Estimating Dietary Protein and Sodium Intake with Sodium Removal in Peritoneal Dialysis Patients
Abstract
1. Introduction
2. Materials and Methods
2.1. Laboratory Method
2.2. Statistical Methods
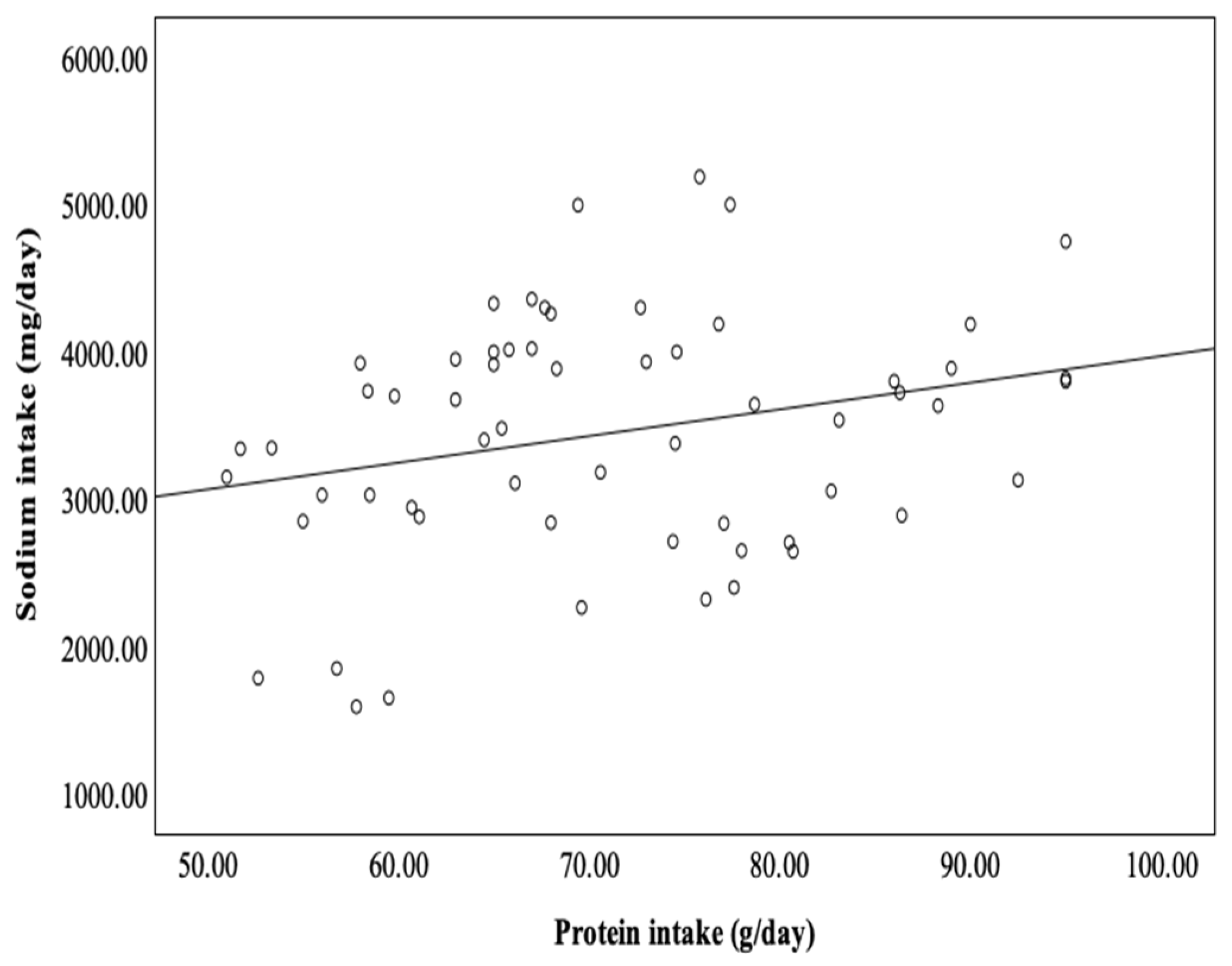
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kircelli, F.; Asci, G.; Yilmaz, M.; Sevinc Ok, E.; Demirci, M.-S.; Toz, H.; Akcicek, F.; Ok, E.; Ozkahya, M. The impact of strict volume control strategy on patient survival and technique failure in peritoneal dialysis patients. Blood Purif. 2011, 32, 30–37. [Google Scholar] [CrossRef]
- Paniagua, R.; Ventura, M.-D.; Avila-Díaz, M.; Hinojosa-Heredia, H.; Méndez-Durán, A.; Cueto-Manzano, A.; Cisneros, A.; Ramos, A.; Madonia-Juseino, C.; Belio-Caro, F.; et al. NT-pro-BNP, fluid volume overload and dialysis modality are independent predictors of mortality in ESRD patients. Nephrol. Dial. Transplant. 2009, 25, 551–557. [Google Scholar] [CrossRef]
- Krediet, R.-T.; Balafa, O. Cardiovascular risk in the peritoneal dialysis patient. Nat. Rev. Nephrol. 2010, 6, 451–460. [Google Scholar] [CrossRef]
- Tangwonglert, T.; Davenport, A. Changes in extracellular water and left ventricular mass in peritoneal dialysis patients. Kidney Res. Clin. Pract. 2021, 40, 135–142. [Google Scholar] [CrossRef]
- Brown, E.-A.; Davies, S.-J.; Rutherford, P.; Meeus, F.; Borras, M.; Riegel, W.; Divino-Filho, J.-C.; Vonesh, E.; van Bree, M.; EAPOS Group. Survival of functionally anuric patients on automated peritoneal dialysis:the European APD Outcome Study. J. Am. Soc. Nephrol. 2003, 14, 2948–2957. [Google Scholar] [CrossRef]
- Boonpheng, B.; Thongprayoon, C.; Cheungpasitporn, W. The comparison of risk of stroke in patients with peritoneal dialysis and hemodialysis: A systemic review and meta-analysis. J. Evid. Based Med. 2018, 11, 158–168. [Google Scholar] [CrossRef]
- Ateş, K.; Nergizoğlu, G.; Keven, K.; Sen, A.; Kutlay, S.; Ertürk, S.; Duman, N.; Karatan, O.; Ertuğ, A.-E. Effect of fluid and sodium removal on mortality in peritoneal dialysis patients. Kidney Int. 2010, 60, 767–776. [Google Scholar] [CrossRef]
- Mok, N.M.; Fan, N.; Finney, H.; Fan, S.L. Relationship between sodium removal, hydration and outcomes in peritoneal dialysis patients. Nephrology 2021, 26, 676–683. [Google Scholar] [CrossRef]
- Gong, N.; Zhou, C.; Hu, J.; Zhong, X.; Yi, Z.; Zhang, T.; Yang, C.; Lin, Y.; Tian, J.; Qin, X.; et al. High-salt diet accelerated the decline of residual renal function in patients with peritoneal dialysis. Front. Med. 2021, 8, 28009. [Google Scholar] [CrossRef]
- Liao, C.-T.; Chen, Y.M.; Shiao, C.C.; Hu, F.-C.; Huang, J.-W.; Kao, T.-W.; Chuang, H.-F.; Hung, K.Y.; Wu, K.D.; Tsai, T.-J. Rate of decline of residual renal function is associated with all-cause mortality and technique failure in patients on long-term peritoneal dialysis. Nephrol. Dial. Transplant. 2009, 24, 2909–2914. [Google Scholar] [CrossRef]
- Wang, A.J.-M.; Brimble, K.S.; Brunier, G.; Holt, S.G.; Jha, V.; Johnson, D.W.; Kang, S.W.; Kooman, J.P.; Lambie, M.; McIntyre, C.; et al. ISPD cardiovascular and metabolic guidelines in adult peritoneal dialysis patients Part I—Assessment and management of various cardiovascular risk factors. Perit. Dial. Int. 2015, 35, 379–387. [Google Scholar] [CrossRef]
- Weir, M.R. Is it the low-protein diet or simply the salt restriction? Kidney Int. 2007, 71, 188–190. [Google Scholar] [CrossRef][Green Version]
- Dong, J.; Li, Y.; Yang, Z.; Luo, J. Low dietary sodium intake increases the death risk in peritoneal dialysis. Clin. J. Am. Soc. Nephrol. 2010, 5, 240–247. [Google Scholar] [CrossRef]
- Fouque, D.; Kalantar-Zadeh, K.; Kopple, J.; Cano, N.; Chauveau, P.; Cuppari, L.; Franch, H.; Guarnieri, G.; Ikizler, T.-A.; Kaysen, G.; et al. A proposed nomenclature and diagnostic criteria for protein-energy wasting in acute and chronic kidney disease. Kidney Int. 2008, 73, 391–398. [Google Scholar] [CrossRef]
- Kiebalo, T.; Holotka, J.; Habura, I.; Pawlaczyk, K. Nutritional status in peritoneal dialysis: Nutritional guidelines, adequacy and the management of malnutrition. Nutrients 2020, 12, 1715. [Google Scholar] [CrossRef]
- Dong, J.; Li, Y.-J.; Lu, X.-H.; Gan, H.-P.; Zuo, L.; Wang, H.-Y. Correlations of lean body mass with nutritional indicators and mortality in patients on peritoneal dialysis. Kidney Int. 2008, 73, 334–340. [Google Scholar] [CrossRef]
- Ikizler, T.A.; Burrowes, J.D.; Byham-Gray, L.; Campbell, K.L.; Carrero, J.J.; Chan, W.; Fouque, D.; Friedman, A.; Ghaddar, S.; Goldstein-Fuchs, D.; et al. KDOQI Clinical Practice Guideline for Nutrition in CKD: 2020 Update. Am. J. Kidney Dis. 2020, 76, S1–S107. [Google Scholar] [CrossRef]
- Bi, S.H.; Wang, X.; Tang, W.; Wang, T.; Li, B.; Sua, C. Longitudinal association between dietary protein intake and survival in peritoneal dialysis patients. Ren. Fail. 2023, 45, 2182605. [Google Scholar] [CrossRef]
- NKF-DOQI clinical practice guidelines for peritoneal dialysis adequacy. Assessment of nutritional status. Am. J. Kidney Dis. 2007, 30, S125–S129.
- Bargnoux, A.S.; Barguil, Y.; Zaoui, E.; Jean, G.; Cristol, J.P. Suivi de la dialyse: Test d’équilibration péritonéale, anticoagulation régionale au citrate, et fonction rénales résiduelle [Dialysis monitoring: Peritoneal equilibrium test, regional citrate anticoagulation and residual renal function]. Ann. Biol. Clin. 2019, 77, 391–396. [Google Scholar]
- Su, C.Y.; Lu, X.H.; Tang, W.; Wang, P.Y.; Wang, T. Peritoneal dialysis can maintain nitrogen balance with low Kt/V in anuric patients. Clin. Nephrol. 2022, 97, 206–214. [Google Scholar] [CrossRef] [PubMed]
- Davies, S.J.; Phillips, L.; Naish, P.F.; Russell, G.I. Quantifying comorbidity in peritoneal dialysis patients and its relationship to other predictors of survival. Nephrol. Dial. Transplant. 2002, 17, 1085–1092. [Google Scholar] [CrossRef] [PubMed]
- Chung, S.H.; Heimbürger, O.; Stenvinkel, P.; Qureshi, A.R.; Lindholm, B. Association between residual renal function, inflammation and patient survival in new peritoneal dialysis patients. Nephrol. Dial. Transplant. 2003, 18, 590–597. [Google Scholar] [CrossRef] [PubMed]
- Shafi, T.; Jaar, B.G.; Plantinga, L.C.; Fink, N.E.; Sadler, J.H.; Parekh, R.S.; Powe, N.R.; Coresh, J. Association of residual urine output with mortality, quality of life, and inflammation in incident hemodialysis patients: The CHOICE (Choices for healthy outcomes in caring for end-stage renal disease) study. Am. J. Kidney Dis. 2010, 56, 348–358. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.-W.; Lien, Y.-C.; Wu, H.-Y.; Yen, C.-J.; Pan, C.-C.; Hung, T.-W.; Su, C.-T.; Chiang, C.-K.; Cheng, H.-T.; Hung, K.-Y. Lean body mass predicts long-term survival in chinese patients on peritoneal dialysis. PLoS ONE 2013, 8, e54976. [Google Scholar] [CrossRef] [PubMed]
- Paniagua, R.; Amato, D.; Vonesh, E.; Correa-Rotter, R.; Ramos, A.; Moran, J.; Mujais, S. Effects of increased peritoneal clearances on mortality rates in peritoneal dialysis: ADEMEX, a prospective, randomized, controlled trial. J. Am. Soc. Nephrol. 2002, 13, 1307–1320. [Google Scholar] [CrossRef] [PubMed]
- Zoccali, C.; Moissl, U.; Chazot, C.; Mallamaci, F.; Tripepi, G.; Arkossy, O.; Wabel, P.; Stuard, S. Chronic Fluid Overload and Mortality in ESRD. J. Am. Soc. Nephrol. 2017, 28, 2491–2497. [Google Scholar] [CrossRef] [PubMed]
- Tsai, Y.C.; Chiu, Y.W.; Tsai, J.C.; Kuo, H.T.; Hung, C.C.; Hwang, S.J.; Chen, T.H.; Kuo, M.C.; Chen, H.C. Association of fluid overload with cardiovascular morbidity and all-cause mortality in stages 4 and 5 CKD. Clin. J. Am. Soc. Nephrol. 2015, 10, 39–46. [Google Scholar] [CrossRef] [PubMed]
- Wang, A.Y.M.; Wang, M.; Woo, J.; Law, M.C.; Chow, K.M.; Li, P.K.T.; Lui, S.F.; Sanderson, J.E. A novel association between residual renal function and left ventricular hypertrophy in peritoneal dialysis patients. Kidney Intern. 2002, 62, 639–647. [Google Scholar] [CrossRef]
- Kopp, C.; Linz, P.; Dahlmann, A.; Hammon, M.; Jantsch, J.; Müller, D.-N.; Schmieder, R.-E.; Cavallaro, A.; Eckardt, K.-U.; Uder, M.; et al. 23Na magnetic resonance imaging-determined tissue sodium in healthy subjects and hypertensive patients. Hypertension 2013, 61, 635–640. [Google Scholar] [CrossRef]
- Schneider, M.P.; Raff, U.; Kopp, C.; Scheppach, J.-B.; Toncar, S.; Wanner, C.; Schlieper, G.; Saritas, T.; Floege, J.; Schmid, M.; et al. Skin Sodium Concentration Correlates with Left Ventricular Hypertrophy in CKD. J. Am. Soc. Nephrol. 2017, 28, 1867–1876. [Google Scholar] [CrossRef] [PubMed]
- Sahinoz, M.; Tintara, S.; Deger, S.M.; Alsouqi, A.; Crescenzi, R.L.; Mambungu, C.; Vincz, A.; Mason, O.; Prigmore, H.L.; Guide, A.; et al. Tissue sodium stores in peritoneal dialysis and hemodialysis patients determined by 23-sodium magnetic resonance imaging. Nephrol. Dial. Transplant. 2020, 36, 1307–1317. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.-M.; Lee, E.K.; Kang, S.; Kim, S.M.; Kim, H.-W.; Kim, S.B. Simple method to estimate daily sodium intake during measurement of dialysis adequacy in chronic peritoneal dialysis patients. Asia Pac. J. Clin. Nutr. 2017, 26, 1001–1006. [Google Scholar]
- Maharjan, S.; Davenport, A. Comparison of sodium removal in peritoneal dialysis patients treated by continuous ambulatory and automated peritoneal dialysis. J. Nephrol. 2019, 32, 1011–1019. [Google Scholar] [CrossRef]
- Avila-Díaz, M.; Ventura, M.-d.-J.; Valle, D.; Vicenté-Martínez, M.; García-González, Z.; Cisneros, A.; Furlong, M.-d.-C.; Gómez, M.; Amato, D.; Paniagua, R. Inflammation and extracellular volume expansion are related to sodium and water removal in patients on peritoneal dialysis. Perit. Dial. Int. 2006, 26, 574–580. [Google Scholar] [CrossRef]
- Abdel-Nabey, M.; Saint-Jacques, C.; Boffa, J.-J.; Frochot, V.; Livrozet, M.; Daudon, M.; Flamant, M.; Letavernier, E.; Haymann, J.-P. 24-h urine collection: A relevant tool in CKD nutrition evaluation. Nutrients 2020, 12, 2615. [Google Scholar] [CrossRef] [PubMed]
- Qin, A.; Liu, X.; Yin, X.; Zhou, H.; Tang, Y.; Qin, W. Normalized protein catabolic rate is a superior nutritional marker associated with dialysis adequacy in continuous am-bulatory peritoneal dialysis patients. Front. Med. 2021, 7, 603725. [Google Scholar] [CrossRef]
- Dong, J.; Li, Y.; Xu, Y.; Xu, R. Daily protein intake and survival in patients on peritoneal dialysis. Nephrol Dial Transplant. 2011, 26, 3715–3721. [Google Scholar] [CrossRef]
- Canada-USA (CANUSA) Peritoneal Dialysis Study Group. Adequacy of dialysis and nutrition in continuous peritoneal dialysis: Association with clinical outcomes. J. Am. Soc. Nephrol. 1996, 7, 198–207. [Google Scholar] [CrossRef]
- Kalantar-Zadeh, K.; Kopple, J.D.; Humphreys, M.H.; Block, G. Comparing outcome predictability of markers of malnutrition-inflammation complex syndrome in haemodialysis patients. Nephrol. Dial. Transplant. 2004, 19, 1507–1519. [Google Scholar] [CrossRef]
- Keshaviah, P.R.; Nolph, K.D.; Moore, H.L.; Prowant, B.; Emerson, P.F.; Meyer, M.; Twardowski, Z.J.; Khanna, R.; Ponferrada, L.; Collins, A. Lean body mass estimation by creatinine kinetics. J. Am. Soc. Nephrol. 1994, 4, 1475–1485. [Google Scholar] [CrossRef] [PubMed]
- Tennankore, K.K.; Bargman, J.M. Nutrition and the Kidney: Recommendations for peritoneal dialysis. Adv. Chronic Kidney Dis. 2013, 20, 190–201. [Google Scholar] [CrossRef] [PubMed]
- Szeto, C.C.; Lai, K.N.; Wong, Y.; Law, M.C.; Leung, C.B.; Yu, A.W.; Li, P.K. Independent effects of residual renal function and dialysis adequacy on nutritional status and patient outcome in continuous ambulatory peritoneal dialysis. Am. J. Kidney Dis. 1999, 34, 1056–1064. [Google Scholar] [CrossRef] [PubMed]
- Wang, A.Y.-M.; Sea, M.M.-M.; Ip, R.; Law, M.C.; Chow, K.M.; Lui, S.F.; Li, P.K.-T.; Woo, J. Independent effects of residual renal function and dialysis adequacy on dietary micronutrient intakes in patients receiving continuous ambulatory peritoneal dialysis. Am. J. Clin. Nutr. 2002, 76, 569–576. [Google Scholar] [CrossRef] [PubMed]
- Heimbürger, O.; Qureshi, A.R.; Blaner, W.S.; Berglund, L.; Stenvinkel, P. Hand-grip muscle strength, lean body mass, and plasma proteins as markers of nutritional status in patients with chronic renal failure close to start of dialysis therapy. Am. J. Kidney Dis. 2000, 36, 1213–1225. [Google Scholar] [CrossRef] [PubMed]
- Davenport, A.; Sayed, R.H.; Fan, S. The effect of racial origin on total body water volume in peritoneal dialysis patients. Clin. J. Am. Soc. Nephrol. 2011, 6, 2492–2498. [Google Scholar] [CrossRef]
- Vongsanim, S.; Davenport, A. Estimating dietary protein intake in peritoneal dialysis patients: The effect of ethnicity. Perit. Dial. Int. 2018, 38, 384–387. [Google Scholar] [CrossRef]

|
RRF < 2 mL/min/1.73 m2 (n = 28) |
RRF > 2 mL/min/1.73 m2 (n = 32) | p-Value | |
|---|---|---|---|
| Age (years) | 56.39 ± 13.25 | 58.28 ± 12.53 | 0.573 |
| Male gender (n; %) | 11 (39.3%) | 17 (53.1%) | 0.208 |
| BMI (kg/m2) | 24.87 ± 3.57 | 24.92 ± 4.73 | 0.930 |
| %LBM | 37.23 ± 7.14 | 51.48 ± 11.41 | <0.001 |
| PD duration (months) | 51 (3–147) | 24.75 (3–67) | 0.001 |
| CAPD vs. CCPD/APD (n; %) | 20 (71.4%) | 22 (68.8%) | 0.523 |
| DM (n; %) | 9 (32.1%) | 7 (21.9%) | 0.272 |
| Davies comorbidity grade low/(medium + high) (n; %) | 8 (28.6%) | 12 (37.5%) | 0.325 |
| D/PCr | 0.67 ± 0.11 | 0.64 ± 0.12 | 0.259 |
| F/FA transporters (n; %) | 19 (67.9%) | 16 (50%) | 0.128 |
| RD (mL/day) | 800 (0–1650) | 1425 (300–3235) | 0.000 |
| UF (mL/day) | 1197.32 ± 440.35 | 866.09 ± 425.65 | <0.004 |
| MAP (mmHg) | 98.28 ± 10.64 | 92.9 ± 9.65 | <0.046 |
| Kt/V | 2.2 (1.3–3.33) | 2.61 (1.6–4.4) | 0.011 |
| CrCl (L/week) | 59.85 (44–115.9) | 95.45 (55.8–233.6) | 0.000 |
| Hb (g/dL) | 8.5 (9–11.8) | 9.75 (8.9–14) | 0.03 |
| Creatinine (mg/dL) | 9.2 ± 2.17 | 6.67 ± 1.7 | <0.001 |
| Albumin (g/L) | 32.61 ± 5.98 | 35.03 ± 5.23 | 0.099 |
| Cholesterol (mg/dL) | 5.46 ± 1.74 | 5.01 ± 1.3 | 0.465 |
| CRP (mg/L) | 5.95 (0.6–23) | 4.98 (0.3–14.6) | 0.101 |
| Sodium intake (g/day) | 3.51 ± 0.84 | 3.46 ± 0.83 | 0.883 |
| Protein intake (g/day) | 66.71 ± 14.13 | 71.65 ± 13.03 | 0.373 |
| DPI (g/kg/day) | 0.96 ± 0.15 | 0.93 ± 0.2 | 0.65 |
| nPCR (g/kg/day) | 0.86 ± 0.23 | 0.84 ± 0.21 | 0.836 |
| DEI (kcal/kg/day) | 29.63 ± 2.7 | 26.81 ± 6.52 | 0.249 |
| DSR (mmol/day) | 171.7 ± 57.97 | 132.77 ± 64.7 | <0.021 |
| USR (mmol/day) | 72.8 (0–165) | 101.15 (25.2–338.1) | 0.000 |
| TSR (mmol/day) | 211.07 ± 62.81 | 253.54 ± 92.56 | <0.046 |
| DCG—Low | DCG—Medium | DCG—High | |
|---|---|---|---|
| (n = 19) | (n = 28) | (n = 13) | |
| Age (years; mean ± SD) | 56.63 ± 11.54 | 55.50 ± 13.25 | 64.08 ± 12.18 |
| PD duration (months; mean ± SD) | 33.42 ± 33.81 | 32.71 ± 41.53 | 27.61 ± 20 |
| Sodium intake (g/day; mean ± SD) | 3.12 ± 0.86 ++ | 4.02 ± 0.58 | 3.15 ± 0.25 |
| Protein intake (g/day; mean ± SD) | 68.85 ± 12.25 | 75.97 ± 13.34 | 59.31 ± 10.30 |
| DPI (g/kg/day; mean ± SD) | 0.91 ± 0.18 | 1 ± 0.21 | 0.82 ± 0.12 |
| nPCR (g/kg/day; mean ± SD) | 0.87 ± 0.23 | 0.83 ± 0.21 | 0.79 ± 0.16 |
| DEI (kcal/kg/day; mean ± SD) | 26.16 ± 5.01 | 30.32 ± 6.49 | 24.34 ± 4.46 |
| DSR (mmol/day; mean ± SD) | 149.45 ± 61.50 | 152.27 ± 64.68 | 151.73 ± 71.72 |
| USR (mmol/day; mean ± SD) | 113.64 ± 83.95 ** | 78.93 ± 67.37 | 43.55 ± 33.86 |
| TSR (mmol/day; mean ± SD) | 263.12 ± 94.25 | 230.68 ± 77.77 | 195.29 ± 54.6 |
| D/PCr (mean ± SD) | 0.61 ± 0.12 | 0.67 ± 0.12 | 0.70 ± 0.09 |
| Kt/V (mean ± SD) | 2.63 ± 0.73 ** | 2.53 ± 0.62 ** | 1.88 ± 0.40 |
| CrCl | |||
| (L/week; mean ± SD) | 90.99 ± 44.3 * | 89.56 ± 28.91 * | 60.32 ± 12.84 |
| UF | |||
| (mL/day; mean ± SD) | 1077.63 ± 443.45 | 970 ± 480.66 | 1046.54 ± 62.84 |
| RD | |||
| (mL/day; mean ± SD) | 1339.74 ± 814.47 ** | 942.86 ± 662.87 | 560.77 ± 428.57 |
| Cr (mg/dL; mean ± SD) | 8.23 ± 2.46 | 7.35 ± 2.34 | 8.37 ± 1.88 |
| Albumin (g/L; mean ± SD) | 37.63 ± 5.76 **+ | 33.36 ± 4.47 | 29.61 ± 4.61 |
| Cholesterol (mg/dL; mean ± SD) | 206.88 ± 58.75 | 173.63 ± 34.42 | 218.1 ± 69.99 |
| Hb (g/dL; mean ± SD) | 11.03 ± 1.27 *+ | 10.27 ± 0.76 | 10 ± 0.89 |
| CRP (mg/L; mean ± SD) | 4.07 ± 3.17 ** | 5.91 ± 3.36 | 10 ± 5.80 |
| MAP (mmHg; mean ± SD) | 95.11 ± 8.50 | 94.43 ± 10.23 | 98.15 ± 13.23 |
| LVH (Yes/No) | 4/15 **++ | 15/13 ** | 12/1 |
| DPI < 0.8 g/kg/day (n = 18) | DPI > 0.8 g/kg/day (n = 42) | p-Value | |
|---|---|---|---|
| Age (years; mean ± SD) | 57.67 ± 9.25 | 63.59 ± 11.61 | 0.184 |
| PD duration (months; mean ± SD) | 9.78 ± 10.18 | 37.11 ± 34.93 | 0.011 |
| BMI (kg/m2; mean ± SD) | 26.19 ± 2.4 | 24.02 ± 3.13 | <0.041 |
| %LBM | 49.39 ± 14.73 | 47.15 ± 11.25 | 0.689 |
| Protein intake (g/day; mean ± SD) | 60.77 ± 6.26 | 74.3 ± 13.44 | <0.008 |
| nPCR (g/kg/day; mean ± SD) | 0.66 ± 0.1 | 0.92 ± 0.2 | <0.001 |
| DEI (kcal/kg/day; mean ± SD) | 24.22 ± 4.91 | 28.89 ± 5.78 | <0.035 |
| Sodium intake (g/day; mean ± SD) | 2.94 ± 0.86 | 3.69 ± 0.71 | <0.018 |
| Kt/V | 2.11 ± 0.51 | 2.35 ± 0.47 | 0.222 |
| CrCl (L/week; mean ± SD) | 74.22 ± 30.93 | 77.36 ± 19.68 | 0.331 |
| RRF (mL/min/1.73 m2; mean ± SD) | 4.53 ± 3.38 | 3.67 ± 2.42 | 0.557 |
| Albumin (g/L; mean ± SD) | 38.44 ± 3.94 | 37.73 ± 4.9 | 0.674 |
| Cholesterol (mg/dL; mean ± SD) | 212.68 ± 47.18 | 191.8 ± 58.78 | 0.367 |
| Peritoneal protein loss (g/day; mean ± SD) | 6.3 ± 2.81 | 8.6 ± 4.99 | 0.242 |
| DPI (g/kg/day) | Protein Intake (g/day) | ||||
|---|---|---|---|---|---|
| R | p-Value | R | p-Value | ||
| BMI (kg/m2) | Male | −0.434 | 0.021 | −0.138 | 0.484 |
| Female | −0.365 | 0.04 | −0.125 | 0.496 | |
| nPCR (g/kg/day) | Male | 0.352 | 0.067 | 0.241 | 0.217 |
| Female | 0.455 | 0.009 | 0.282 | 0.117 | |
| LBM (%) | Male | −0.204 | 0.297 | −0.053 | 0.788 |
| Female | −0.124 | 0.439 | −0.063 | 0.734 | |
| Total energy intake (kcal/day) | Male | 0.243 | 0.213 | 0.409 | 0.031 |
| Female | 0.255 | 0.160 | 0.476 | 0.006 | |
| DEI (kcal/kg/day) | Male | 0.557 | 0.002 | 0.355 | 0.064 |
| Female | 0.655 | 0.00 | 0.343 | 0.054 | |
| Albumin (g/L) | Male | 0.042 | 0.831 | −0.048 | 0.808 |
| Female | −0.002 | 0.991 | 0.059 | 0.746 | |
| LBM% | ||
|---|---|---|
| R | p-Value | |
| Female gender | −0.433 | 0.015 |
| Age (years) | −0.261 | 0.157 |
| BMI (kg/m2) | 0.462 | 0.009 |
| nPCR (g/kg/day) | 0.186 | 0.316 |
| Davies comorbidity grade | −0.193 | 0.299 |
| RRF (mL/min/1.73 m2) | 0.616 | 0.000 |
| Kt/V | 0.09 | 0.636 |
| CrCl (L/week) | 0.482 | 0.007 |
| Albumin (g/L) | 0.515 | 0.003 |
| Peritoneal protein loss (g/day) | 0.133 | 0.501 |
| DPI (g/kg/day) | Total Protein Intake (g/day) | nPCR (g/kg/day) | ||||
|---|---|---|---|---|---|---|
| R | p-Value | R | p-Value | R | p-Value | |
| TSR (mmol/day) | 0.154 | 0.408 | 0.267 | 0.147 | 0.179 | 0.336 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bontić, A.; Kezić, A.; Pavlović, J.; Baralić, M.; Gajić, S.; Petrovic, K.; Ristanović, V.K.; Petrović, O.; Stjepanović, V.; Stanković, S.; et al. Estimating Dietary Protein and Sodium Intake with Sodium Removal in Peritoneal Dialysis Patients. Metabolites 2024, 14, 460. https://doi.org/10.3390/metabo14080460
Bontić A, Kezić A, Pavlović J, Baralić M, Gajić S, Petrovic K, Ristanović VK, Petrović O, Stjepanović V, Stanković S, et al. Estimating Dietary Protein and Sodium Intake with Sodium Removal in Peritoneal Dialysis Patients. Metabolites. 2024; 14(8):460. https://doi.org/10.3390/metabo14080460
Chicago/Turabian StyleBontić, Ana, Aleksandra Kezić, Jelena Pavlović, Marko Baralić, Selena Gajić, Kristina Petrovic, Vidna Karadžić Ristanović, Olga Petrović, Vera Stjepanović, Sanja Stanković, and et al. 2024. "Estimating Dietary Protein and Sodium Intake with Sodium Removal in Peritoneal Dialysis Patients" Metabolites 14, no. 8: 460. https://doi.org/10.3390/metabo14080460
APA StyleBontić, A., Kezić, A., Pavlović, J., Baralić, M., Gajić, S., Petrovic, K., Ristanović, V. K., Petrović, O., Stjepanović, V., Stanković, S., & Radović, M. (2024). Estimating Dietary Protein and Sodium Intake with Sodium Removal in Peritoneal Dialysis Patients. Metabolites, 14(8), 460. https://doi.org/10.3390/metabo14080460








