Zhen, T.; Xing, K.; Zhu, S.; Luo, M. RNA-seq analyses the effect of high-salt diet in hypertension. Gene 2018, 677, 245–250. [Google Scholar] [CrossRef] [PubMed]
- He, F.J.; Tan, M.; Ma, Y.; MacGregor, G.A. Salt Reduction to Prevent Hypertension and Cardiovascular Disease: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2020, 75, 632–647. [Google Scholar] [CrossRef]
- Jankowich, M.; Choudhary, G. Endothelin-1 levels and cardiovascular events. Trends Cardiovasc. Med. 2020, 30, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Infante, T.; Costa, D.; Napoli, C. Novel Insights Regarding Nitric Oxide and Cardiovascular Diseases. Angiology 2021, 72, 411–425. [Google Scholar] [CrossRef]
- Haffke, M.; Freitag, H.; Rudolf, G.; Seifert, M.; Doehner, W.; Scherbakov, N.; Hanitsch, L.; Wittke, K.; Bauer, S.; Konietschke, F.; et al. Endothelial dysfunction and altered endothelial biomarkers in patients with post-COVID-19 syndrome and chronic fatigue syndrome (ME/CFS). J. Transl. Med. 2022, 20, 138. [Google Scholar] [CrossRef]
- Moriya, J. Critical roles of inflammation in atherosclerosis. J. Cardiol. 2019, 73, 22–27. [Google Scholar] [CrossRef]
- Xu, S.; Zhang, J.; Liu, J.; Ye, J.; Xu, Y.; Wang, Z.; Yu, J.; Ye, D.; Zhao, M.; Feng, Y.; et al. The role of interleukin-10 family members in cardiovascular diseases. Int. Immunopharmacol. 2021, 94, 107475. [Google Scholar] [CrossRef] [PubMed]
- Yntema, T.; Koonen, D.; Kuipers, F. Emerging Roles of Gut Microbial Modulation of Bile Acid Composition in the Etiology of Cardiovascular Diseases. Nutrients 2023, 15, 1850. [Google Scholar] [CrossRef]
- Chakraborty, S.; Lulla, A.; Cheng, X.; Yeo, J.Y.; Mandal, J.; Yang, T.; Mei, X.; Saha, P.; Golonka, R.M.; Yeoh, B.S.; et al. Conjugated bile acids are nutritionally re-programmable antihypertensive metabolites. J. Hypertens. 2023, 41, 979–994. [Google Scholar] [CrossRef] [PubMed]
- Cai, J.; Sun, L.; Gonzalez, F.J. Gut microbiota-derived bile acids in intestinal immunity, inflammation, and tumorigenesis. Cell Host Microbe 2022, 30, 289–300. [Google Scholar] [CrossRef]
- Xu, R.H.; Shen, J.N.; Lu, J.B.; Liu, Y.J.; Song, Y.; Cao, Y.; Wang, Z.H.; Zhang, J. Bile acid profiles and classification model accuracy for inflammatory bowel disease diagnosis. Medicine 2024, 103, e38457. [Google Scholar] [CrossRef]
- Ge, X.; Huang, S.; Ren, C.; Zhao, L. Taurocholic Acid and Glycocholic Acid Inhibit Inflammation and Activate Farnesoid X Receptor Expression in LPS-Stimulated Zebrafish and Macrophages. Molecules 2023, 28, 2005. [Google Scholar] [CrossRef]
- Yao, S.; Ren, S.; Cai, C.; Cao, X.; Shi, Y.; Wu, P.; Ye, Y. Glycocholic acid supplementation improved growth performance and alleviated tissue damage in the liver and intestine in Pelteobagrus fulvidraco fed a high-pectin diet. Fish Physiol. Biochem. 2022, 50, 41–57. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Romero-Ramírez, L.; Mey, J. Taurolithocholic acid but not tauroursodeoxycholic acid rescues phagocytosis activity of bone marrow-derived macrophages under inflammatory stress. J. Cell. Physiol. 2022, 237, 1455–1470. [Google Scholar] [CrossRef]
- Lee, P.C.; Wu, C.J.; Hung, Y.W.; Lee, C.J.; Chi, C.T.; Lee, I.C.; Yu-Lun, K.; Chou, S.H.; Luo, J.C.; Hou, M.C.; et al. Gut microbiota and metabolites associate with outcomes of immune checkpoint inhibitor-treated unresectable hepatocellular carcinoma. J. Immunother. Cancer 2022, 10, e004779. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, M.; Cheng, S. The application of experimental animal models in the study of acupuncture and moxibustion treatment of cancer. J. Shanghai Univ. Tradit. Chin. Med. 2024, 38, 82–88. [Google Scholar]
- Zhang, L.; Xu, W.; Zuo, Q. A Brief Discussion on the Development and Utilization of Experimental Rat Resources Abroad. Chin. J. Exp. Anim. 2023, 31, 1512–1518. [Google Scholar]
- Qu, Y.; Liu, L.; Yong, R.; Xue, Y.; Deng, T.; Wang, J.; Zhang, L. Research on the Mechanism of Buyang Huanwu Tang on the Balance of RAS System in Renal Tissue of Hypertensive Model Rats. Chin. J. Tradit. Chin. Med. 2019, 37, 1729–1733. [Google Scholar]
- Li, M.; Liu, X. Pitavastatin maintains MAPK7 expression and alleviates angiotensin II-induced vascular endothelial cell inflammation and injury. Exp. Ther. Med. 2022, 23, 132. [Google Scholar] [CrossRef] [PubMed]
- Nishimoto, M.; Griffin, K.A.; Wynne, B.M.; Fujita, T. Salt-Sensitive Hypertension and the Kidney. Hypertension 2024, 81, 1206–1217. [Google Scholar] [CrossRef] [PubMed]
- Cahill, P.A.; Redmond, E.M. Vascular endothelium—Gatekeeper of vessel health. Atherosclerosis 2016, 248, 97–109. [Google Scholar] [CrossRef] [PubMed]
- Félétou, M.; Köhler, R.; Vanhoutte, P.M. Endothelium-derived vasoactive factors and hypertension: Possible roles in pathogenesis and as treatment targets. Curr. Hypertens. Rep. 2010, 12, 267–275. [Google Scholar] [CrossRef]
- Sandoo, A.; van Zanten, J.J.C.S.V.; Metsios, G.S.; Carroll, D.; Kitas, G.D. The endothelium and its role in regulating vascular tone. Open Cardiovasc. Med. J. 2010, 4, 302–312. [Google Scholar] [CrossRef] [PubMed]
- Jukema, R.A.; de Winter, R.W.; van Diemen, P.A.; Driessen, R.S.; Danser, A.H.J.; Garrelds, I.M.; Raijmakers, P.G.; van de Ven, P.M.; Knaapen, P.; Danad, I.; et al. The relation of RAAS activity and endothelin-1 levels to coronary atherosclerotic burden and microvascular dysfunction in chest pain patients. Atherosclerosis 2022, 347, 47–54. [Google Scholar] [CrossRef]
- Li, W.; Liu, Q.; Shi, J.; Xu, X.; Xu, J. The role of TNF-alpha in the fate regulation and functional reprogramming of mesenchymal stem cells in an inflammatory microenvironment. Front. Immunol. 2023, 14, 1074863. [Google Scholar]
- Zhang, Z.; Zhao, L.; Zhou, X.; Meng, X.; Zhou, X. Role of inflammation, immunity, and oxidative stress in hypertension: New insights and potential therapeutic targets. Front. Immunol. 2022, 13, 1098725. [Google Scholar] [CrossRef] [PubMed]
- Guo, Q.; Tang, Y.; Li, Y.; Xu, Z.; Zhang, D.; Liu, J.; Wang, X.; Xia, W.; Xu, S. Perinatal High-Salt Diet Induces intestinal flora Dysbiosis, Bile Acid Homeostasis Disbalance, and NAFLD in Weanling Mice Offspring. Nutrients 2021, 13, 2135. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Li, M.; Cui, B.; Chen, X. Antibiotic Disruption of the intestinal flora Enhances the Murine Hepatic Dysfunction Associated with a High-Salt Diet. Front. Pharmacol. 2022, 13, 829686. [Google Scholar]
- Higarza, S.G.; Arboleya, S.; Arias, J.L.; Gueimonde, M.; Arias, N. Akkermansia muciniphila and environmental enrichment reverse cognitive impairment associated with high-fat high-cholesterol consumption in rats. Gut Microbes 2021, 13, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Tsai, C.H.; Huang, P.J.; Lee, I.T.; Chen, C.M.; Wu, M.H. Correction for: Endothelin-1-mediated miR-let-7g-5p triggers interlukin-6 and TNF-alpha to cause myopathy and chronic adipose inflammation in elderly patients with diabetes mellitus. Aging 2023, 15, 287. [Google Scholar] [CrossRef]
- Tang, J.; Xie, Q.; Ma, D.; Wang, W. Effects of ET-1 and TNF-alpha levels on the cardiac function and prognosis in rats with chronic heart failure. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 11004–11010. [Google Scholar]






Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zeng, B.; Peng, X.; Chen, L.; Liu, J.; Xia, L. Bile Acid Metabolism Analysis Provides Insights into Vascular Endothelial Injury in Salt-Sensitive Hypertensive Rats. Metabolites 2024, 14, 452. https://doi.org/10.3390/metabo14080452
Zeng B, Peng X, Chen L, Liu J, Xia L. Bile Acid Metabolism Analysis Provides Insights into Vascular Endothelial Injury in Salt-Sensitive Hypertensive Rats. Metabolites. 2024; 14(8):452. https://doi.org/10.3390/metabo14080452
Chicago/Turabian StyleZeng, Baihan, Xile Peng, Li Chen, Jiao Liu, and Lina Xia. 2024. "Bile Acid Metabolism Analysis Provides Insights into Vascular Endothelial Injury in Salt-Sensitive Hypertensive Rats" Metabolites 14, no. 8: 452. https://doi.org/10.3390/metabo14080452



