Drug or Toxic? A Brief Understanding of the Edible Corolla of Rhododendron decorum Franch. by Bai Nationality with Comparative Metabolomics Analysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Collection
2.2. Metabolite Extraction and Identification by UPLC-ESI-MS/MS
2.3. Data Analysis
3. Results
3.1. Metabolite Identification and Annotation
3.2. Differential Metabolomic Profiling
3.2.1. Differential Metabolomic Profiling of Floral Organs
3.2.2. Differential Metabolomic Profiling in Distinct Geographical Regions
3.3. Comparison of Selected Discriminated Metabolites
3.4. Functional Annotation and Enrichment Analysis of Differential Metabolites
3.4.1. Classification of Differential Metabolite Pathways
3.4.2. Enrichment Analysis of Differential Metabolites
3.4.3. Network Analysis for Enrichment of DAMs
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Li, Z.; Yang, Q.; Dong, X.; Zhu, Y.; Zhao, S.; Zhang, W.; Wang, S. Transcriptome analysis of flower color variation in five Rhododendron species (Ericaceae). Braz. J. Bot. 2021, 44, 685–695. [Google Scholar] [CrossRef]
- Mo, Z.; Fu, C.; Zhu, M.; Milne, R.; Yang, J.; Cai, J.; Qin, H.; Zheng, W.; Hollingsworth, P.; Li, D.; et al. Resolution, conflict and rate shifts: Insights from a densely sampled plastome phylogeny for Rhododendron (Ericaceae). Ann. Bot. 2022, 130, 687–701. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.N.; Zhang, H.P.; Wang, L.Q.; Bao, G.H.; Qin, G.W. Diterpenoids from the flowers of Rhododendron molle. J. Nat. Prod. 2004, 67, 1903–1906. [Google Scholar] [CrossRef]
- Zhang, Z.R.; Zhong, J.D.; Li, H.M.; Li, H.Z.; Li, R.T.; Deng, X.L. Two new grayanane diterpenoids from the flowers of Rhododendron molle. J. Asian Nat. Prod. Res. 2012, 14, 764–768. [Google Scholar] [CrossRef]
- Zhu, Y.X.; Zhang, Z.X.; Zhang, H.P.; Chai, L.S.; Li, L.; Ma, S.G.; Li, Y. A new ascorbic acid derivative and two new terpenoids from the leaves and twigs of Rhododendron decorum. J. Asian Nat. Prod. Res. 2019, 21, 579–586. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.X.; Zhang, Z.X.; Yan, H.M.; Lu, D.; Zhang, H.P.; Li, L.; Liu, Y.B.; Li, Y. Antinociceptive Diterpenoids from the Leaves and Twigs of Rhododendron decorum. J. Nat. Prod. 2018, 81, 1183–1192. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.J.; Su, H.G.; Peng, X.R.; Bi, H.C.; Qiu, M.H. An updated review of the genus Rhododendron since 2010: Traditional uses, phytochemistry, and pharmacology. Phytochemistry 2024, 217, 113899. [Google Scholar] [CrossRef]
- Vengryte, M.; Raudone, L. Phytochemical Profiling and Biological Activities of Rhododendron Subsect. Ledum: Discovering the Medicinal Potential of Labrador Tea Species in the Northern Hemisphere. Plants 2024, 13, 901. [Google Scholar] [CrossRef]
- Song, Z.F.; Zheng, X.W.; Xue, Q.W.; Qin, J.X.; Fang, X.; Liang, S. The basic chemical substances of three medicinal parts from Rhododendron molle G. Don. Nat. Prod. Res. 2022, 36, 4498–4501. [Google Scholar] [CrossRef]
- Deepak, H.V.; Swamy, M.M.M.; Murai, Y.; Suga, Y.; Anetai, M.; Yo, T.; Kuragano, M.; Uwai, K.; Tokuraku, K.; Monde, K. Daurichromenic Acid from the Chinese Traditional Medicinal Plant Rhododendron dauricum Inhibits Sphingomyelin Synthase and Aβ Aggregation. Molecules 2020, 25, 4077. [Google Scholar] [CrossRef]
- Rapinski, M.; Musallam, L.; Arnason, J.T.; Haddad, P.; Cuerrier, A. Adipogenic Activity of Wild Populations of Rhododendron groenlandicum, a Medicinal Shrub from the James Bay Cree Traditional Pharmacopeia. Evid-Based Compl Alt. 2015, 2015, 492458. [Google Scholar] [CrossRef]
- Eid, H.M.; Ouchfoun, M.; Saleem, A.; Guerrero-Analco, J.A.; Walshe-Roussel, B.; Musallam, L.; Rapinski, M.; Cuerrier, A.; Martineau, L.C.; Arnason, J.T.; et al. A combination of (+)-catechin and (−)-epicatechin underlies the in vitro adipogenic action of Labrador tea (Rhododendron groenlandicum), an antidiabetic medicinal plant of the Eastern James Bay Cree pharmacopeia. J. Ethnopharmacol. 2016, 178, 251–257. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.X.; Zhou, M.; Zhang, Y.; Fu, Y.; Li, J.W.; Yang, X.F. Poisonous delicacy: Market-oriented surveys of the consumption of Rhododendron flowers in Yunnan, China. J. Ethnopharmacol. 2021, 265, 113320. [Google Scholar] [CrossRef] [PubMed]
- Baral, S.; Baral, B.K.; Sharma, P.; Shrestha, S.L. Dried rhododendron flower ingestion presenting with bradycardia and hypotension: A case report. J. Med. Case Rep. 2022, 16, 189. [Google Scholar] [CrossRef]
- Li-Beisson, Y.; Hirai, M.Y.; Nakamura, Y. Plant metabolomics. J. Exp. Bot. 2024, 75, 1651–1653. [Google Scholar] [CrossRef] [PubMed]
- Horn, P.J.; Chapman, K.D. Imaging plant metabolism in situ. J. Exp. Bot. 2024, 75, 1654–1670. [Google Scholar] [CrossRef] [PubMed]
- Elattar, M.M.; Hammoda, H.M.; Ghareeb, D.A.; Abdulmalek, S.A.; Abdelrahim, F.A.; Seif, I.A.K.; Dawood, H.M.; Darwish, R.S. Insights into bioactive constituents of onion (Allium cepa L.) waste: A comparative metabolomics study enhanced by chemometric tools. BMC Complement. Med. Ther. 2024, 24, 271. [Google Scholar] [CrossRef]
- Monari, S.; Ferri, M.; Salinitro, M.; Tassoni, A. New Insights on Primary and Secondary Metabolite Contents of Seven Italian Wild Food Plants with Medicinal Applications: A Comparative Study. Plants 2023, 12, 12183180. [Google Scholar] [CrossRef]
- Guo, Y.; Gao, C.Y.; Wang, M.K.; Fu, F.F.; El-Kassaby, Y.A.; Wang, T.L.; Wang, G.B. Metabolome and transcriptome analyses reveal flavonoids biosynthesis differences in Ginkgo biloba associated with environmental conditions. Ind. Crop Prod. 2020, 1, 158–169. [Google Scholar] [CrossRef]
- Brennan, L. Metabolomics in nutrition research—A powerful window into nutritional metabolism. Essays Biochem. 2016, 60, 451–458. [Google Scholar] [CrossRef]
- Li, B.B.; He, X.Y.; Jia, W.; Li, H.K. Novel Applications of Metabolomics in Personalized Medicine: A Mini-Review. Molecules 2017, 22, 1173. [Google Scholar] [CrossRef]
- Duan, L.; Guo, L.; Wang, L.; Yin, Q.; Zhang, C.M.; Zheng, Y.G.; Liu, E.H. Application of metabolomics in toxicity evaluation of traditional Chinese medicines. Chin. Med. 2018, 13, 60. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Zhang, J.-L.; Li, F. Application of Metabolomics in the Study of Natural Products. Nat. Prod. Bioprosp. 2018, 8, 321–334. [Google Scholar] [CrossRef]
- González-Peña, D.; Brennan, L. Recent Advances in the Application of Metabolomics for Nutrition and Health. Annu. Rev. Food Sci. 2019, 10, 479–519. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.H.; Zhang, L.Z.; Zheng, X.; Huang, Q.H.; Farag, M.A.; Zhu, R.Y.; Zhao, C. Emerging applications of metabolomics in food science and future trends. Food Chem. 2022, 16, 100500. [Google Scholar] [CrossRef] [PubMed]
- Fraga, C.G.; Clowers, B.H.; Moore, R.J.; Zink, E.M. Signature-Discovery Approach for Sample Matching of a Nerve-Agent Precursor Using Liquid Chromatography-Mass Spectrometry, XCMS, and Chemometrics. Anal. Biochem. 2010, 82, 4165–4173. [Google Scholar] [CrossRef]
- Gong, F.; Yu, W.; Zeng, Q.; Dong, J.; Cao, K.; Xu, H.; Zhou, X. Rhododendron chrysanthum’s Primary Metabolites Are Converted to Phenolics More Quickly When Exposed to UV-B Radiation. Biomolecules 2023, 13, 1700. [Google Scholar] [CrossRef]
- Kanehisa, M.; Goto, S. KEGG: Kyoto encyclopedia of genes and genomes. Nucleic Acids Res. 2000, 28, 27–30. [Google Scholar] [CrossRef]
- David, S.W.; Yannick, D.F.; Ana, M.; An, C.G.; Kevin, L.; Rosa, V.F.; Tanvir, S.; Daniel, J.; Carin, L.; Naama, K.; et al. HMDB 4.0: The human metabolome database for 2018. Nucleic Acids Res. 2018, 46, D608–D617. [Google Scholar] [CrossRef]
- Conroy, M.J.; Andrews, R.M.; Andrews, S.; Cockayne, L.; Dennis, E.A.; Fahy, E.; Gaud, C.; Griffiths, W.J.; Jukes, G.; Kolchin, M.; et al. LIPID MAPS: Update to databases and tools for the lipidomics community. Nucleic Acids Res. 2024, 52, D1677–D1682. [Google Scholar] [CrossRef]
- Thévenot, E.A.; Roux, A.; Xu, Y.; Ezan, E.; Junot, C. Analysis of the Human Adult Urinary Metabolome Variations with Age, Body Mass Index, and Gender by Implementing a Comprehensive Workflow for Univariate and OPLS Statistical Analyses. J. Proteome Res. 2015, 14, 3322–3335. [Google Scholar] [CrossRef]
- Yu, G.C.; Wang, L.G.; Han, Y.Y.; He, Q.Y. clusterProfiler: An R Package for Comparing Biological Themes Among Gene Clusters. Omics 2012, 16, 284–287. [Google Scholar] [CrossRef] [PubMed]
- Hakimi, A.A.; Reznik, E.; Lee, C.H.; Creighton, C.J.; Brannon, A.R.; Luna, A.; Aksoy, B.A.; Liu, E.M.; Shen, R.L.; Lee, W.; et al. An Integrated Metabolic Atlas of Clear Cell Renal Cell Carcinoma. Cancer Cell 2016, 29, 104–116. [Google Scholar] [CrossRef]
- Popescu, R.; Kopp, B. The genus Rhododendron: An ethnopharmacological and toxicological review. J. Ethnopharmacol. 2013, 147, 42–62. [Google Scholar] [CrossRef] [PubMed]
- Tasdemir, D.; Cartagena, M.; Carballeira, N.M. Comparative analyses on fatty acid composition of bioactive organic extracts from different anatomical organs of five Turkish Rhododendron species. Planta Med. 2007, 73, 948. [Google Scholar] [CrossRef]
- Ye, L.J.; Möller, M.; Luo, Y.H.; Zou, J.Y.; Zheng, W.; Liu, J.; Li, D.Z.; Gao, L.M. Variation in gene expression along an elevation gradient of Rhododendron sanguineum var. haemaleum assessed in a comparative transcriptomic analysis. Front. Plant Sci. 2023, 14, 1133065. [Google Scholar] [CrossRef]
- Yokoyama, K.; Araki, Y.; Ito, E. Biosynthesis of poly (Galactosylglycerol phosphate) in bacillus-coagulans. Eur. J. Biochem. 1987, 165, 47–53. [Google Scholar] [CrossRef]
- Sweeney, R.P.; Lowary, T.L. A Route to Polyprenol Pyrophosphate-Based Probes of O-Polysaccharide Biosynthesis in Klebsiella pneumoniae O2a. Org. Lett. 2019, 21, 1050–1053. [Google Scholar] [CrossRef]
- Hendrich, A.B. Flavonoid-membrane interactions: Possible consequences for biological effects of some polyphenolic compounds. Acta Pharmacol. Sin. 2006, 27, 27–40. [Google Scholar] [CrossRef]
- Beekmann, K.; Actis-Goretta, L.; van Bladeren, P.J.; Dionisi, F.; Destaillats, F.; Rietjens, I. A state-of-the-art overview of the effect of metabolic conjugation on the biological activity of flavonoids. Food Funct. 2012, 3, 1008–1018. [Google Scholar] [CrossRef]
- Li, C.H.; Dai, T.T.; Chen, J.; Chen, M.S.; Liang, R.H.; Liu, C.M.; Du, L.Q.; McClements, D.J. Modification of flavonoids: Methods and influences on biological activities. Crit. Rev. Food Sci. 2023, 63, 10637–10658. [Google Scholar] [CrossRef]
- Yao, Z.J.; Wu, Q.; Sheng, W.B.; Zhou, X.D.; Cheng, L.D.; Tian, X.; Yuan, H.W.; Gong, L.M.; Wang, W.; Li, B.; et al. Flavonoidal alkaloids: Emerging targets for drug discovery from Nature's bounty. Fitoterapia 2024, 177, 106099. [Google Scholar] [CrossRef] [PubMed]
- Gu, J.Y.; Dong, Y.C. Preparing Multi-Functional Protein Textiles by the Flavonoid-Rich Ginkgo biloba L. Leaf Extracts. J. Nat. Fibers 2024, 21, 2296908. [Google Scholar] [CrossRef]
- Gowd, V.; Kass, J.D.; Sarkar, N.; Ramakrishnan, P. Role of Sam68 as an adaptor protein in inflammatory signaling. Cell Mol. Life Sci. 2024, 81, 89. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.R.; Chen, M.; Lee, J.D.; Zhang, J.F.; Lin, S.Y.; Fu, T.M.; Chen, H.; Ishikawa, T.; Chiang, S.Y.; Katon, J.; et al. Reactivation of PTEN tumor suppressor for cancer treatment through inhibition of a MYC-WWPI inhibitory pathway. Science 2019, 364, 651. [Google Scholar] [CrossRef] [PubMed]
- Liou, C.S.; Sirk, S.J.; Diaz, C.A.C.; Klein, A.P.; Fischer, C.R.; Higginbottom, S.K.; Erez, A.; Donia, M.S.; Sonnenburg, J.L.; Sattely, E.S. A Metabolic Pathway for Activation of Dietary Glucosinolates by a Human Gut Symbiont. Cell 2020, 180, 717. [Google Scholar] [CrossRef]
- Kim, Y.C.; Cha, A.; Hussain, M.; Lee, K.; Lee, S. Impact of Agrobacterium-infiltration and transient overexpression of BroMYB28 on glucoraphanin biosynthesis in broccoli leaves. Plant Biotechnol. Rep. 2020, 14, 373–380. [Google Scholar] [CrossRef]
- Tsuji, P.A.; Walle, T. Cytotoxic effects of the dietary flavones chrysin and apigenin in a normal trout liver cell line. Chem-Biol. Interact. 2008, 171, 37–44. [Google Scholar] [CrossRef]
- Li, N.; Liu, J.H.; Zhang, J.; Yu, B.Y. Comparative evaluation of cytotoxicity and antioxidative activity of 20 flavonoids. J. Agric. Food Chem. 2008, 56, 3876–3883. [Google Scholar] [CrossRef]
- Tang, C.Y.; Fan, Y.J.; Wang, T.; Wang, J.; Xiao, M.J.; He, M.; Chang, X.Y.; Li, Y.L.; Li, X.Z. Metabolomic Profiling of Floccularia luteovirens from Different Geographical Regions Proposes a Novel Perspective on Their Antioxidative Activities. Antioxidants 2024, 13, 620. [Google Scholar] [CrossRef]
- Fattorini, R.; Egan, P.A.; Rosindell, J.; Farrell, I.W.; Stevenson, P.C. Grayanotoxin I variation across tissues and species of Rhododendron suggests pollinator-herbivore defence trade-offs. Phytochemistry 2023, 212, 113707. [Google Scholar] [CrossRef] [PubMed]
- Rezk, A.; Al-Hashimi, A.; John, W.; Schepker, H.; Ullrich, M.S.; Brix, K. Assessment of cytotoxicity exerted by leaf extracts from plants of the genus Rhododendron towards epidermal keratinocytes and intestine epithelial cells. BMC Complem. Altern. 2015, 15, 364. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Huang, L.Q. Molecular Pharmacognosy in Daodi herbs. Chin. Sci. B-Chin. 2020, 65, 1093–1102. [Google Scholar] [CrossRef]
- Zhang, C.; Zhao, M.; Jiang, B.; Yu, J.; Hao, Q.F.; Liu, W.X.; Hu, Z.; Zhang, Y.M.; Song, C. Extraction optimization, structural characterization and potential alleviation of hyperuricemia by flavone glycosides from celery seeds. Food Funct. 2022, 13, 9832–9846. [Google Scholar] [CrossRef]
- Epifanio, N.M.D.; Cavalcanti, L.R.I.; dos Santos, K.F.; Duarte, P.S.C.; Kachlicki, P.; Ozarowski, M.; Riger, C.J.; Chaves, D.S.D. Chemical characterization and in vivo antioxidant activity of parsley (Petroselinum crispum) aqueous extract. Food Funct. 2020, 11, 5346–5356. [Google Scholar] [CrossRef]
- Prajapati, K.P.; Singh, A.P.; Dubey, K.; Ansari, M.; Temgire, M.; Anand, B.G.; Kar, K. Myricetin inhibits amyloid fibril formation of globular proteins by stabilizing the native structures. Colloid. Surf. B 2020, 186, 110640. [Google Scholar] [CrossRef]
- Nakazato, T.; Ito, K.; Ikeda, Y.; Kizaki, M. Green tea component, catechin, induces apoptosis of human malignant B cells via production of reactive oxygen species. Clin. Cancer Res. 2005, 11, 6040–6049. [Google Scholar] [CrossRef]
- Wang, N.; He, J.W.; Chang, A.K.; Wang, Y.; Xu, L.N.; Chong, X.Y.; Lu, X.; Sun, Y.H.; Xia, X.C.; Li, H.; et al. (−)-Epigallocatechin-3-gallate Inhibits Fibrillogenesis of Chicken Cystatin. J. Agric. Food Chem. 2015, 63, 1347–1351. [Google Scholar] [CrossRef]
- Lee, P.; Tan, K.S. Effects of Epigallocatechin gallate against Enterococcus faecalis biofilm and virulence. Arch. Oral. Biol. 2015, 60, 393–399. [Google Scholar] [CrossRef]
- Chung, S.S.; Vadgama, J.V. Curcumin and Epigallocatechin Gallate Inhibit the Cancer Stem Cell Phenotype via Down-regulation of STAT3-NFκB Signaling. Anticancer Res. 2015, 35, 39–46. [Google Scholar]
- Li, G.Y.; Xie, C.Y.; Wang, Q.H.; Wan, D.; Zhang, Y.; Wu, X.; Yin, Y.L. Uridine/UMP metabolism and their function on the gut in segregated early weaned piglets. Food Funct. 2019, 10, 4081–4089. [Google Scholar] [CrossRef] [PubMed]
- Mehra, R.; Kumar, H.; Kumar, N.; Kaushik, R. Red rice conjugated with barley and rhododendron extracts for new variant of beer. J. Food Sci. Technol. Mys 2020, 57, 4152–4159. [Google Scholar] [CrossRef] [PubMed]



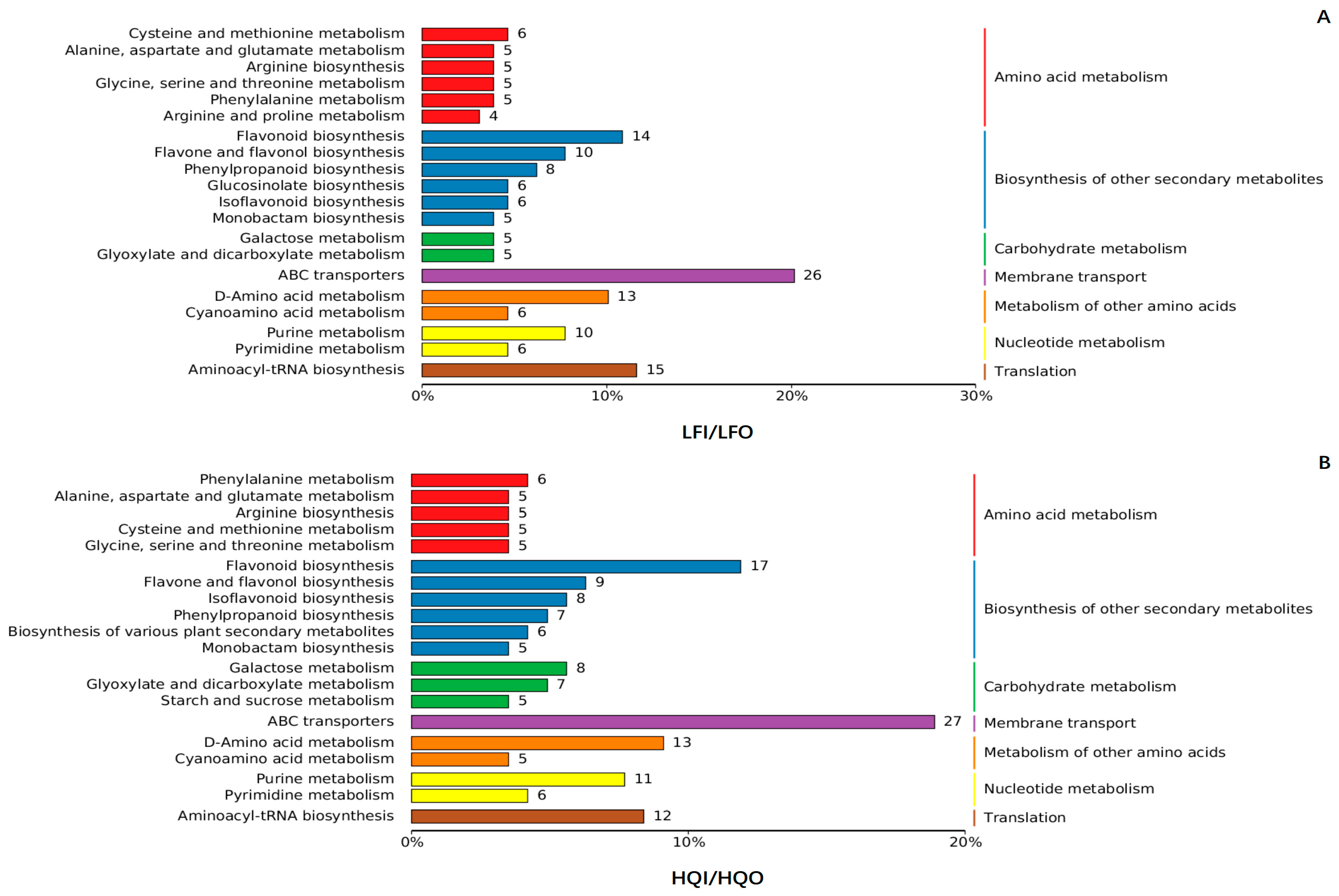
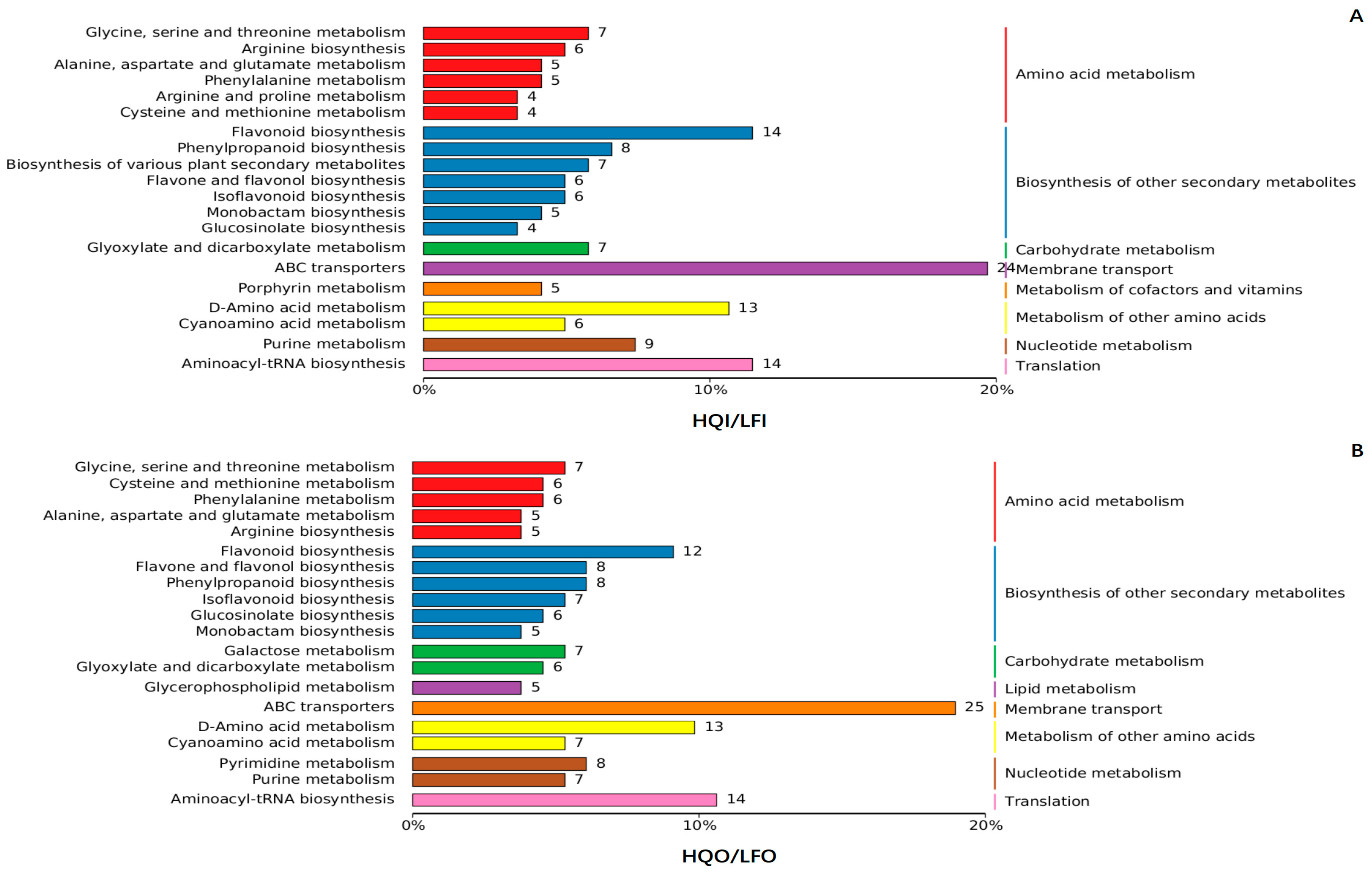
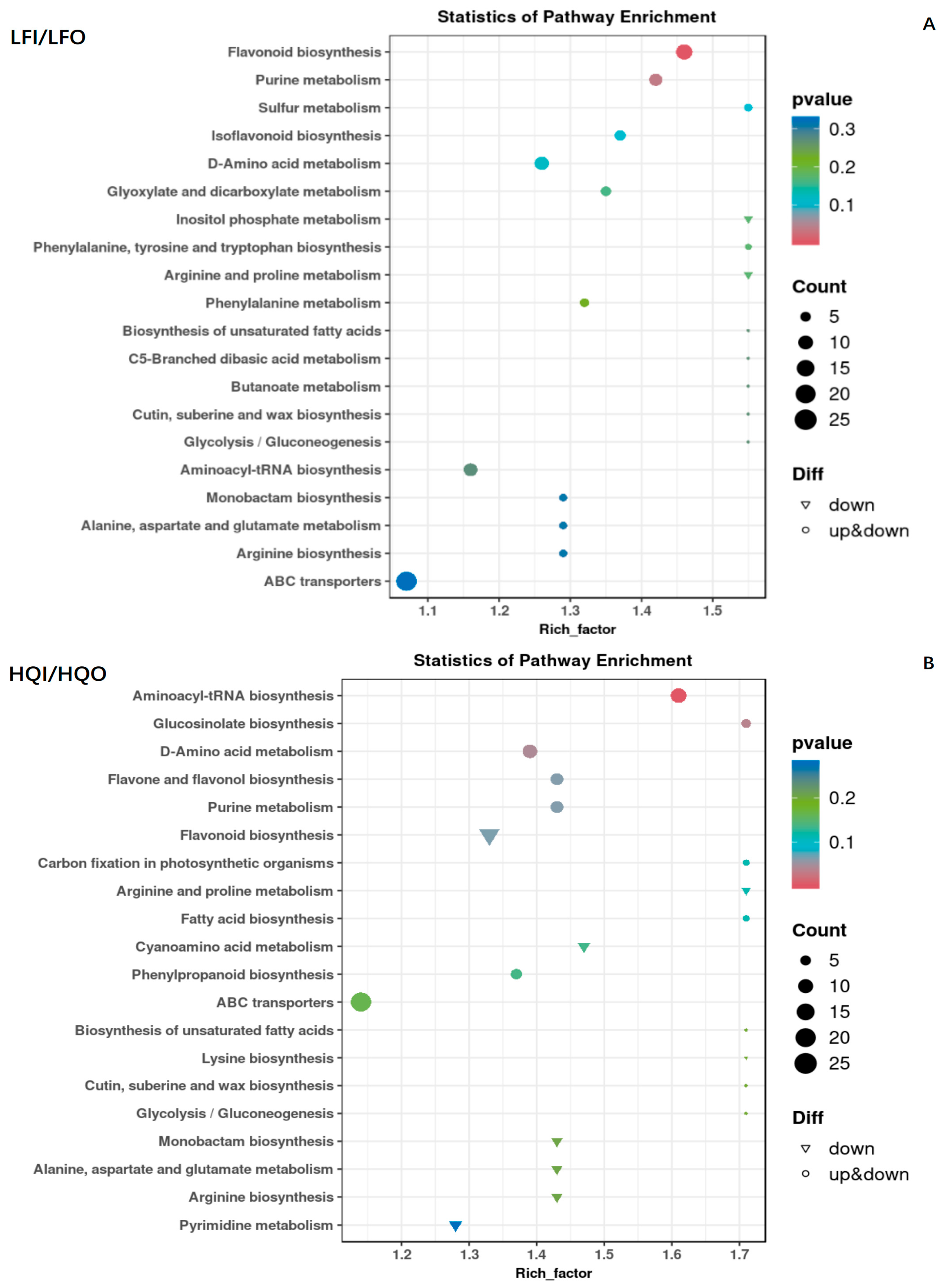



| Superclass | Class (HMDB_Taxonomy) | Metabolite | HQI/HQO | LFI/LFO | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| FC (ABS) | p-Value | VIP | Regulated | FC (ABS) | p-Value | VIP | Regulated | |||
| Alkaloids and derivatives (3) | Riddelline | 13.84 | 0.01 | 1.18 | up | 16.09 | 0.00 | 1.15 | up | |
| Acetyl-L-Carnitine (Hydrochloride) | 13.92 | 0.00 | 1.15 | up | ||||||
| Salsolidine | 14.72 | 0.02 | 1.12 | down | ||||||
| Benzenoids (7) | Benzene and substituted derivatives | Ethyl Pivaloylacetate | 17.06 | 0.00 | 1.20 | down | 14.58 | 0.00 | 1.16 | down |
| Dibenzyl Disulfide | 17.55 | 0.00 | 1.20 | down | ||||||
| 2,6-Dihydroxybenzoic Acid | 17.04 | 0.00 | 1.20 | down | ||||||
| 4-Acetoxy-3,5-Dimethoxybenzoic Acid | 11.08 | 0.03 | 1.09 | up | ||||||
| Ethyl 2,4,6-Trihydroxybenzoate | 10.62 | 0.04 | 1.07 | down | ||||||
| Naphthalenes | Apiin | 13.26 | 0.05 | 1.09 | up | |||||
| Phenols | Syringylpropane | 15.94 | 0.02 | 1.12 | down | |||||
| Lipids and lipid-like molecules (15) | Fatty acyls | 2,6,4′-Trihydroxy-4-Methoxybenzophenone | 15.80 | 0.00 | 1.20 | up | 16.97 | 0.00 | 1.16 | up |
| 9(S)-Hotre | 13.03 | 0.00 | 1.20 | down | 12.98 | 0.00 | 1.16 | down | ||
| Suberic Acid | 20.33 | 0.00 | 1.16 | up | ||||||
| Dodecanedioic Acid | 15.37 | 0.00 | 1.16 | down | ||||||
| Glycerophospholipids | (2S,3R,4S)-4-Hydroxyisoleucine | 10.37 | 0.00 | 1.20 | up | |||||
| Prenol lipids | Menaquinone-4 | 15.40 | 0.00 | 1.20 | down | 15.06 | 0.00 | 1.16 | down | |
| Isopulegol | 14.93 | 0.01 | 1.18 | down | 15.84 | 0.00 | 1.15 | down | ||
| Shanzhiside | 13.50 | 0.00 | 1.20 | down | 10.54 | 0.01 | 1.13 | down | ||
| Monotropein | 20.81 | 0.00 | 1.20 | down | ||||||
| Carvacrol | 15.04 | 0.00 | 1.20 | down | ||||||
| Oleoside | 11.40 | 0.00 | 1.20 | down | ||||||
| (R)-(+)-Citronellal | 16.13 | 0.00 | 1.16 | up | ||||||
| Geniposidic Acid | 11.86 | 0.05 | 1.06 | down | ||||||
| Steroids and steroid derivatives | 2-Phenylethylamine (Hydrochloride) | 11.45 | 0.00 | 1.20 | down | |||||
| Ambolic acid | 10.63 | 0.00 | 1.15 | up | ||||||
| Nucleosides, nucleotides and analogues (3) | Uridine 5′-Monophosphate Disodium Salt | 14.09 | 0.00 | 1.15 | down | |||||
| Purine nucleosides | 8-Hydroxyguanosine | 10.38 | 0.02 | 1.16 | down | |||||
| Pyrimidine nucleotides | Uridine 5′-Monophosphate | 13.62 | 0.00 | 1.20 | down | |||||
| Organic acids and derivatives (6) | Carboxylic acids and derivatives | D-Ornithine (Hydrochloride) | 15.27 | 0.00 | 1.20 | down | ||||
| L-Phenylalanyl-L-Tryptophan (Phenylalanyltryptophan) | 12.06 | 0.02 | 1.16 | down | ||||||
| H-D-Cis-Hyp-OH | 15.04 | 0.01 | 1.14 | down | ||||||
| Triglochinic Acid | 12.06 | 0.00 | 1.16 | down | ||||||
| Cinnamoylglycine | 10.55 | 0.00 | 1.16 | down | ||||||
| Hydroxy acids and derivatives | 3-Hydroxyglutaric Acid | 13.91 | 0.00 | 1.20 | up | |||||
| Organic oxygen compounds (11) | Organooxygen compounds | Quininic Acid | 12.24 | 0.03 | 1.14 | up | 12.77 | 0.00 | 1.15 | up |
| Nicotinamide Riboside (Chloride) | 20.58 | 0.02 | 1.15 | down | 18.02 | 0.04 | 1.08 | down | ||
| Cornuside | 12.04 | 0.00 | 1.20 | down | 14.94 | 0.01 | 1.14 | down | ||
| Sequoyitol | 17.14 | 0.00 | 1.20 | down | ||||||
| Acetylpyrazine | 16.11 | 0.00 | 1.16 | up | ||||||
| 4-Hydroxyacetophenone | 15.66 | 0.00 | 1.16 | up | ||||||
| Fructose | 11.82 | 0.03 | 1.09 | up | ||||||
| Inositol | 18.63 | 0.02 | 1.12 | down | ||||||
| Primin-1 | 14.43 | 0.01 | 1.14 | down | ||||||
| 3-Hydroxy-4-Methoxyacetophenone | 14.29 | 0.00 | 1.15 | down | ||||||
| Styrene-Cis-2,3-Dihydrodiol | 12.73 | 0.00 | 1.19 | down | ||||||
| Organoheterocyclic compounds (2) | Pyridines and derivatives | Nicotinamide | 14.43 | 0.00 | 1.20 | up | 15.78 | 0.00 | 1.16 | up |
| Quinolines and derivatives | (2S,3S)-2-(3,4,5-trihydroxyphenyl)-3,4-dihydro-2H-chromene-3,5,7-triol | 10.34 | 0.00 | 1.16 | down | |||||
| Phenylpropanoids and polyketides (36) | 2-Arylbenzofuran flavonoids | Moracin C | 12.14 | 0.01 | 1.18 | down | ||||
| Cinnamic acids and derivatives | (E)-M-Coumaric Acid | 16.70 | 0.00 | 1.20 | up | 17.01 | 0.00 | 1.15 | up | |
| p-Coumaryl Alcohol | 11.37 | 0.02 | 1.16 | up | 14.24 | 0.00 | 1.16 | up | ||
| Ferulamide | 17.32 | 0.00 | 1.19 | up | ||||||
| (E)-Ferulic Acid | 19.47 | 0.00 | 1.20 | down | ||||||
| Calceolarioside B | 13.67 | 0.03 | 1.10 | down | ||||||
| p-Hydroxyphenethyl Trans-Ferulate | 10.64 | 0.00 | 1.15 | down | ||||||
| Coumarins and derivatives | Cichoriin | 12.72 | 0.00 | 1.19 | down | |||||
| Norbraylin | 14.61 | 0.00 | 1.15 | up | ||||||
| Herniarin | 14.55 | 0.00 | 1.16 | up | ||||||
| 5,7,8-Trimethoxycoumarin | 15.17 | 0.03 | 1.09 | down | ||||||
| Flavonoids | Magnolioside | 13.82 | 0.00 | 1.20 | up | 16.84 | 0.00 | 1.16 | up | |
| Aristolone-2 | 16.49 | 0.01 | 1.17 | up | 16.80 | 0.01 | 1.13 | up | ||
| Tricetin | 17.65 | 0.01 | 1.19 | down | 18.88 | 0.00 | 1.15 | down | ||
| Herbacetin | 16.70 | 0.03 | 1.15 | down | 16.85 | 0.01 | 1.15 | down | ||
| (+)-Gallocatechin | 14.66 | 0.00 | 1.20 | down | 16.57 | 0.00 | 1.16 | down | ||
| 5-Hydroxy-7,8-Dimethoxy (2R)-Flavanone-5-O-β-D-Glucopyranoside | 13.90 | 0.00 | 1.20 | down | 13.06 | 0.00 | 1.15 | down | ||
| Isoquercetin | 20.58 | 0.02 | 1.16 | up | ||||||
| 5,7-Dimethoxyluteolin | 16.40 | 0.00 | 1.20 | up | ||||||
| Neoisoastilbin | 24.03 | 0.00 | 1.20 | down | ||||||
| Quercetagitrin | 20.24 | 0.01 | 1.17 | down | ||||||
| (+)-Taxifolin | 17.20 | 0.00 | 1.20 | down | ||||||
| Taxifolin | 17.01 | 0.00 | 1.20 | down | ||||||
| Quercetin 3-O-Neohesperidoside | 15.51 | 0.00 | 1.20 | down | ||||||
| Velutin | 13.82 | 0.01 | 1.19 | down | ||||||
| Vicenin 2 | 12.47 | 0.01 | 1.18 | down | ||||||
| Methyllinderone | 11.55 | 0.00 | 1.20 | down | ||||||
| 2-Phenylethylamine | 10.62 | 0.00 | 1.20 | down | ||||||
| (−)-Epigallocatechin | 16.37 | 0.00 | 1.15 | down | ||||||
| Cyanidin 3-Rutinoside | 15.88 | 0.02 | 1.11 | down | ||||||
| Methylnissolin-3-O-Glucoside | 14.49 | 0.00 | 1.16 | down | ||||||
| Tiliroside | 13.98 | 0.01 | 1.13 | down | ||||||
| Theaflavin-3-Gallate | 11.74 | 0.01 | 1.13 | down | ||||||
| Isoflavonoids | Genistein | 10.69 | 0.01 | 1.17 | up | |||||
| Linear 1,3-diarylpropanoids | Loureirin C | 12.21 | 0.00 | 1.16 | down | |||||
| Phenylpropanoic acids | (−)-Catechin Gallate | 19.60 | 0.01 | 1.17 | down | 22.03 | 0.00 | 1.16 | down | |
| Unknown (2) | 2-Hydroxy-3-Methylbenzalpyruvate | 14.97 | 0.01 | 1.14 | up | |||||
| Nudifloside D | 10.27 | 0.01 | 1.13 | down | ||||||
| Superclass | Class (HMDB_Taxonomy) | Metabolite | HQI/LFI | HQO/LFO | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| log2FC (ABS) | p-Value | VIP | Regulated | log2FC (ABS) | p-Value | VIP | Regulated | |||
| Alkaloids and derivatives (2) | Acetyl-L-Carnitine (Hydrochloride) | 11.71 | 0.00 | 1.24 | down | |||||
| Salsolidine | 12.21 | 0.04 | 1.11 | down | ||||||
| Benzenoids (5) | Benzene and substituted derivatives | 4-Methoxycinnamyl Alcohol | 20.72 | 0.00 | 1.23 | up | 21.06 | 0.00 | 1.20 | up |
| 4-Acetoxy-3,5-Dimethoxybenzoic Acid | 20.23 | 0.00 | 1.24 | down | ||||||
| 2,6-Dihydroxybenzoic Acid | 16.09 | 0.00 | 1.20 | up | ||||||
| Dibenzyl Disulfide | 13.90 | 0.00 | 1.20 | up | ||||||
| Phenols | Syringylpropane | 15.94 | 0.02 | 1.20 | up | |||||
| Lipids and lipid-like molecules (11) | Fatty acyls | Suberic Acid | 20.54 | 0.00 | 1.24 | down | ||||
| Dodecanedioic Acid | 14.58 | 0.01 | 1.17 | down | ||||||
| Glycerophospholipids | (2S,3R,4S)-4-Hydroxyisoleucine | 12.66 | 0.01 | 1.22 | up | |||||
| Prenol lipids | (R)-(+)-Citronellal | 15.01 | 0.00 | 1.24 | down | |||||
| Isopimaric Acid | 13.86 | 0.00 | 1.24 | down | ||||||
| α-Boswellic Acid | 13.39 | 0.00 | 1.20 | up | ||||||
| Carvacrol | 12.06 | 0.00 | 1.20 | up | ||||||
| Monotropein | 11.58 | 0.01 | 1.19 | up | ||||||
| Steroids and steroid derivatives | Isomangiferolic Acid | 14.26 | 0.00 | 1.24 | up | 12.35 | 0.00 | 1.20 | up | |
| Estradiol | 10.55 | 0.00 | 1.24 | down | ||||||
| 2-Phenylethylamine (Hydrochloride) | 13.01 | 0.00 | 1.20 | up | ||||||
| Nucleosides, nucleotides and analogues (2) | Pyrimidine nucleotides | Uridine 5′-Monophosphate | 14.64 | 0.00 | 1.20 | up | ||||
| Uridine 5′-Monophosphate Disodium Salt | 13.37 | 0.00 | 1.20 | down | ||||||
| Organic acids and derivatives (6) | Carboxylic acids and derivatives | D-Ornithine (Hydrochloride) | 12.62 | 0.03 | 1.13 | up | ||||
| L-Phenylalanyl-L-Tryptophan | 12.19 | 0.00 | 1.20 | up | ||||||
| H-D-Cis-Hyp-OH | 13.50 | 0.00 | 1.19 | down | ||||||
| Triglochinic Acid | 13.06 | 0.00 | 1.20 | down | ||||||
| Cinnamoylglycine | 12.44 | 0.01 | 1.19 | down | ||||||
| Hydroxy acids and derivatives | 3-Hydroxyglutaric Acid | 14.26 | 0.00 | 1.24 | up | |||||
| Organic nitrogen compounds (1) | Organonitrogen compounds | D-Erythro-Sphingosine | 15.27 | 0.00 | 1.20 | up | ||||
| Organic oxygen compounds (7) | Organooxygen compounds | Sequoyitol | 17.14 | 0.00 | 1.23 | down | ||||
| Acetylpyrazine | 12.07 | 0.00 | 1.24 | down | ||||||
| 4-Hydroxyacetophenone | 11.81 | 0.00 | 1.24 | down | ||||||
| Inositol | 15.95 | 0.02 | 1.17 | down | ||||||
| 3-Hydroxy-4-Methoxyacetophenone | 12.83 | 0.00 | 1.19 | down | ||||||
| Primin-1 | 12.64 | 0.00 | 1.20 | down | ||||||
| Styrene-Cis-2,3-Dihydrodiol | 11.77 | 0.02 | 1.16 | up | ||||||
| Organoheterocyclic compounds (1) | Indoles and derivatives | 2-(4-Hydroxy-2-Oxoindolin-3-Yl) Acetonitrile | 14.24 | 0.00 | 1.23 | down | 14.36 | 0.00 | 1.20 | down |
| Phenylpropanoids and polyketides (29) | Cinnamic acids and derivatives | Ferulamide | 10.37 | 0.00 | 1.24 | up | ||||
| (E)-Ferulic Acid | 15.83 | 0.01 | 1.19 | up | ||||||
| Calceolarioside B | 13.34 | 0.01 | 1.18 | down | ||||||
| Coumarins and derivatives | Herniarin | 14.96 | 0.02 | 1.19 | down | |||||
| Norbraylin | 13.78 | 0.00 | 1.23 | down | ||||||
| Cichoriin | 12.72 | 0.00 | 1.23 | down | ||||||
| 5,7,8-Trimethoxycoumarin | 11.02 | 0.01 | 1.19 | down | ||||||
| Flavonoids | 5-Hydroxy-7,8-Dimethoxyflavanone | 21.19 | 0.00 | 1.24 | down | 22.04 | 0.00 | 1.20 | down | |
| Farrerol | 11.35 | 0.01 | 1.22 | down | 18.44 | 0.00 | 1.20 | down | ||
| Padmatin | 15.79 | 0.00 | 1.23 | down | 16.45 | 0.00 | 1.20 | down | ||
| 4′-Hydroxy-5,7-Dimethoxyflavanone | 12.15 | 0.00 | 1.24 | down | 13.54 | 0.00 | 1.20 | down | ||
| Gossypin | 15.01 | 0.03 | 1.17 | down | 11.90 | 0.00 | 1.20 | down | ||
| Norwogonin | 12.55 | 0.00 | 1.24 | down | 11.75 | 0.03 | 1.13 | down | ||
| Isoquercetin | 21.72 | 0.01 | 1.21 | up | ||||||
| (−)-Epigallocatechin | 16.37 | 0.00 | 1.23 | up | ||||||
| Neoisoastilbin | 23.78 | 0.00 | 1.20 | up | ||||||
| (+)-Taxifolin | 19.75 | 0.00 | 1.20 | up | ||||||
| Taxifolin | 19.44 | 0.01 | 1.19 | up | ||||||
| Velutin | 16.52 | 0.00 | 1.20 | up | ||||||
| 2-Phenylethylamine | 12.49 | 0.05 | 1.10 | up | ||||||
| Methyllinderone | 11.54 | 0.00 | 1.20 | up | ||||||
| Methylnissolin-3-O-Glucoside | 17.69 | 0.01 | 1.18 | down | ||||||
| Tiliroside | 16.82 | 0.00 | 1.20 | down | ||||||
| 5,7-Dimethoxyluteolin | 16.40 | 0.00 | 1.20 | down | ||||||
| Cyanidin 3-Rutinoside | 12.76 | 0.01 | 1.18 | down | ||||||
| Isoflavonoids | Genistein | 10.62 | 0.00 | 1.24 | up | |||||
| Phenylpropanoic acids | Piscidic Acid | 10.40 | 0.00 | 1.20 | up | |||||
| Tannins | 3,8-Di-O-Methylellagic Acid | 18.34 | 0.00 | 1.24 | up | 16.22 | 0.00 | 1.20 | up | |
| Linear 1,3-diarylpropanoids | Loureirin C | 11.77 | 0.00 | 1.20 | down | |||||
| Unknown (2) | 2-Hydroxy-3-Methylbenzalpyruvate | 14.69 | 0.00 | 1.24 | down | |||||
| H-Gly-Pro-OH | 12.51 | 0.00 | 1.19 | up | ||||||
| Metabolite ID | Metabolite | KEGG_Annotation | KEGG_Pathway_Annotation |
|---|---|---|---|
| HQI/HQO | |||
| POS_q83 | Apiin | C04858 | Flavone and flavonol biosynthesis (ko00944) |
| NEG_q313 | Myricetin | C10107 | Flavonoid biosynthesis (ko00941) Flavone and flavonol biosynthesis (ko00944) Biosynthesis of secondary metabolites (ko01110) |
| POS_t44 | 5-Hydroxyisourate | C11821 | Purine metabolism (ko00230) Metabolic pathways (ko01100) |
| NEG_t83 | S-(4-Methylthiobutylthiohydroximoyl)-L-cysteine | C17242 | Glucosinolate biosynthesis (ko00966) Biosynthesis of secondary metabolites (ko01110) 2-Oxocarboxylic acid metabolism (ko01210) |
| POS_q213 | L-Arginine | C00062 | Arginine biosynthesis (ko00220) Monobactam biosynthesis (ko00261) Arginine and proline metabolism (ko00330) D-Amino acid metabolism (ko00470) Aminoacyl-tRNA biosynthesis (ko00970) Metabolic pathways (ko01100) Biosynthesis of secondary metabolites (ko01110) Biosynthesis of amino acids (ko01230) ABC transporters (ko02010) |
| LFI/LFO | |||
| NEG_q424 | Tricetin | C10192 | Flavonoid biosynthesis (ko00941) |
| NEG_q207 | (+)-Gallocatechin | C12127 | Flavonoid biosynthesis (ko00941) Biosynthesis of secondary metabolites (ko01110) |
| POS_q148 | (−)-Epigallocatechin | C12136 | Flavonoid biosynthesis (ko00941) Biosynthesis of secondary metabolites (ko01110) |
| NEG_q160 | Dihydromyricetin | C02906 | Flavonoid biosynthesis (ko00941) Biosynthesis of secondary metabolites (ko01110) |
| NEG_q313 | Myricetin | C10107 | Flavonoid biosynthesis (ko00941) Flavone and flavonol biosynthesis (ko00944) Biosynthesis of secondary metabolites (ko01110) |
| HQI/LFI | |||
| POS_q148 | (−)-Epigallocatechin | C12136 | Flavonoid biosynthesis (ko00941) Biosynthesis of secondary metabolites (ko01110) |
| HQO/LFO | |||
| NEG_q431 | Uridine 5′-Monophosphate | C00105 | Pyrimidine metabolism (ko00240) Metabolic pathways (ko01100) Nucleotide metabolism (ko01232) Biosynthesis of cofactors (ko01240) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, W.; Wang, L.; Yu, C.; Fan, Z.; Yang, K.; Mo, X. Drug or Toxic? A Brief Understanding of the Edible Corolla of Rhododendron decorum Franch. by Bai Nationality with Comparative Metabolomics Analysis. Metabolites 2024, 14, 484. https://doi.org/10.3390/metabo14090484
Liu W, Wang L, Yu C, Fan Z, Yang K, Mo X. Drug or Toxic? A Brief Understanding of the Edible Corolla of Rhododendron decorum Franch. by Bai Nationality with Comparative Metabolomics Analysis. Metabolites. 2024; 14(9):484. https://doi.org/10.3390/metabo14090484
Chicago/Turabian StyleLiu, Weiwei, Ling Wang, Chenghua Yu, Zhongyu Fan, Kaiye Yang, and Xinchun Mo. 2024. "Drug or Toxic? A Brief Understanding of the Edible Corolla of Rhododendron decorum Franch. by Bai Nationality with Comparative Metabolomics Analysis" Metabolites 14, no. 9: 484. https://doi.org/10.3390/metabo14090484
APA StyleLiu, W., Wang, L., Yu, C., Fan, Z., Yang, K., & Mo, X. (2024). Drug or Toxic? A Brief Understanding of the Edible Corolla of Rhododendron decorum Franch. by Bai Nationality with Comparative Metabolomics Analysis. Metabolites, 14(9), 484. https://doi.org/10.3390/metabo14090484





