Combining the Vaginal Microbiome and Serum Metabolome to Screen for Potential Biomarkers of Early Pregnancy in Cows
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Animals and Groups
2.2. Collection and Preparation of Samples
2.3. Vaginal Flora Diversity Analysis
2.3.1. 16S rDNA Gene Sequencing Analysis
2.3.2. Statistical Analysis of 16S rDNA Vaginal Flora
2.4. Serum Untargeted Metabolomics Sample Preparation and Analysis
2.4.1. Sample Preparation
2.4.2. Chromatography-Mass Spectrometry (MS)
2.4.3. Serum Metabolomics Data Processing and Statistical Analysis
2.5. Metabolic Pathway Marker Validation
2.6. Histological Correlation Analysis
3. Results
3.1. Analysis of Vaginal Flora Diversity
3.1.1. Abundance Composition of the Vaginal Flora and Diversity of Vaginal Microorganisms
3.1.2. Vaginal Microbiota Analysis and Functional Prediction
3.2. Serum Untargeted Metabolomics Analysis
3.2.1. Multivariate Statistical Analyses
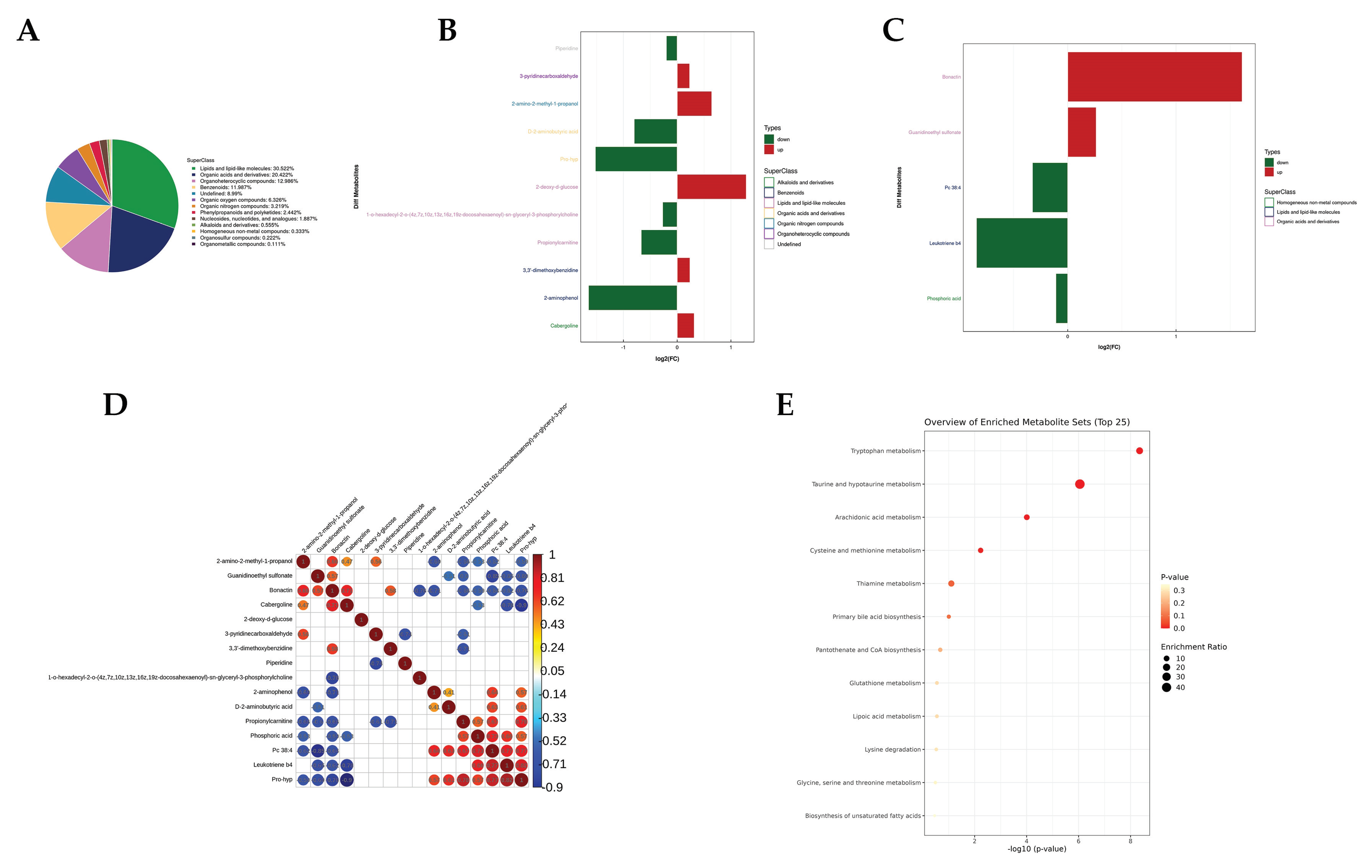
3.2.2. Serum Metabolomics Analysis
3.3. Metabolic Pathway Marker Validation
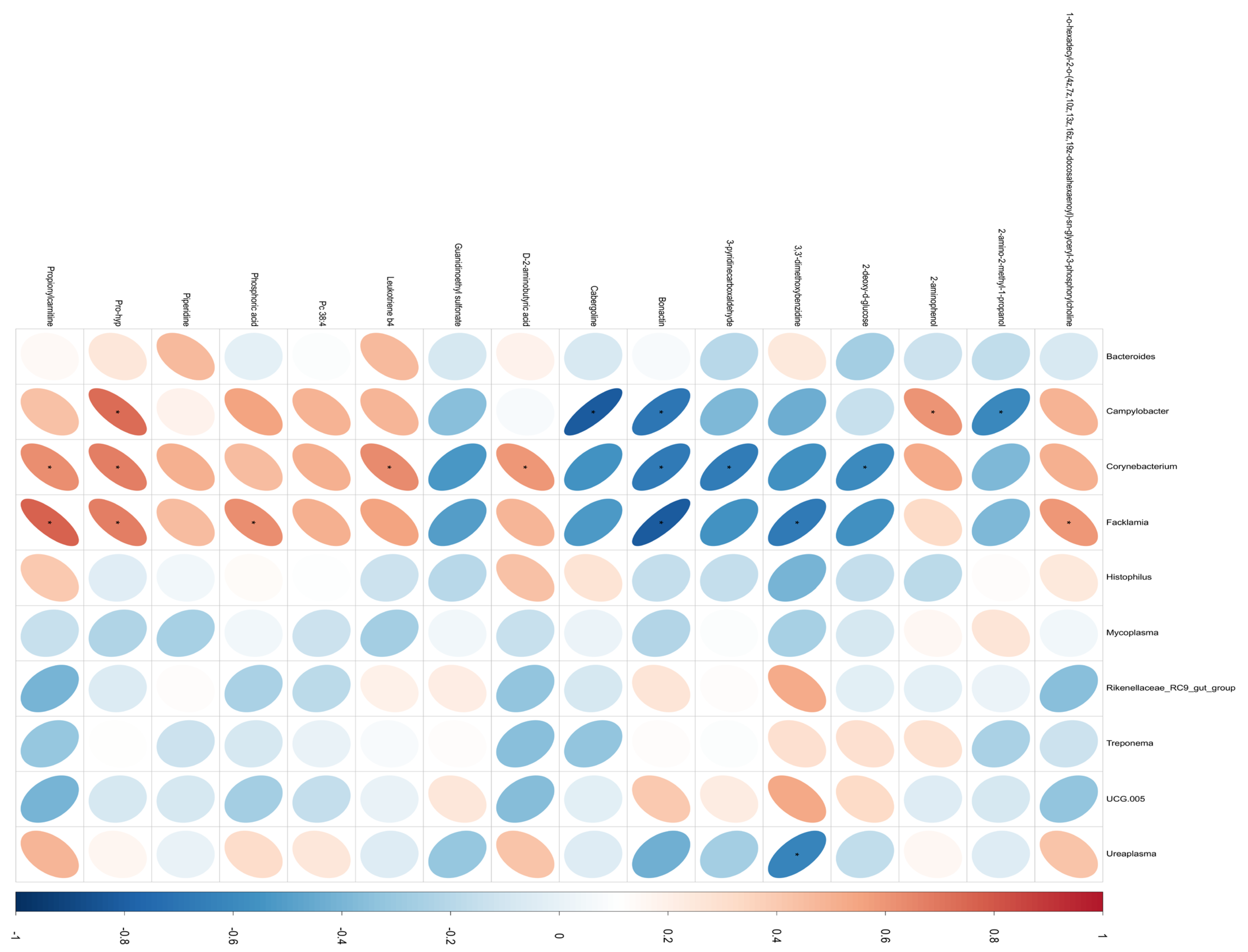
3.4. Spearman Correlation Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gnemmi, G.M.; Maraboli, C.V.A.; Gnemmi, B.; Saleri, R.; De Rensis, F. Use and Adequacy of Non-Pregnancy Diagnosis in Cow. Which Future? Reprod. Domest. Anim. 2022, 57 (Suppl. S5), 45–52. [Google Scholar] [CrossRef] [PubMed]
- Romano, J.E.; Thompson, J.A.; Kraemer, D.C.; Westhusin, M.E.; Forrest, D.W.; Tomaszweski, M.A. Early Pregnancy Diagnosis by Palpation per Rectum: Influence on Embryo/Fetal Viability in Dairy Cattle. Theriogenology 2007, 67, 486–493. [Google Scholar] [CrossRef]
- Fricke, P.M.; Ricci, A.; Giordano, J.O.; Carvalho, P.D. Methods for and Implementation of Pregnancy Diagnosis in Dairy Cows. Vet. Clin. N. Am. Food Anim. Pr. 2016, 32, 165–180. [Google Scholar] [CrossRef]
- Viana, J.H.M.; Vargas, M.S.B.; Siqueira, L.G.B.; Camargo, L.S.A.; Figueiredo, A.C.S.; Fernandes, C.A.C.; Palhao, M.P. Efficacy of Induction of Luteolysis in Superovulated Cows Is Dependent on Time of Prostaglandin F2alpha Analog Treatment: Effects on Plasma Progesterone and Luteinizing Hormone Profiles. Theriogenology 2016, 86, 934–939. [Google Scholar] [CrossRef] [PubMed]
- Giordano, J.O.; Guenther, J.N.; Lopes, G.; Fricke, P.M. Changes in Serum Pregnancy-Associated Glycoprotein, Pregnancy-Specific Protein B, and Progesterone Concentrations before and after Induction of Pregnancy Loss in Lactating Dairy Cows. J. Dairy Sci. 2012, 95, 683–697. [Google Scholar] [CrossRef]
- Green, J.A.; Parks, T.E.; Avalle, M.P.; Telugu, B.P.; McLain, A.L.; Peterson, A.J.; McMillan, W.; Mathialagan, N.; Hook, R.R.; Xie, S.; et al. The Establishment of an ELISA for the Detection of Pregnancy-Associated Glycoproteins (PAGs) in the Serum of Pregnant Cows and Heifers. Theriogenology 2005, 63, 1481–1503. [Google Scholar] [CrossRef]
- Zoli, A.P.; Guilbault, L.A.; Delahaut, P.; Ortiz, W.B.; Beckers, J.F. Radioimmunoassay of a Bovine Pregnancy-Associated Glycoprotein in Serum: Its Application for Pregnancy Diagnosis. Biol. Reprod. 1992, 46, 83–92. [Google Scholar] [CrossRef]
- Barbato, O.; Menchetti, L.; Brecchia, G.; Barile, V.L. Using Pregnancy-Associated Glycoproteins (PAGs) to Improve Reproductive Management: From Dairy Cows to Other Dairy Livestock. Animals 2022, 12, 2033. [Google Scholar] [CrossRef]
- Abdel Aziz, R.L.; Hussein, M.M.; Mohamed, M.A.A.; Elsaid, H.; Abdel-Wahab, A. Heat Stress during Critical Windows of the Oestrous Cycle and Risk of Pregnancy Establishment in Embryo-Recipient Dairy Heifers. Reprod. Domest. Anim. 2022, 57, 856–863. [Google Scholar] [CrossRef]
- Sun, Y.; Liu, T.; Si, Y.; Cao, B.; Zhang, Y.; Zheng, X.; Feng, W. Integrated Metabolomics and 16S rRNA Sequencing to Investigate the Regulation of Chinese Yam on Antibiotic-Induced Intestinal Dysbiosis in Rats. Artif. Cells Nanomed. Biotechnol. 2019, 47, 3382–3390. [Google Scholar] [CrossRef]
- Diboun, I.; Ramanjaneya, M.; Majeed, Y.; Ahmed, L.; Bashir, M.; Butler, A.E.; Abou-Samra, A.B.; Atkin, S.L.; Mazloum, N.A.; Elrayess, M.A. Metabolic Profiling of Pre-Gestational and Gestational Diabetes Mellitus Identifies Novel Predictors of Pre-Term Delivery. J. Transl. Med. 2020, 18, 366. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, P.; Rodning, S.P.; Diniz, W.J.S.; Dyce, P.W. Co-Expression Network and Integrative Analysis of Metabolome and Transcriptome Uncovers Biological Pathways for Fertility in Beef Heifers. Metabolites 2022, 12, 708. [Google Scholar] [CrossRef] [PubMed]
- Ziklo, N.; Vidgen, M.E.; Taing, K.; Huston, W.M.; Timms, P. Dysbiosis of the Vaginal Microbiota and Higher Vaginal Kynurenine/Tryptophan Ratio Reveals an Association with Chlamydia Trachomatis Genital Infections. Front. Cell Infect. Microbiol. 2018, 8, 1. [Google Scholar] [CrossRef]
- Zhai, Y.; Xia, F.; Shi, L.; Ma, W.; Lv, X.; Sun, W.; Ji, P.; Gao, S.; Machaty, Z.; Liu, G.; et al. Early Pregnancy Markers in the Serum of Ewes Identified via Proteomic and Metabolomic Analyses. Int. J. Mol. Sci. 2023, 24, 14054. [Google Scholar] [CrossRef] [PubMed]
- Baud, A.; Hillion, K.-H.; Plainvert, C.; Tessier, V.; Tazi, A.; Mandelbrot, L.; Poyart, C.; Kennedy, S.P. Microbial Diversity in the Vaginal Microbiota and Its Link to Pregnancy Outcomes. Sci. Rep. 2023, 13, 9061. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Liu, M.; Ke, S.; Huang, X.; Fang, S.; He, M.; Fu, H.; Chen, C.; Huang, L. Gut and Vagina Microbiota Associated with Estrus Return of Weaning Sows and Its Correlation with the Changes in Serum Metabolites. Front. Microbiol. 2021, 12, 690091. [Google Scholar] [CrossRef]
- Deng, F.; McClure, M.; Rorie, R.; Wang, X.; Chai, J.; Wei, X.; Lai, S.; Zhao, J. The Vaginal and Fecal Microbiomes Are Related to Pregnancy Status in Beef Heifers. J. Anim. Sci. Biotechnol. 2019, 10, 92. [Google Scholar] [CrossRef]
- Wu, Y.; Zhang, H.; Zhang, R.; Cao, G.; Li, Q.; Zhang, B.; Wang, Y.; Yang, C. Serum Metabolome and Gut Microbiome Alterations in Broiler Chickens Supplemented with Lauric Acid. Poult. Sci. 2021, 100, 101315. [Google Scholar] [CrossRef]
- Rasmussen, M.A.; Thorsen, J.; Dominguez-Bello, M.G.; Blaser, M.J.; Mortensen, M.S.; Brejnrod, A.D.; Shah, S.A.; Hjelmsø, M.H.; Lehtimäki, J.; Trivedi, U.; et al. Ecological Succession in the Vaginal Microbiota during Pregnancy and Birth. ISME J. 2020, 14, 2325–2335. [Google Scholar] [CrossRef]
- Zhao, F.; Hu, X.; Ying, C. Advances in Research on the Relationship between Vaginal Microbiota and Adverse Pregnancy Outcomes and Gynecological Diseases. Microorganisms 2023, 11, 991. [Google Scholar] [CrossRef]
- Koester, L.R.; Petry, A.L.; Youngs, C.R.; Schmitz-Esser, S. Ewe Vaginal Microbiota: Associations with Pregnancy Outcome and Changes during Gestation. Front. Microbiol. 2021, 12, 745884. [Google Scholar] [CrossRef] [PubMed]
- Serrano, M.G.; Parikh, H.I.; Brooks, J.P.; Edwards, D.J.; Arodz, T.J.; Edupuganti, L.; Huang, B.; Girerd, P.H.; Bokhari, Y.A.; Bradley, S.P.; et al. Racioethnic Diversity in the Dynamics of the Vaginal Microbiome during Pregnancy. Nat. Med. 2019, 25, 1001–1011. [Google Scholar] [CrossRef]
- Ault, T.B.; Clemmons, B.A.; Reese, S.T.; Dantas, F.G.; Franco, G.A.; Smith, T.P.L.; Edwards, J.L.; Myer, P.R.; Pohler, K.G. Uterine and Vaginal Bacterial Community Diversity Prior to Artificial Insemination between Pregnant and Nonpregnant Postpartum Cows1. J. Anim. Sci. 2019, 97, 4298–4304. [Google Scholar] [CrossRef] [PubMed]
- Appiah, M.O.; Wang, J.; Lu, W. Microflora in the Reproductive Tract of Cattle: A Review. Agriculture 2020, 10, 232. [Google Scholar] [CrossRef]
- Chen, S.-Y.; Deng, F.; Zhang, M.; Jia, X.; Lai, S.-J. Characterization of Vaginal Microbiota Associated with Pregnancy Outcomes of Artificial Insemination in Dairy Cows. J. Microbiol. Biotechnol. 2020, 30, 804–810. [Google Scholar] [CrossRef] [PubMed]
- Fei, N.; Zhao, L. An Opportunistic Pathogen Isolated from the Gut of an Obese Human Causes Obesity in Germfree Mice. ISME J. 2013, 7, 880–884. [Google Scholar] [CrossRef]
- Ansari, A.; You, Y.-A.; Lee, G.; Kim, S.M.; Park, S.W.; Hur, Y.M.; Kim, Y.J. Dysbiotic Vaginal Microbiota Induces Preterm Birth Cascade via Pathogenic Molecules in the Vagina. Metabolites 2024, 14, 45. [Google Scholar] [CrossRef]
- Ni, J.; Wang, J.; Zhao, K.; Chen, Y.; Xia, S.; Lai, S. Vaginal Microbiome Dynamics of Cows in Different Parities. Animals 2023, 13, 2880. [Google Scholar] [CrossRef]
- Lu, H.; Xu, X.; Fu, D.; Gu, Y.; Fan, R.; Yi, H.; He, X.; Wang, C.; Ouyang, B.; Zhao, P.; et al. Butyrate-Producing Eubacterium Rectale Suppresses Lymphomagenesis by Alleviating the TNF-Induced TLR4/MyD88/NF-κB Axis. Cell Host Microbe 2022, 30, 1139–1150.e7. [Google Scholar] [CrossRef]
- Zeng, X.; Li, S.; Ye, Q.; Cai, S.; Quan, S.; Liu, L.; Zhang, S.; Chen, F.; Cai, C.; Wang, F.; et al. The Combined Use of Medium- and Short-Chain Fatty Acids Improves the Pregnancy Outcomes of Sows by Enhancing Ovarian Steroidogenesis and Endometrial Receptivity. Nutrients 2022, 14, 4405. [Google Scholar] [CrossRef]
- Webb, E.M.; Holman, D.B.; Schmidt, K.N.; Pun, B.; Sedivec, K.K.; Hurlbert, J.L.; Bochantin, K.A.; Ward, A.K.; Dahlen, C.R.; Amat, S. 188 Characterization of the Vagino-Uterine Microbiota in Pregnant and Non-Pregnant Beef Cattle Using 16S Rrna Gene Sequencing and Culturing. J. Anim. Sci. 2023, 101, 6–7. [Google Scholar] [CrossRef]
- Bowman, C.E.; Arany, Z.; Wolfgang, M.J. Regulation of Maternal-Fetal Metabolic Communication. Cell Mol. Life Sci. 2021, 78, 1455–1486. [Google Scholar] [CrossRef]
- Thiyagarajamoorthy, D.K.; Arulanandam, C.D.; Dahms, H.-U.; Murugaiah, S.G.; Krishnan, M.; Rathinam, A.J. Marine Bacterial Compounds Evaluated by In Silico Studies as Antipsychotic Drugs against Schizophrenia. Mar. Biotechnol. 2018, 20, 639–653. [Google Scholar] [CrossRef]
- Schumacher, R.W.; Talmage, S.C.; Miller, S.A.; Sarris, K.E.; Davidson, B.S.; Goldberg, A. Isolation and Structure Determination of an Antimicrobial Ester from a Marine Sediment-Derived Bacterium. J. Nat. Prod. 2003, 66, 1291–1293. [Google Scholar] [CrossRef]
- Rabby, S.M.F.; Chakraborty, M.; Gupta, D.R.; Rahman, M.; Paul, S.K.; Mahmud, N.U.; Rahat, A.A.M.; Jankuloski, L.; Islam, T. Bonactin and Feigrisolide C Inhibit Magnaporthe oryzae Triticum Fungus and Control Wheat Blast Disease. Plants 2022, 11, 2108. [Google Scholar] [CrossRef]
- Islam, M.T.; Laatsch, H.; von Tiedemann, A. Inhibitory Effects of Macrotetrolides from Streptomyces spp. On Zoosporogenesis and Motility of Peronosporomycete Zoospores Are Likely Linked with Enhanced ATPase Activity in Mitochondria. Front. Microbiol. 2016, 7, 1824. [Google Scholar] [CrossRef] [PubMed]
- Sato, K.; Asai, T.T.; Jimi, S. Collagen-Derived Di-Peptide, Prolylhydroxyproline (Pro-Hyp): A New Low Molecular Weight Growth-Initiating Factor for Specific Fibroblasts Associated with Wound Healing. Front. Cell Dev. Biol. 2020, 8, 548975. [Google Scholar] [CrossRef]
- Breeveld-Dwarkasing, V.N.A.; Te Koppele, J.M.; Bank, R.A.; Van Der Weijden, G.C.; Taverne, M.A.M.; Van Dissel-Emiliani, F.M.F. Changes in Water Content, Collagen Degradation, Collagen Content, and Concentration in Repeated Biopsies of the Cervix of Pregnant Cows. Biol. Reprod. 2003, 69, 1608–1614. [Google Scholar] [CrossRef] [PubMed]
- Kimira, Y.; Sato, T.; Sakamoto, M.; Osawa, Y.; Mano, H. Collagen-Derived Dipeptide Pro-Hyp Enhanced ATDC5 Chondrocyte Differentiation under Hypoxic Conditions. Molecules 2023, 28, 4664. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.S.; Tao, J.Z. Metabolomics and Pathway Analyses to Characterize Metabolic Alterations in Pregnant Dairy Cows on D 17 and D 45 after AI. Sci. Rep. 2018, 8, 5973. [Google Scholar] [CrossRef]
- Groebner, A.E.; Schulke, K.; Schefold, J.C.; Fusch, G.; Sinowatz, F.; Reichenbach, H.D.; Wolf, E.; Meyer, H.H.D.; Ulbrich, S.E. Immunological Mechanisms to Establish Embryo Tolerance in Early Bovine Pregnancy. Reprod. Fertil. Dev. 2011, 23, 619–632. [Google Scholar] [CrossRef] [PubMed]
- Ye, Q.; Zeng, X.; Cai, S.; Qiao, S.; Zeng, X. Mechanisms of Lipid Metabolism in Uterine Receptivity and Embryo Development. Trends Endocrinol. Metab. 2021, 32, 1015–1030. [Google Scholar] [CrossRef] [PubMed]
- Crawford, M.A.; Sinclair, A.J.; Hall, B.; Ogundipe, E.; Wang, Y.; Bitsanis, D.; Djahanbakhch, O.B.; Harbige, L.; Ghebremeskel, K.; Golfetto, I.; et al. The Imperative of Arachidonic Acid in Early Human Development. Prog. Lipid Res. 2023, 91, 101222. [Google Scholar] [CrossRef]
- Hanna, V.S.; Hafez, E.A.A. Synopsis of Arachidonic Acid Metabolism: A Review. J. Adv. Res. 2018, 11, 23–32. [Google Scholar] [CrossRef]
- Murphy, R.C.; Gijón, M.A. Biosynthesis and Metabolism of Leukotrienes. Biochem. J. 2007, 405, 379–395. [Google Scholar] [CrossRef]
- Jian, F.; Ma, Y.; Liu, Z.; Wang, L.; Zhang, Y. The Change of LTB4 and 5-LO during Pregnancy. Arch. Gynecol. Obstet. 2013, 288, 769–773. [Google Scholar] [CrossRef] [PubMed]
- Korzekwa, A.J.; Bah, M.M.; Kurzynowski, A.; Lukasik, K.; Groblewska, A.; Skarzynski, D.J. Leukotrienes Modulate Secretion of Progesterone and Prostaglandins during the Estrous Cycle and Early Pregnancy in Cattle: An in Vivo Study. Reproduction 2010, 140, 767–776. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Fu, C.; Mao, W.; Gao, R.; Deng, Y.; Gao, L.; Wu, J.; Zhang, S.; Shen, Y.; Liu, K.; Li, Q.; et al. Prostaglandin F2α-PTGFR Signaling Promotes Proliferation of Endometrial Epithelial Cells of Cattle through Cell Cycle Regulation. Anim. Reprod. Sci. 2020, 213, 106276. [Google Scholar] [CrossRef]
- Cui, L.; Wang, H.; Lin, J.; Wang, Y.; Dong, J.; Li, J.; Li, J. Progesterone Inhibits Inflammatory Response in E.Coli- or LPS-Stimulated Bovine Endometrial Epithelial Cells by NF-κB and MAPK Pathways. Dev. Comp. Immunol. 2020, 105, 103568. [Google Scholar] [CrossRef]
- Ulbrich, S.E.; Meyer, S.U.; Zitta, K.; Hiendleder, S.; Sinowatz, F.; Bauersachs, S.; Büttner, M.; Fröhlich, T.; Arnold, G.J.; Reichenbach, H.-D.; et al. Bovine Endometrial Metallopeptidases MMP14 and MMP2 and the Metallopeptidase Inhibitor TIMP2 Participate in Maternal Preparation of Pregnancy. Mol. Cell. Endocrinol. 2011, 332, 48–57. [Google Scholar] [CrossRef]
- Kaczynski, P.; Goryszewska, E.; Baryla, M.; Waclawik, A. Prostaglandin F2α Stimulates Angiogenesis at the Embryo-Maternal Interface during Early Pregnancy in the Pig. Theriogenology 2020, 142, 169–176. [Google Scholar] [CrossRef] [PubMed]
- Ballas, P.; Rückert, C.; Wagener, K.; Drillich, M.; Kämpfer, P.; Busse, H.-J.; Ehling-Schulz, M. Corynebacterium endometrii sp. Nov., Isolated from the Uterus of a Cow with Endometritis. Int. J. Syst. Evol. Microbiol. 2020, 70, 146–152. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Tang, H.; Xu, Z.; Tang, H.; Fan, Z.; Jiao, X.; Huang, J. Prevalence and Characteristics of Campylobacter from the Genital Tract of Primates and Ruminants in Eastern China. Transbound. Emerg. Dis. 2022, 69, e1892–e1898. [Google Scholar] [CrossRef]
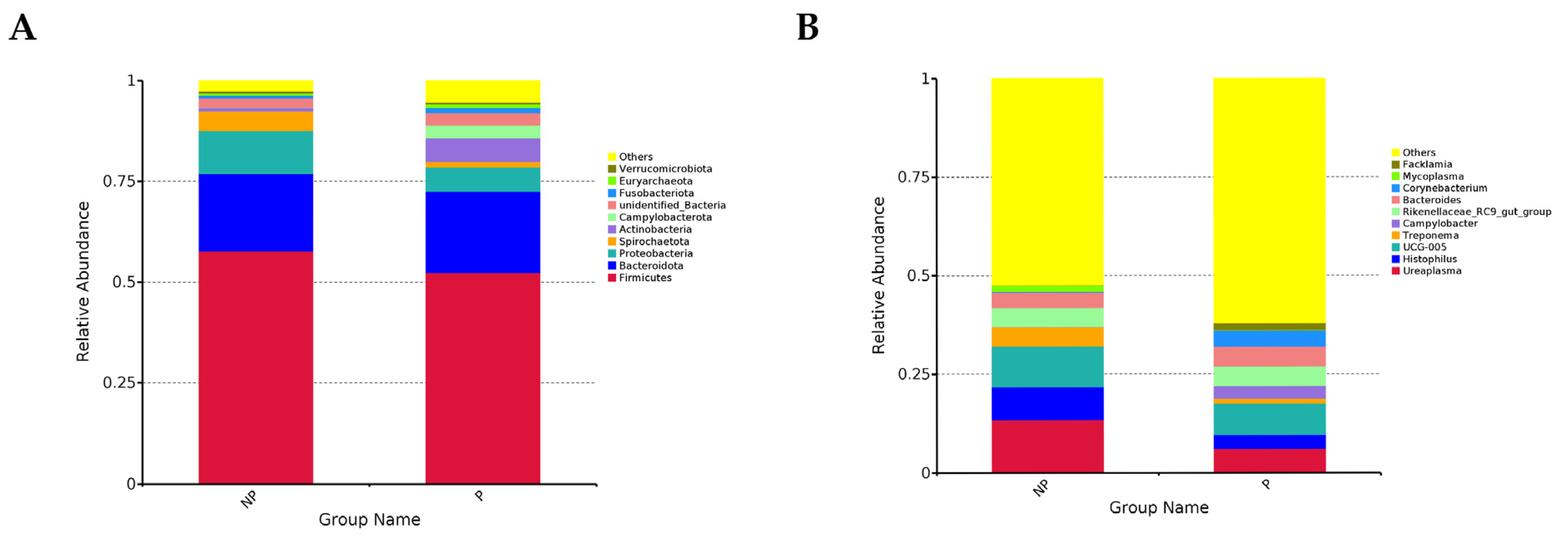
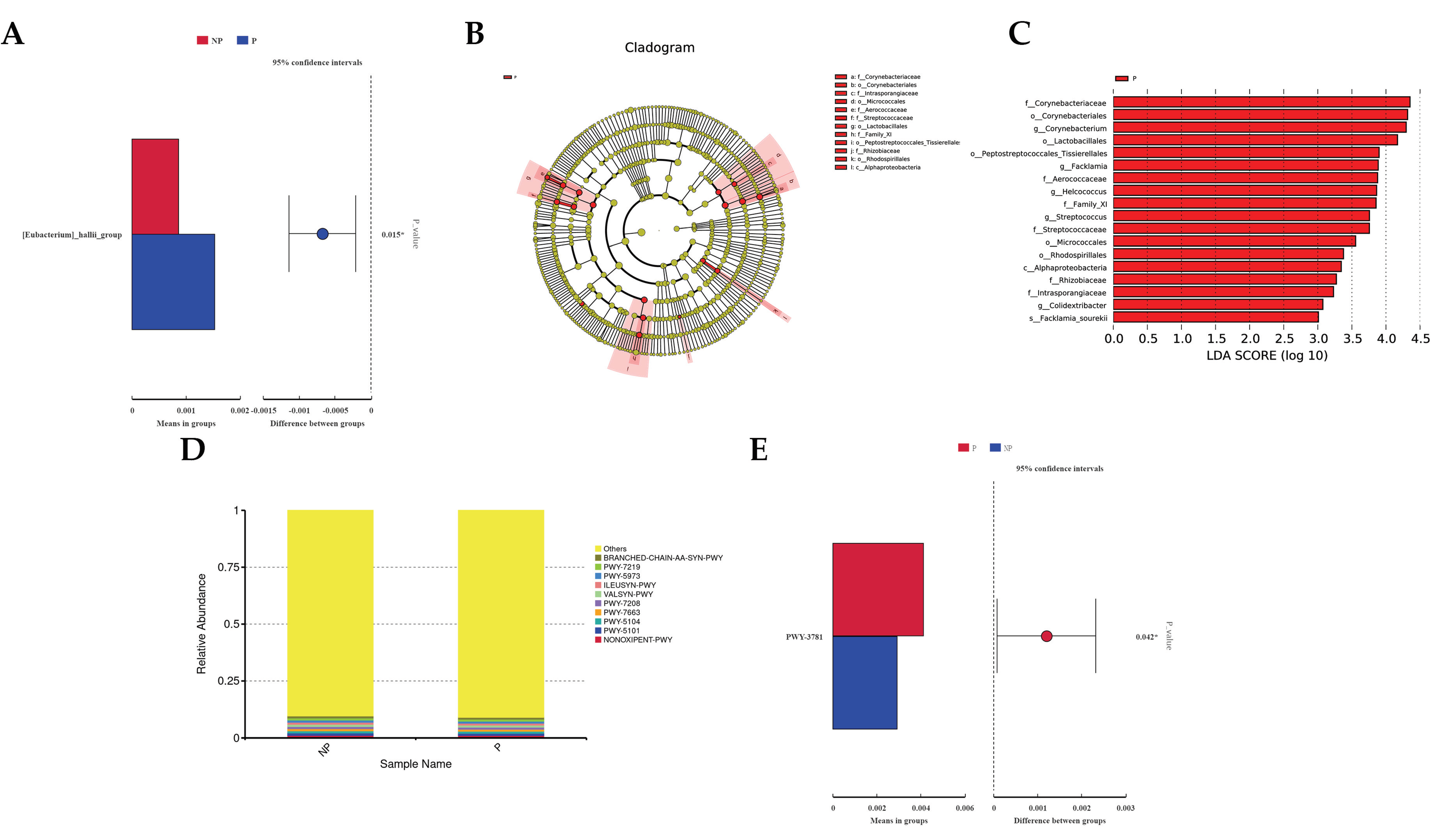

| Groups | Chao1 | Shannon | Simpson |
|---|---|---|---|
| NP | 1776.93 ± 215.56 | 8.52 ± 0.62 | 0.98 ± 0.02 |
| P | 1706.72 ± 209.55 | 7.69 ± 2.47 | 0.93 ± 0.11 |
| p-value | 0.71 | 0.60 | 0.48 |
| Number | Metabolite | m/z | RT | VIP | p-Value | Fold Change | AUC |
|---|---|---|---|---|---|---|---|
| 1 | Bonactin | 399.22 | 30.396 | 1.472 | 0.007 | 3.053 | 0.972 |
| 2 | 2-deoxy-d-glucose | 187.048 | 262.733 | 1.544 | 0.047 | 2.438 | 0.806 |
| 3 | 2-amino-2-methyl-1-propanol | 90.091 | 594.255 | 3.729 | 0.006 | 1.562 | 0.833 |
| 4 | Cabergoline | 452.314 | 218.311 | 3.398 | 0.049 | 1.246 | 0.917 |
| 5 | Guanidinoethyl sulfonate | 166.018 | 207.754 | 1.752 | 0.042 | 1.2 | 0.833 |
| 6 | 3,3′-dimethoxybenzidine | 245.15 | 328.868 | 1.606 | 0.039 | 1.18 | 0.861 |
| 7 | 3-pyridinecarboxaldehyde | 108.055 | 366.259 | 1.073 | 0.03 | 1.175 | 0.889 |
| 8 | Phosphoric acid | 96.96 | 445.631 | 3.755 | 0.019 | 0.927 | 0.861 |
| 9 | Piperidine | 86.097 | 290.788 | 3.835 | 0.039 | 0.869 | 0.806 |
| 10 | 1-o-hexadecyl-2-o-(4z,7z,10z,13z,16z,19z-docosahexaenoyl) -sn-glyceryl-3-phosphorylcholine | 792.59 | 38.166 | 1.688 | 0.029 | 0.828 | 0.861 |
| 11 | Pc 38:4 | 868.606 | 167.263 | 1.316 | 0.038 | 0.798 | 0.861 |
| 12 | Propionylcarnitine | 218.139 | 307.294 | 7.282 | 0.005 | 0.629 | 0.944 |
| 13 | D-2-aminobutyric acid | 104.071 | 504.695 | 3.585 | 0.044 | 0.574 | 0.806 |
| 14 | Leukotriene b4 | 339.232 | 30.839 | 2.312 | 0.019 | 0.558 | 0.917 |
| 15 | Pro-hyp | 229.118 | 448.612 | 3.526 | 0 | 0.348 | 1 |
| 16 | 2-aminophenol | 110.071 | 446.875 | 3.163 | 0.014 | 0.319 | 0.806 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luo, Y.; Wang, Z.; Zhao, X.; Xing, J.; Chen, Z.; Zhao, W.; Long, X.; Zhang, Y.; Shao, Y. Combining the Vaginal Microbiome and Serum Metabolome to Screen for Potential Biomarkers of Early Pregnancy in Cows. Metabolites 2024, 14, 469. https://doi.org/10.3390/metabo14090469
Luo Y, Wang Z, Zhao X, Xing J, Chen Z, Zhao W, Long X, Zhang Y, Shao Y. Combining the Vaginal Microbiome and Serum Metabolome to Screen for Potential Biomarkers of Early Pregnancy in Cows. Metabolites. 2024; 14(9):469. https://doi.org/10.3390/metabo14090469
Chicago/Turabian StyleLuo, Yan, Zhen Wang, Xin Zhao, Jiankang Xing, Zhiliang Chen, Wenxue Zhao, Xiaoqing Long, Yanbing Zhang, and Yongbin Shao. 2024. "Combining the Vaginal Microbiome and Serum Metabolome to Screen for Potential Biomarkers of Early Pregnancy in Cows" Metabolites 14, no. 9: 469. https://doi.org/10.3390/metabo14090469
APA StyleLuo, Y., Wang, Z., Zhao, X., Xing, J., Chen, Z., Zhao, W., Long, X., Zhang, Y., & Shao, Y. (2024). Combining the Vaginal Microbiome and Serum Metabolome to Screen for Potential Biomarkers of Early Pregnancy in Cows. Metabolites, 14(9), 469. https://doi.org/10.3390/metabo14090469







