Local and Systemic Micro-Rheological Changes during Intestinal Anastomosis Operation: A Metabolic Dependence in an Experimental Model
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Animals
2.2. Surgical Protocol
2.3. Blood Sampling
2.4. Hematological Measurements
2.5. Hemorheological Measurements
2.6. Blood Gas, Acid-Base Parameters, Metabolites and Electrolytes
2.7. Statistical Analysis
3. Results
3.1. Hematological Parameters
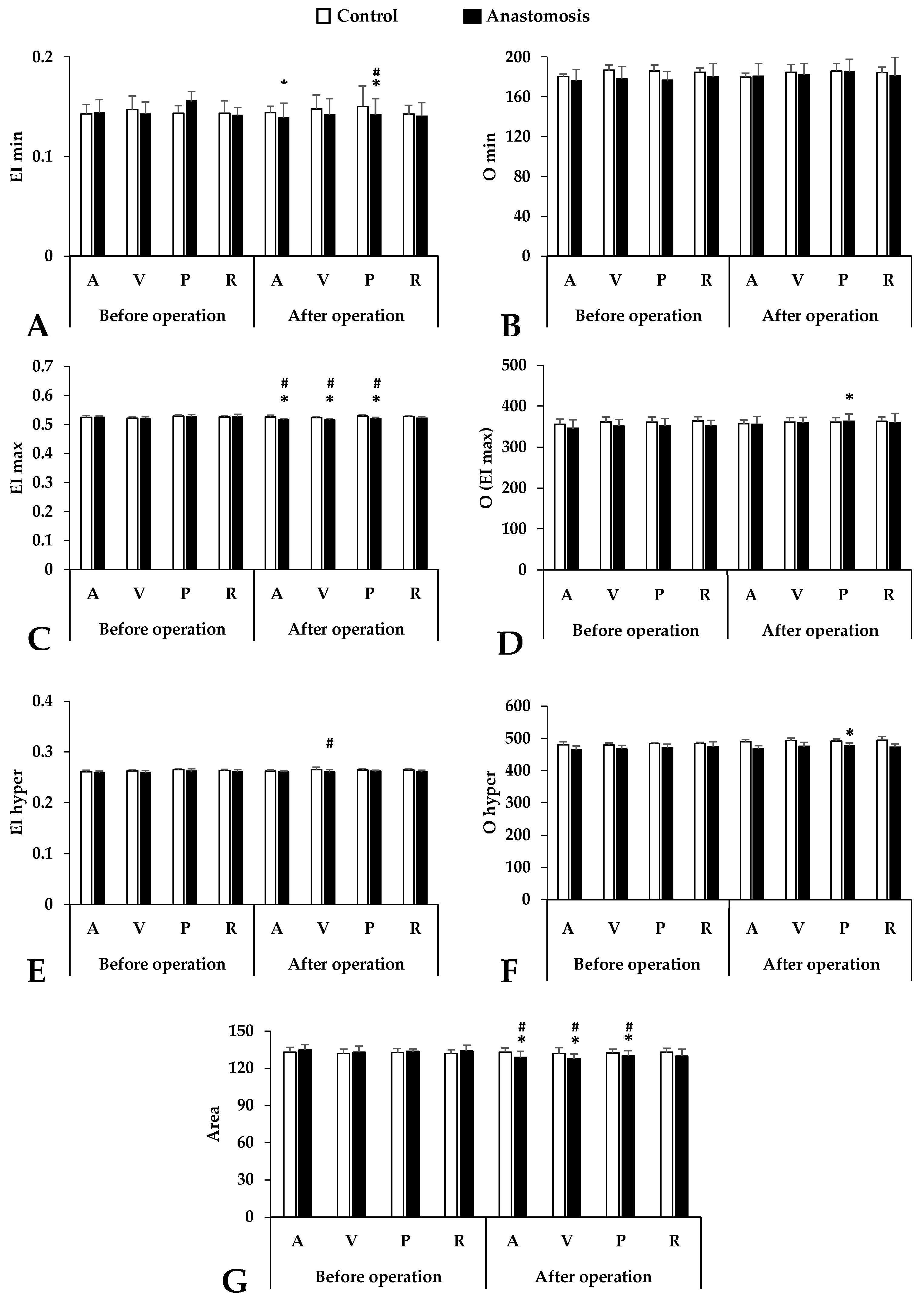
3.2. Red Blood Cell Deformability
3.3. Osmotic Gradient Ektacytometry (Osmoscan)
3.4. Red Blood Cell Aggregation
3.5. Blood Gas, Acid-Base and Metabolites
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Copley, A.L. The rheology of blood. A survey. J. Colloid. Sci. 1952, 7, 323–333. [Google Scholar] [CrossRef]
- Baskurt, O.K.; Meiselman, H.J. Blood rheology and hemodynamics. Semin. Thromb. Hemost. 2003, 29, 435–450. [Google Scholar] [CrossRef] [PubMed]
- Windberger, U.; Baskurt, O.K. Comparative Hemorheology. In Handbook of Hemorheology and Hemodynamics; Baskurt, O.K., Hardeman, M.R., Rampling, M.W., Meiselman, H.J., Eds.; IOS Press: Amsterdam, The Netherlands, 2007; pp. 267–285. [Google Scholar]
- Jung, F.; Mrowietz, C.; Hiebl, B.; Franke, R.P.; Pindur, G.; Sternitzky, R. Influence of rheological parameters on the velocity of erythrocytes passing nailfold capillaries in humans. Clin. Hemorheol. Microcirc. 2011, 48, 129–139. [Google Scholar] [CrossRef]
- Sosa, J.M.; Nielsen, N.D.; Vignes, S.M.; Chen, T.G.; Shevkoplyas, S.S. The relationship between red blood cell deformability metrics and perfusion of an artificial microvascular network. Clin. Hemorheol. Microcirc. 2014, 57, 275–289. [Google Scholar] [CrossRef]
- Silva, J.M.; Oliveira, A.M.; Marti, Y.N.; Gonzaga, T.B.; Ferreira, A.M.; Maia, V.P.; Rezend, E. Outcome of surgical patients who present acidosis postoperatively. Crit. Care 2011, 15, 64. [Google Scholar] [CrossRef][Green Version]
- Arzanipour, G.; Heitz, J.W. Hypoxia. In Post-Anesthesia Care: Symptoms, Diagnosis and Management; Heitz, J.W., Ed.; Cambridge University Press: Cambridge, UK, 2016; pp. 66–73. ISBN 978-1-139-51955-7. [Google Scholar]
- Yagi, K.; Fujii, T. Management of acute metabolic acidosis in the ICU: Sodium bicarbonate and renal replacement therapy. Crit. Care 2021, 25, 314. [Google Scholar] [CrossRef] [PubMed]
- Bölke, E.; Jehle, P.M.; Graf, M.; Baier, A.; Wiedeck, H.; Steinbach, G.; Storck, M.; Orth, K. Inflammatory response during abdominal and thyroid surgery: A prospective clinical trial on mediator release. Shock 2001, 16, 334–339. [Google Scholar] [CrossRef] [PubMed]
- Sido, B.; Teklote, J.-R.; Hartel, M.; Friess, H.; Büchler, M.W. Inflammatory response after abdominal surgery. Best Pract. Res. Clin. Anaesthesiol. 2004, 18, 439–454. [Google Scholar] [CrossRef]
- Buttenschoen, K.; Fathimani, K.; Buttenschoen, D.C. Effect of major abdominal surgery on the host immune response to infection. Curr. Opin. Infect. Dis. 2010, 23, 259–267. [Google Scholar] [CrossRef]
- Badani, S.; Na, S. Chronic concern over an acute-phase reactant: A teachable moment. JAMA Internal. Med. 2014, 174, 319. [Google Scholar] [CrossRef][Green Version]
- Oelsner, W.K.; Engstrom, S.M.; Benvenuti, M.A.; An, T.J.; Jacobson, R.A.; Polkowski, G.G.; Schoenecker, J.G. Characterizing the acute phase response in healthy patients following total joint arthroplasty: Predictable and consistent. J. Arthroplast. 2017, 32, 309–314. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.-C.; Ahearn, J.M. Chapter 10—Acute-Phase Proteins and Inflammation: Immunological and Clinical Implications. In Measuring Immunity; Lotze, M.T., Thomson, A.W., Eds.; Academic Press: London, UK, 2005; pp. 131–143. ISBN 978-0-12-455900-4. [Google Scholar]
- Holzer-Geissler, J.C.J.; Schwingenschuh, S.; Zacharias, M.; Einsiedler, J.; Kainz, S.; Reisenegger, P.; Holecek, C.; Hofmann, E.; Wolff-Winiski, B.; Fahrngruber, H.; et al. The impact of prolonged inflammation on wound healing. Biomedicines 2022, 10, 856. [Google Scholar] [CrossRef]
- Mantovani, A.; Garlanda, C. Humoral innate immunity and acute-phase proteins. N. Eng. J. Med. 2023, 388, 439–452. [Google Scholar] [CrossRef]
- Hedberg, S.; Xiao, Y.; Klasson, A.; Maleckas, A.; Wirén, M.; Thorell, A.; Laurenius, A.; Engström, M.; Olbers, T. The jejunojejunostomy: An achilles heel of the Roux-en-Y gastric bypass construction. Obes. Surg. 2021, 31, 5141–5147. [Google Scholar] [CrossRef] [PubMed]
- Noun, R.; Zeidan, S.; Safa, N. Laparoscopic latero-lateral jejuno-jejunostomy as a rescue procedure after complicated mini-gastric bypass. Obes. Surg. 2006, 16, 1539–1541. [Google Scholar] [CrossRef]
- Sacks, B.C.; Mattar, S.G.; Qureshi, F.G.; Eid, G.M.; Collins, J.L.; Barinas-Mitchell, E.J.; Schauer, P.R.; Ramanathan, R.C. Incidence of marginal ulcers and the use of absorbable anastomotic sutures in laparoscopic Roux-en-Y gastric bypass. Surg. Obes. Relat. Dis. 2006, 2, 11–16. [Google Scholar] [CrossRef]
- Clatterbuck, B.; Moore, L. Small Bowel Resection. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2024. [Google Scholar]
- Nemeth, N.; Kiss, F.; Klarik, Z.; Toth, E.; Mester, A.; Furka, I.; Miko, I. Simultaneous investigation of hemodynamic, microcirculatory and arterio-venous micro-rheological parameters in infrarenal or suprarenal aortic cross-clamping model in the rat. Clin. Hemorheol. Microcirc. 2014, 57, 339–353. [Google Scholar] [CrossRef]
- Klarik, Z.; Kiss, F.; Miko, I.; Nemeth, N. Aorto-porto-caval micro-rheological differences of red blood cells in laboratory rats: Further deformability and ektacytometrial osmoscan data. Clin. Hemorheol. Microcirc. 2013, 53, 217–229. [Google Scholar] [CrossRef]
- Son, K.H.; Lim, C.H.; Song, E.J.; Sun, K.; Son, H.S.; Lee, S.H. Inter-species hemorheologic differences in arterial and venous blood. Clin Hemorheol Microcirc 2010, 44, 27–33. [Google Scholar] [CrossRef] [PubMed]
- Kayar, E.; Mat, F.; Meiselman, H.J.; Baskurt, O.K. Red Blood Cell Rheological Alterations in a Rat Model of Ischemia-Reperfusion Injury. Biorheology 2001, 38, 405–414. [Google Scholar]
- Baskurt, O.K.; Boynard, M.; Cokelet, G.C.; Connes, P.; Cooke, B.M.; Forconi, S.; Liao, F.; Hardeman, M.R.; Jung, F.; Meiselman, H.J.; et al. International Expert Panel for Standardization of Hemorheological Methods. New Guidelines for hemorheological laboratory techniques. Clin. Hemorheol. Microcirc. 2009, 42, 75–97. [Google Scholar] [CrossRef] [PubMed]
- Baskurt, O.K.; Hardeman, M.R.; Uyuklu, M.; Ulker, P.; Cengiz, M.; Nemeth, N.; Shin, S.; Alexy, T.; Meiselman, H.J. Parameterization of red blood cell elongation index--shear stress curves obtained by ektacytometry. Scand. J. Clin. Lab. Invest. 2009, 69, 777–788. [Google Scholar] [CrossRef]
- Nemeth, N.; Kiss, F.; Miszti-Blasius, K. Interpretation of osmotic gradient ektacytometry (osmoscan) data: A comparative study for methodological standards. Scand. J. Clin. Lab. Invest. 2015, 75, 213–222. [Google Scholar] [CrossRef] [PubMed]
- Vayá, A.; Falcó, C.; Fernández, P.; Contreras, T.; Valls, M.; Aznar, J. Erythrocyte aggregation determined with the myrenne aggregometer at two modes (M0, M1) and at two times (5 and 10 sec). Clin. Hemorheol. Microcirc. 2003, 29, 119–127. [Google Scholar] [PubMed]
- Nemeth, N.; Soukup, J.; Menzel, M.; Henze, D.; Clausen, T.; Rieger, A.; Holz, C.; Scharf, A.; Hanisch, F.; Furka, I.; et al. Local and systemic hemorheological effects of cerebral hyper- and hypoperfusion in a porcine model. Clin. Hemorheol. Microcirc. 2006, 35, 59–65. [Google Scholar] [PubMed]
- Klarik, Z.; Toth, E.; Kiss, F.; Miko, I.; Furka, I.; Nemeth, N. A modified microsurgical model for end-to-side selective portacaval shunt in the rat: Intraoperative microcirculatory investigations. Acta Cirúrgica Bras. 2013, 28, 625–631. [Google Scholar] [CrossRef]
- Mester, A.; Magyar, Z.; Molnar, A.; Somogyi, V.; Tanczos, B.; Peto, K.; Nemeth, N. Age- and gender-related hemorheological alterations in intestinal ischemia-reperfusion in the rat. J. Surg. Res. 2018, 225, 68–75. [Google Scholar] [CrossRef] [PubMed]
- Simmonds, M.J.; Baskurt, O.K.; Meiselman, H.J.; Marshall-Gradisnik, S.M. A comparison of capillary and venous blood sampling methods for the use in haemorheology studies. Clin. Hemorheol. Microcirc. 2011, 47, 111–119. [Google Scholar] [CrossRef]
- Joint Working Group on Refinement. First Report of the BVA/FRAME/RSPCA/UFAW Joint Working Group on Refinement. Lab. Anim. 1993, 27, 1–22. [Google Scholar] [CrossRef]
- Waters, J.H.; Miller, L.R.; Clack, S.; Kim, J.V. Cause of metabolic acidosis in prolonged surgery. Crit. Care Med. 1999, 27, 2142–2146. [Google Scholar] [CrossRef]
- Skeith, L.; Baumann Kreuziger, L.; Crowther, M.A.; Warkentin, T.E. A practical approach to evaluating postoperative thrombocytopenia. Blood Adv. 2020, 4, 776–783. [Google Scholar] [CrossRef]
- Cicco, G.; Pirrelli, A. Red blood cell (RBC) deformability, RBC aggregability and tissue oxygenation in hypertension. Clin. Hemorheol. Microcirc. 1999, 21, 169–177. [Google Scholar]
- Straat, M.; van Bruggen, R.; de Korte, D.; Juffermans, N.P. Red blood cell clearance in inflammation. Transfus. Med. Hemother. 2012, 39, 353–361. [Google Scholar] [CrossRef]
- Pretorius, E. Erythrocyte deformability and eryptosis during inflammation, and impaired blood rheology. Clin. Hemorheol. Microcirc. 2018, 69, 545–550. [Google Scholar] [CrossRef]
- Czaja, B.; Gutierrez, M.; Závodszky, G.; de Kanter, D.; Hoekstra, A.; Eniola-Adefeso, O. The influence of red blood cell deformability on hematocrit profiles and platelet margination. PLoS Comput. Biol. 2020, 16, e1007716. [Google Scholar] [CrossRef]
- Taraconat, P.; Gineys, J.-P.; Isebe, D.; Nicoud, F.; Mendez, S. Red blood cell rheology during a complete blood count: A proof of concept. PLoS ONE 2023, 18, e0280952. [Google Scholar] [CrossRef]
- Nader, E.; Skinner, S.; Romana, M.; Fort, R.; Lemonne, N.; Guillot, N.; Gauthier, A.; Antoine-Jonville, S.; Renoux, C.; Hardy-Dessources, M.D.; et al. Blood rheology: Key parameters, impact on blood flow, role in sickle cell disease and effects of exercise. Front. Physiol. 2019, 10, 1329. [Google Scholar] [CrossRef] [PubMed]
- Hakim, T.S.; Macek, A.S. Effect of hypoxia on erythrocyte deformability in different species. Biorheology 1988, 25, 857–868. [Google Scholar] [CrossRef] [PubMed]
- Reinhart, W.H.; Piety, N.Z.; Shevkoplyas, S.S. Influence of red blood cell aggregation on perfusion of an artificial microvascular network. Microcirculation 2017, 24, e12317. [Google Scholar] [CrossRef] [PubMed]
- Lazari, D.; Freitas Leal, J.K.; Brock, R.; Bosman, G. The relationship between aggregation and deformability of red blood cells in health and disease. Front. Physiol. 2020, 11, 288. [Google Scholar] [CrossRef] [PubMed]
- Nemeth, N.; Miko, I.; Furka, A.; Kiss, F.; Furka, I.; Koller, A.; Szilasi, M. Concerning the importance of changes in hemorheological parameters caused by acid-base and blood gas alterations in experimental surgical models. Clin. Hemorheol. Microcirc. 2012, 51, 43–50. [Google Scholar] [CrossRef]
- Nemeth, N.; Deak, A.; Szentkereszty, Z.; Peto, K. Effects and influencing factors on hemorheological variables taken into consideration in surgical pathophysiology research. Clin. Hemorheol. Microcirc. 2018, 69, 133–140. [Google Scholar] [CrossRef] [PubMed]
- Cicha, I.; Suzuki, Y.; Tateishi, N.; Maeda, N. Changes of RBC aggregation in oxygenation-deoxygenation: pH dependency and cell morphology. Am. J. Physiol. Heart. Circ. 2003, 284, H2335–H2342. [Google Scholar] [CrossRef] [PubMed]
- Uyuklu, M.; Meiselman, H.J.; Baskurt, O.K. Effect of hemoglobin oxygenation level on red blood cell deformability and aggregation parameters. Clin. Hemorheol. Microcirc. 2009, 41, 179–188. [Google Scholar] [CrossRef] [PubMed]
- Brun, J.-F.; Varlet-Marie, E.; Myzia, J.; Raynaud de Mauverger, E.; Pretorius, E. Metabolic influences modulating erythrocyte deformability and eryptosis. Metabolites 2021, 12, 4. [Google Scholar] [CrossRef]
- Toth, K.; Kesmarky, G.; Alexy, T. Clinical Significance of Hemorheological Alterations. In Handbook of Hemorheology and Hemodynamics; Baskurt, O.K., Hardeman, M.R., Rampling, M.W., Meiselman, H.J., Eds.; IOS Press: Amsterdam, The Netherlands, 2007; pp. 392–432. [Google Scholar]
- Alexy, T.; Detterich, J.; Connes, P.; Toth, K.; Nader, E.; Kenyeres, P.; Arriola-Montenegro, J.; Ulker, P.; Simmonds, M.J. Physical properties of blood and their relationship to clinical conditions. Front Physiol. 2022, 13, 906768. [Google Scholar] [CrossRef]



| Variable | Group | Before Operation | After Operation | ||||||
|---|---|---|---|---|---|---|---|---|---|
| A | V | P | R | A | V | P | R | ||
| WBC [109/L] | Control | 15.86 ± 4.06 | 15.52 ± 2.89 | 14.78 ± 6.18 | 12.51 ± 4.52 | 15.89 ± 3.12 | 15.33 ± 3.02 | 15.19 ± 4.28 | 13.5 ± 4.5 |
| Anastomosis | 19.19 ± 7.25 | 18.6 ± 7.6 | 19.11 ± 8.08 | 19.22 ± 6.65 | 13.24 ± 2.47 *# | 12.74 ± 2.49 *# | 14.12 ± 3.85 * | 14.28 ± 2.96 * | |
| RBC [1012/L] | Control | 5.79 ± 0.44 | 5.81 ± 0.7 | 6.55 ± 1.1 | 6.37 ± 0.83 | 6.17 ± 0.71 | 6.32 ± 0.86 * | 6.63 ± 0.48 | 6.52 ± 0.74 |
| Anastomosis | 6.7 ± 0.55 | 6.93 ± 0.62 | 7.23 ± 0.55 | 6.85 ± 0.43 | 6.89 ± 0.34 # | 7.37 ± 1.27 *# | 8.05 ± 1.32 *# | 7.37 ± 1.52 *# | |
| Hgb [g/L] | Control | 108.2 ± 11.3 | 111.8 ± 10.9 | 116.2 ± 10.2 | 111.1 ± 8.1 | 111.6 ± 7.2 | 112.8 ± 7.7 | 129.8 ± 20.1 * | 117.9 ± 23.9 |
| Anastomosis | 103.9 ± 9.2 | 103.9 ± 8.5 | 107.8 ± 33.9 | 113.8 ± 13.5 | 120.4 ± 26.5 *# | 125.6 ± 24.8 *# | 118.3 ± 16.6 * | 116.6 ± 16.7 | |
| Hct [%] | Control | 33.28 ± 2.78 | 33.48 ± 2.5 | 39.59 ± 6.27 | 36.98 ± 4.55 | 34.39 ± 3.87 | 34.02 ± 3.32 | 39.04 ± 3.37 | 36.89 ± 3.4 |
| Anastomosis | 36.08 ± 3.77 | 37.56 ± 3.93 | 39.34 ± 3.5 | 37.17 ± 2.54 | 37.65 ± 2.34 | 38.29 ± 2.52 | 46.93 ± 6.63 *# | 45.37 ± 7.8 *# | |
| MCV [fL] | Control | 57.47 ± 2.35 | 57.61 ± 2.35 | 58.52 ± 2.41 | 57.66 ± 2.07 | 57.8 ± 2.81 | 57.85 ± 2.75 | 58.04 ± 2.77 | 57.72 ± 2.7 |
| Anastomosis | 53.75 ± 1.78 | 54.14 ± 1.67 | 54.37 ± 1.47 | 54.26 ± 1.39 | 55.53 ± 1.48 *# | 55.88 ± 1.63 *# | 56.02 ± 1.59 *# | 56.39 ± 1.34 *# | |
| MCH [pg] | Control | 17.94 ± 0.88 | 17.91 ± 0.98 | 17.89 ± 0.97 | 17.94 ± 1.09 | 17.99 ± 0.83 | 18.08 ± 0.9 | 17.85 ± 0.83 | 17.84 ± 0.97 |
| Anastomosis | 16.11 ± 0.6 | 16.13 ± 0.59 | 16.06 ± 0.6 | 16.21 ± 0.57 | 16.14 ± 0.63 | 16.14 ± 0.54 | 16.15 ± 0.6 | 16.01 ± 0.37 | |
| MCHC [g/L] | Control | 312.13 ± 6.23 | 310.31 ± 6.58 | 305.94 ± 8.59 | 308.56 ± 9.49 | 310.81 ± 3.37 | 310.31 ± 6.44 | 305.56 ± 4.4 | 308.63 ± 5.63 |
| Anastomosis | 300 ± 4.7 | 297.8 ± 7.1 | 295.6 ± 6.9 | 298.7 ± 4.7 | 300 ± 2.4 # | 293.4 ± 8.4 # | 291.4 ± 8.3 # | 292.4 ± 6.5 # | |
| Plt [109/L] | Control | 465.4 ± 107.9 | 472.6 ± 129.4 | 456 ± 132 | 423.6 ± 137.7 | 393.3 ± 123.1 * | 376.1 ± 141.8 * | 305.6 ± 95.8 * | 418.8 ± 121.3 |
| Anastomosis | 504.6 ± 107.7 | 464.1 ± 141.9 | 478.6 ± 139.6 | 478.8 ± 124.6 | 393.7 ± 126.3 * | 409.9 ± 136.1 *# | 393.2 ± 161.6 *# | 396.67 ± 155.1 * | |
| Variable | Group | Before Operation | After Operation | ||||||
|---|---|---|---|---|---|---|---|---|---|
| A | V | P | R | A | V | P | R | ||
| ΔEI | Control | 0.383 ± 0.01 | 0.375 ± 0.02 | 0.385 ± 0.01 | 0.383 ± 0.02 | 0.382 ± 0.01 | 0.375 ± 0.01 | 0.379 ± 0.02 * | 0.385 ± 0.01 |
| Anast. | 0.381 ± 0.01 | 0.379 ± 0.01 | 0.372 ± 0.04 | 0.386 ± 0.01 | 0.379 ± 0.01 | 0.374 ± 0.02 * | 0.378 ± 0.02 * | 0.382 ± 0.01 * | |
| ΔO | Control | 175.63 ± 13.5 | 175.75 ± 8.2 | 175 ± 8.9 | 178.75 ± 9.6 | 176.88 ± 7.8 | 176.13 ± 5.1 | 175.25 ± 5 | 179.25 ± 6.9 |
| Anast. | 169.57 ± 13.3 | 173.43 ± 9.1 | 175.43 ± 1.7 | 171.71 ± 7.1 | 175.71 ± 6.8 * | 177.86 ± 10 * | 178.14 ± 7.3 | 179.43 ± 13.3 * | |
| ΔEI /ΔO | Control | 0.002 ± 0.0001 | 0.002 ± 0.0001 | 0.002 ± 0.0001 | 0.002 ± 0.0001 | 0.002 ± 0.0001 | 0.002 ± 0.0001 | 0.002 ± 0.0001 | 0.002 ± 0.0001 |
| Anast. | 0.002 ± 0.0001 | 0.002 ± 0.0001 | 0.002 ± 0.0001 | 0.002 ± 0.0001 | 0.002 ± 0.0001 | 0.002 ± 0.0001 | 0.002 ± 0.0001 | 0.002 ± 0.0001 | |
| rEI | Control | 3.69 ± 0.21 | 3.59 ± 0.43 | 3.69 ± 0.19 | 3.7 ± 0.36 | 3.66 ± 0.07 | 3.56 ± 0.32 | 3.57 ± 0.42 | 3.72 ± 0.24 |
| Anast. | 3.67 ± 0.32 | 3.67 ± 0.3 | 3.54 ± 0.68 | 3.74 ± 0.2 | 3.74 ± 0.26 *# | 3.68 ± 0.47 | 3.7 ± 0.45 *# | 3.75 ± 0.36 | |
| rO | Control | 1.98 ± 0.08 | 1.94 ± 0.04 | 1.94 ± 0.04 | 1.97 ± 0.05 | 1.98 ± 0.05 | 1.96 ± 0.04 | 1.94 ± 0.04 | 1.97 ± 0.13 |
| Anast. | 1.95 ± 0.07 | 1.98 ± 0.08 | 1.99 ± 0.12 | 1.96 ± 0.09 | 1.98 ± 0.05 | 1.98 ± 0.09 | 1.97 ± 0.06 | 2.01 ± 0.14 | |
| rEI /rO | Control | 1.87 ± 0.12 | 1.85 ± 0.23 | 1.9 ± 0.1 | 1.88 ± 0.17 | 1.84 ± 0.06 | 1.82 ± 0.18 | 1.83 ± 0.21 | 1.88 ± 0.14 |
| Anast. | 1.87 ± 0.16 | 1.86 ± 0.22 | 1.79 ± 0.41 | 1.92 ± 0.18 | 1.89 ± 0.15 | 1.87 ± 0.28 | 1.89 ± 0.27 *# | 1.89 ± 0.29 | |
| Variable | Group | Before Operation | After Operation | ||||||
|---|---|---|---|---|---|---|---|---|---|
| A | V | P | R | A | V | P | R | ||
| pO2 [mmHg] | Control | 82.1 ± 8.7 | 45.4 ± 13.8 | 61.5 ± 19.5 | 50.7 ± 11.1 | 82.5 ± 6.5 | 45.1 ± 9.7 | 60.2 ± 7.9 | 55.6 ± 11 |
| Anastomosis | 89.2 ± 1.6 | 49.9 ± 8.8 | 47.7 ± 11.7 | 44.9 ± 10.9 | 75.9 ± 5.5 *# | 46.1 ± 7 | 36.6 ± 5.1 *# | 42.1 ± 3.9 | |
| pCO2 [mmHg] | Control | 48.3 ± 6.1 | 53.2 ± 4.1 | 62 ± 6.8 | 55.8 ± 8.3 | 49.1 ± 8.3 | 54 ± 6.9 | 61.8 ± 8.2 | 54.1 ± 9 |
| Anastomosis | 53.3 ± 3 | 57.1 ± 3.7 | 65.5 ± 8.9 | 58.8 ± 9 | 54.1 ± 15.6 | 63.7 ± 2.6 *# | 75.1 ± 7.6 *# | 69.3 ± 7 *# | |
| pH | Control | 7.37 ± 0.02 | 7.33 ± 0.02 | 7.28 ± 0.04 | 7.33 ± 0.04 | 7.36 ± 0.05 | 7.33 ± 0.04 | 7.3 ± 0.06 | 7.35 ± 0.05 |
| Anastomosis | 7.38 ± 0.05 | 7.36 ± 0.06 | 7.33 ± 0.05 | 7.38 ± 0.03 | 7.34 ± 0.01 | 7.25 ± 0.02 *# | 7.25 ± 0.02 | 7.36 ± 0.01 | |
| cHCO3− [mmol/L] | Control | 27.94 ± 2.33 | 28.13 ± 1.93 | 29.26 ± 2.99 | 29.11 ± 2.64 | 27.29 ± 2.26 | 28.03 ± 1.88 | 28.96 ± 2.1 | 28.75 ± 2.04 |
| Anastomosis | 31.31 ± 2.97 | 32.65 ± 3.33 | 36.2 ± 2.6 | 34.55 ± 3.12 | 28.84 ± 5.07 | 30.15 ± 5.16 | 31.12 ± 4.41 * | 27.3 ± 6.72 * | |
| Variable | Group | Before Operation | After Operation | ||||||
|---|---|---|---|---|---|---|---|---|---|
| A | V | P | R | A | V | P | R | ||
| Na+ [mmol/L] | Control | 141.8 ± 2.2 | 142 ± 5.9 | 143 ± 2.6 | 142 ± 2.4 | 143.1 ± 3.2 | 143.1 ± 3.9 | 142.1 ± 3.4 | 142.6 ± 2.5 |
| Anastomosis | 146.3 ± 4.2 | 149.5 ± 4.4 | 145.6 ± 1.9 | 148.6 ± 3.2 | 148.3 ± 1.2 *# | 152.2 ± 2.2 # | 149.8 ± 3.6 *# | 149.1 ± 6.2 # | |
| K+ [mmol/L] | Control | 4.13 ± 0.6 | 4.29 ± 0.9 | 4.54 ± 0.5 | 4.01 ± 0.4 | 4.09 ± 0.3 | 4.24 ± 0.4 | 4.74 ± 0.2 | 4.1 ± 0.3 |
| Anastomosis | 3.65 ± 0.5 | 3.66 ± 0.5 | 4.83 ± 0.9 | 4.38 ± 09 | 3.67 ± 0.7 | 3.62 ± 0.6 | 3.96 ± 0.5 *# | 3.74 ± 0.5 # | |
| Ca2+ [mmol/L] | Control | 1.42 ± 0.1 | 1.44 ± 0.1 | 1.43 ± 0.1 | 1.43 ± 0.1 | 1.41 ± 0.1 | 1.43 ± 0.1 | 1.43 ± 0.3 | 1.43 ± 0.2 |
| Anastomosis | 1.41 ± 0.1 | 1.4 ± 0.1 | 1.37 ± 0.1 | 1.38 ± 0.2 | 1.38 ± 0.1 | 1.38 ± 0.1 | 1.37 ± 0.2 | 1.38 ± 0.3 | |
| Cl− [mmol/L] | Control | 100.75 ± 3.2 | 100.38 ± 4.1 | 100.5 ± 1.2 | 100.25 ± 2 | 101.75 ± 1.8 | 101.25 ± 2.3 | 101.75 ± 1.5 | 100.75 ± 1.8 |
| Anastomosis | 99.4 ± 3.3 | 98 ± 3.6 | 99.8 ± 4 | 98.8 ± 3.9 | 101.78 ± 6.3 | 101.2 ± 5.5 | 101.6 ± 4.3 | 101.88 ± 4.5 | |
| Variable | Group | Before Operation | After Operation | ||||||
|---|---|---|---|---|---|---|---|---|---|
| A | V | P | R | A | V | P | R | ||
| Glu [mmol/L] | Control | 7.3 ± 2.3 | 6.9 ± 2.5 | 9 ± 2.4 | 7.7 ± 2.4 | 7.5 ± 2.2 | 7.1 ± 1.6 | 9.4 ± 2.4 | 8 ± 2.6 |
| Anastomosis | 6.1 ± 3.4 | 6.2 ± 3.2 | 5.8 ± 3.3 | 6.1 ± 3.1 | 6.5 ± 3.5 | 7 ± 3.4 | 8.7 ± 2.1 * | 8.1 ± 2.8 * | |
| Lac [mmol/L] | Control | 0.96 ± 0.23 | 0.84 ± 0.19 | 1.83 ± 1.06 | 1.02 ± 0.52 | 1.03 ± 0.36 | 0.94 ± 0.2 | 1.71 ± 0.65 | 0.97 ± 0.48 |
| Anastomosis | 1.13 ± 1 | 1.22 ± 1.15 | 0.87 ± 0.4 | 0.82 ± 0.41 | 1.72 ± 1.4 *# | 2.05 ± 1.79 *# | 2.16 ± 1.18 * | 1.96 ± 1.32 *# | |
| Crea [µmol/L] | Control | 106.5 ± 13.8 | 102.9 ± 18.5 | 111.4 ± 12.7 | 91.8 ± 22.6 | 109.5 ± 14.8 | 102.6 ± 18.1 | 110.7 ± 17.1 | 89.9 ± 16.2 |
| Anastomosis | 94.5 ± 10.1 | 95.3 ± 17.8 | 106.4 ± 25.2 | 86.5 ± 26.9 | 100.4 ± 20.1 | 103.8 ± 27.2 | 114.4 ± 29.5 | 88.8 ± 19 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Varga, A.; Matrai, A.A.; Bedocs-Barath, B.; Fazekas, L.A.; Brasil, F.S.; Mehta, A.; Vanyolos, E.; Deak, A.; Lesznyak, T.; Peto, K.; et al. Local and Systemic Micro-Rheological Changes during Intestinal Anastomosis Operation: A Metabolic Dependence in an Experimental Model. Metabolites 2024, 14, 458. https://doi.org/10.3390/metabo14080458
Varga A, Matrai AA, Bedocs-Barath B, Fazekas LA, Brasil FS, Mehta A, Vanyolos E, Deak A, Lesznyak T, Peto K, et al. Local and Systemic Micro-Rheological Changes during Intestinal Anastomosis Operation: A Metabolic Dependence in an Experimental Model. Metabolites. 2024; 14(8):458. https://doi.org/10.3390/metabo14080458
Chicago/Turabian StyleVarga, Adam, Adam Attila Matrai, Barbara Bedocs-Barath, Laszlo Adam Fazekas, Felipe Salignac Brasil, Aashna Mehta, Erzsebet Vanyolos, Adam Deak, Tamas Lesznyak, Katalin Peto, and et al. 2024. "Local and Systemic Micro-Rheological Changes during Intestinal Anastomosis Operation: A Metabolic Dependence in an Experimental Model" Metabolites 14, no. 8: 458. https://doi.org/10.3390/metabo14080458
APA StyleVarga, A., Matrai, A. A., Bedocs-Barath, B., Fazekas, L. A., Brasil, F. S., Mehta, A., Vanyolos, E., Deak, A., Lesznyak, T., Peto, K., & Nemeth, N. (2024). Local and Systemic Micro-Rheological Changes during Intestinal Anastomosis Operation: A Metabolic Dependence in an Experimental Model. Metabolites, 14(8), 458. https://doi.org/10.3390/metabo14080458









