Heterologous and High Production of Ergothioneine in Bacillus licheniformis by Using Genes from Anaerobic Bacteria
Abstract
1. Introduction
2. Materials and Methods
2.1. Strains and Plasmids
2.2. Reagents
2.3. Media
2.4. Construction of Recombinant Plasmids
2.5. Shake Flask Fermentation
2.6. Whole-Cell Transformation
2.7. Sample Processing for Ergothioneine
2.8. Quantitative Determination of Ergothioneine
2.9. Other Detection Methods (OD600, Amino Acids)
3. Results and Discussion
3.1. Biosynthetic Pathways of Ergothioneine and Strategies for Yield Optimization
| Strain | Key Strategy | Growth Conditions | Yield | Fermantation Period/h | Reference |
|---|---|---|---|---|---|
| E. coli E1-A1-thrA-serAc.g * | Histidine supply and methyl donor were optimized, and CaCl2 was added to improve cell membrane permeability | Shake flask | 243.06 mg/L | 108 | [25] |
| Escherichia coli | egtB from Methylobacterium pseudosasicola, egtDE from Methylobacterium strain | Shake flask | 657 mg/L | 192 | [18] |
| S. cerevisiae | egt1 of Neurospora crassa and egt2 of C. purpurea | Fed-batch | 598 mg/L | 84 | [22] |
| E. coli BL21 (DE3) | expression of egtEMs gene; semi-rational design and random mutations of EgtD and TNcEgt1 | Fed-batch | 5.4 g/L | 96 | [23] |
| B. subtilis 168 | The active methyl cycle (AMC) and tricarboxylic acid (TCA) cycles were reconstituted | Fed-batch | 619.42 mg/L | 120 | [26] |
| E. coli BW25113 | Overexpression of Tregt1 and Tregt2 genes | Fed-batch | 4.34 g/L | 143 | [27] |
3.2. Mining of Ergothioneine Synthase Genes
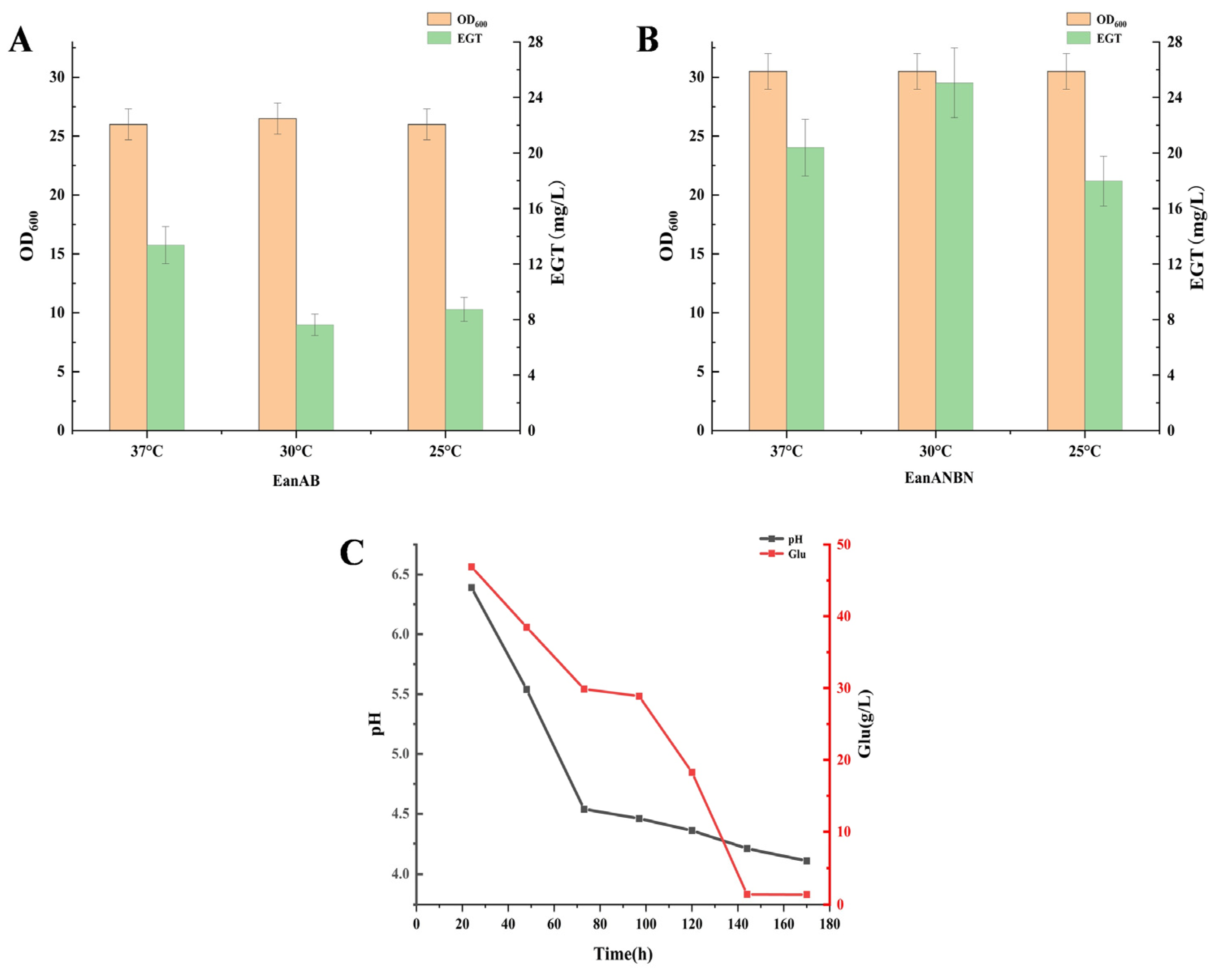
3.3. Expression of Ergothioneine Synthase Genes
3.4. Whole-Cell Conversion for Ergothioneine Production
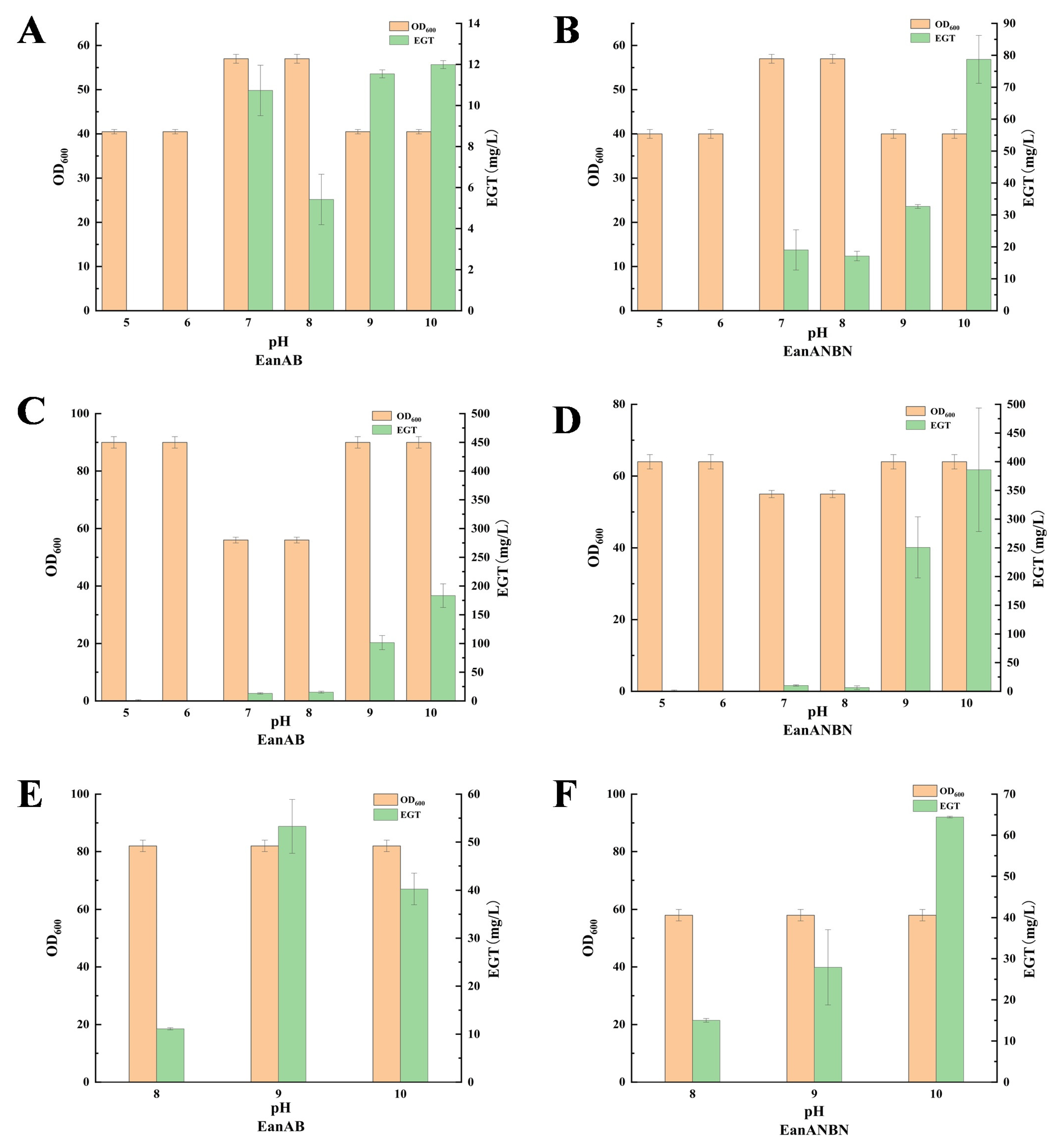
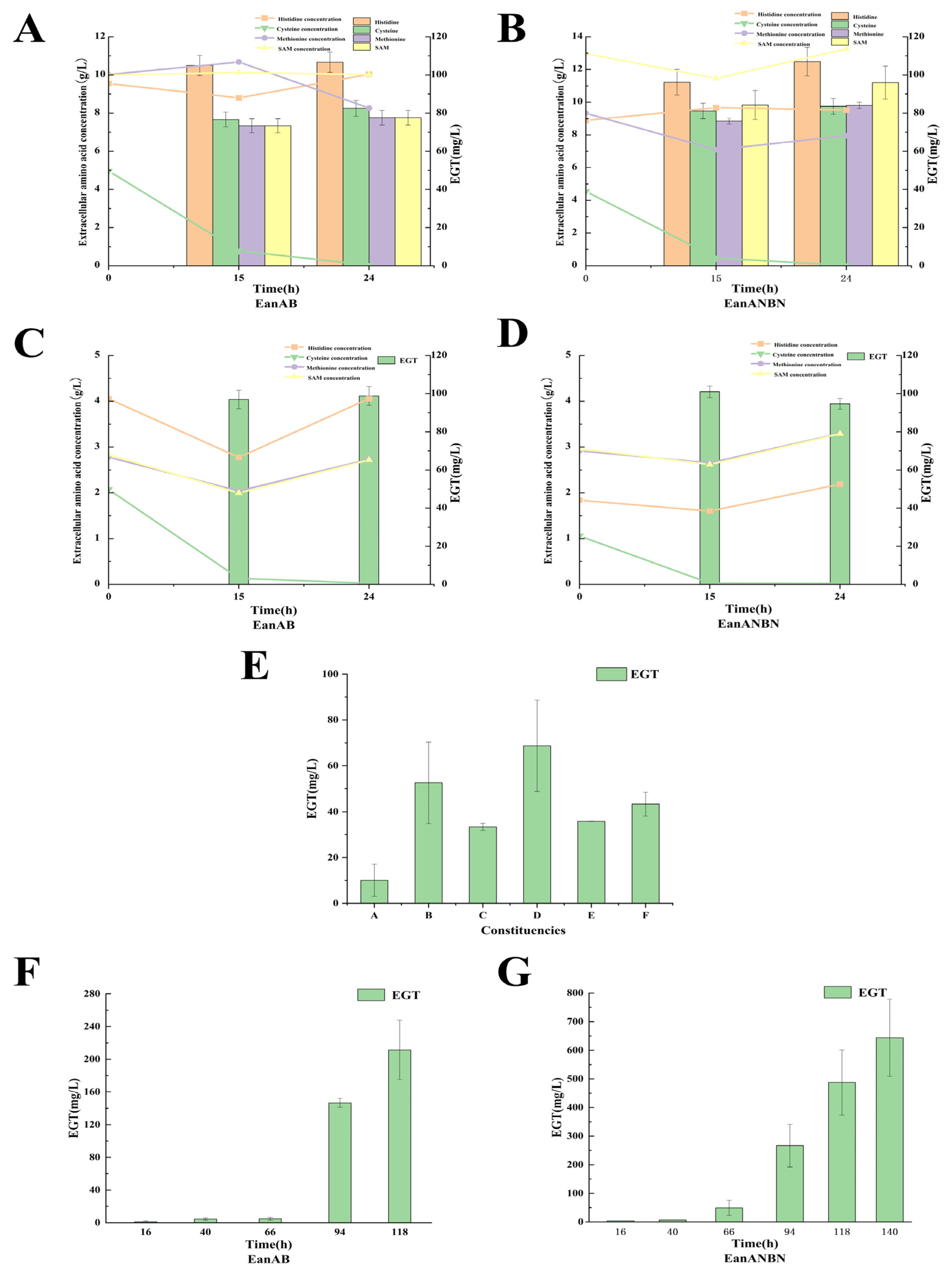
3.5. Effect of Precursor Amino Acid Addition on EGT Yield
3.6. Optimization of Precursor Amino Acid Composition to Enhance EGT Production
3.7. Commercial Feasibility and Scalability of Producing Ergothioneine by Bacillus licheniformis
3.7.1. Growth Characteristics and Fermentation Conditions
3.7.2. Advantages in the Scale-Up Process
3.7.3. Cost-Effectiveness
3.7.4. Sustainability
3.7.5. Resource Utilization and Circular Economy
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Genghof, D.S.; Inamine, E.; Kovalenko, V.; Melville, D.B. Ergothioneine in microorganisms. J. Biol. Chem. 1956, 223, 9–17. [Google Scholar] [PubMed]
- Pan, H.; Guo, L.; Lin, J. Recent Advances in Understanding the In Vivo Distribution and Metabolism of Ergothioneine and Its Roles in Disease Prevention. Food Sci. 2019, 40, 334–340. [Google Scholar]
- Xiong, K.; Xue, S.; Guo, H.; Dai, Y.; Ji, C.; Dong, L.; Zhang, S. Ergothioneine: New functional factor in fermented foods. Crit. Rev. Food Sci. Nutr. 2024, 64, 7505–7516. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.M.; Tang, W.; Wang, X.Y.; Jiang, J.J.; Zhang, W.; Wang, W. Safe and Effective Antioxidant: The Biological Mechanism and Potential Pathways of Ergothioneine in the Skin. Molecules 2023, 28, 1648. [Google Scholar] [CrossRef]
- Maihemuti, M.; Chen, J.; Jiao, C.; Xie, Y. Progress in biosynthesis and application of L-ergothioneine. Nat. Product Res. Dev. 2022, 34, 713–721. [Google Scholar] [CrossRef]
- Feng, L.; E, H.; Zhang, Y.; Li, X.; Zhou, C.; Zhao, X.; Ren, J. Research Progress on Extraction, Separation and Detection of Ergothionine in Edible Fungi. Acta Edulis Fungi 2021, 28, 115–123. [Google Scholar] [CrossRef]
- Ma, X.; Chen, X.; Wu, Z.; Yang, Y.; Qi, S.; Ge, C.; Wang, X.; Sui, Q. Process Research of Natural Antioxidant Ergothioneine. Chin. J. Synth. Chem. 2022, 30, 743–748. [Google Scholar] [CrossRef]
- Osawa, R.; Kamide, T.; Satoh, Y.; Kawano, Y.; Ohtsu, I.; Dairi, T. Heterologous and High Production of Ergothioneine in Escherichia coli. J. Agric. Food Chem. 2018, 66, 1191–1196. [Google Scholar] [CrossRef]
- Wang, J.; Guleria, S.; Koffas, M.A.; Yan, Y. Microbial production of value-added nutraceuticals. Curr. Opin. Biotechnol. 2016, 37, 97–104. [Google Scholar] [CrossRef]
- Burn, R.; Misson, L.; Meury, M.; Seebeck, F.P. Anaerobic Origin of Ergothioneine. Angew. Chem. Int. Ed. Engl. 2017, 56, 12508–12511. [Google Scholar] [CrossRef]
- Cheng, R.; Lai, R.; Peng, C.; Lopez, J.; Li, Z.; Naowarojna, N.; Li, K.; Wong, C.; Lee, N.; Whelan, S.A.; et al. Implications for an imidazol-2-yl carbene intermediate in the rhodanase-catalyzed C-S bond formation reaction of anaerobic ergothioneine biosynthesis. ACS Catal. 2021, 11, 3319–3334. [Google Scholar] [CrossRef] [PubMed]
- Cheng, R.; Wu, L.; Lai, R.; Peng, C.; Naowarojna, N.; Hu, W.; Li, X.; Whelan, S.A.; Lee, N.; Lopez, J.; et al. Single-step Replacement of an Unreactive C-H Bond by a C-S Bond Using Polysulfide as the Direct Sulfur Source in Anaerobic Ergothioneine Biosynthesis. ACS Catal. 2020, 10, 8981–8994. [Google Scholar] [CrossRef] [PubMed]
- Leisinger, F.; Burn, R.; Meury, M.; Lukat, P.; Seebeck, F.P. Structural and Mechanistic Basis for Anaerobic Ergothioneine Biosynthesis. J. Am. Chem. Soc. 2019, 141, 6906–6914. [Google Scholar] [CrossRef] [PubMed]
- Muras, A.; Romero, M.; Mayer, C.; Otero, A. Biotechnological applications of Bacillus licheniformis. Crit. Rev. Biotechnol. 2021, 41, 609–627. [Google Scholar] [CrossRef]
- Liu, X.; Li, Y.; Zhang, L.; Ding, Z.; Xu, S.; Gu, Z.; Shi, G. The transcriptional regulation characteristics of xylose-inducible promoter in Bacillus licheniformis. Chin. J. Appl. Environ. Biol. 2019, 25, 695–701. [Google Scholar] [CrossRef]
- Miller, G.L. Use of Dinitrosalicylic Acid Reagent for Determination of Reducing Sugar. Anal. Chem. 1959, 31, 426–428. [Google Scholar] [CrossRef]
- Liu, Q.; Mao, Y.; Liao, X.; Luo, J.; Ma, H.; Jiang, W. Recent progress in ergothioneine biosynthesis: A review Chin. J. Biotechnol. 2022, 38, 1408–1420. [Google Scholar] [CrossRef]
- Kamide, T.; Takusagawa, S.; Tanaka, N.; Ogasawara, Y.; Kawano, Y.; Ohtsu, I.; Satoh, Y.; Dairi, T. High Production of Ergothioneine in Escherichia coli using the Sulfoxide Synthase from Methylobacterium strains. J. Agric. Food Chem. 2020, 68, 6390–6394. [Google Scholar] [CrossRef]
- Seebeck, F.P. In vitro reconstitution of Mycobacterial ergothioneine biosynthesis. J. Am. Chem. Soc. 2010, 132, 6632–6633. [Google Scholar] [CrossRef]
- Bello, M.H.; Barrera-Perez, V.; Morin, D.; Epstein, L. The Neurospora crassa mutant NcΔEgt-1 identifies an ergothioneine biosynthetic gene and demonstrates that ergothioneine enhances conidial survival and protects against peroxide toxicity during conidial germination. Fungal Genet. Biol. 2012, 49, 160–172. [Google Scholar] [CrossRef]
- Han, Y.; Tang, X.; Zhang, Y.; Hu, X.; Ren, L.J. The current status of biotechnological production and the application of a novel antioxidant ergothioneine. Crit. Rev. Biotechnol. 2021, 41, 580–593. [Google Scholar] [CrossRef] [PubMed]
- van der Hoek, S.A.; Darbani, B.; Zugaj, K.E.; Prabhala, B.K.; Biron, M.B.; Randelovic, M.; Medina, J.B.; Kell, D.B.; Borodina, I. Engineering the Yeast Saccharomyces cerevisiae for the Production of L-(+)-Ergothioneine. Front. Bioeng. Biotechnol. 2019, 7, 262. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Tang, J.; Feng, M.; Chen, S. Engineering Methyltransferase and Sulfoxide Synthase for High-Yield Production of Ergothioneine. J. Agric. Food Chem. 2023, 71, 671–679. [Google Scholar] [CrossRef]
- Li, L.; Xu, S.; Jiang, Y. Research Progress in the Production of Ergothioneine by Biosynthesis. Biotechnol. Bull. 2024, 40, 86–99. [Google Scholar] [CrossRef]
- Chen, J.; Wang, Y.; Du, G.; Kang, Z. Enhancement of Ergothioneine Synthesis in Escherichia coli via Optimization of Precursor Supply and Cell Membrane Permeability. J. Food Sci. Biotechnol. 2022, 41, 43–52. [Google Scholar]
- Li, G. Reprogramming Bacillus Subtilis for the Production of Ergothioneine. Master’s Thesis, Nanjing University, Nanjing, China, 2020. [Google Scholar]
- Chen, Z.; He, Y.; Wu, X.; Wang, L.; Dong, Z.; Chen, X. Toward more efficient ergothioneine production using the fungal ergothioneine biosynthetic pathway. Microb. Cell Fact. 2022, 21, 76. [Google Scholar] [CrossRef]
- Shen, J.; Li, Y.; Shi, G. Whole-cell biosynthesis of (R)-citramalate by recombinant Bacillus licheniformis. Food Ferment. Ind. 2023, 49, 9–15. [Google Scholar] [CrossRef]
- Ma, D.; Qiu, L.; Wang, X.; Li, L.; Peng, S.; Liao, Y.; Li, K. L-arabinose isomerase from Lactobacillus fermentum C6: Enzymatic characteristics and its recombinant Bacillus subtilis whole cells achieving a significantly increased production of D-tagatose. Int. J. Biol. Macromol. 2024, 278, 134753. [Google Scholar] [CrossRef]
- Wang, H. Effect of Amino Acid Transporters in Bacillus licheniformis on β-Alanine Synthesis by Whole-Cell Catalyst. Master’s Thesis, Jiangnan University, Wuxi, China, 2022. [Google Scholar]
- Wang, Q.; Lin, W.; Ni, Y.; Zhou, J.; Xu, G.; Han, R. Engineering of Methionine Adenosyltransferase toward Mitigated Product Inhibition for Efficient Production of S-Adenosylmethionine. J. Agric. Food Chem. 2024, 72, 16900–16910. [Google Scholar] [CrossRef]
- Dong, Z.; Chen, Z.; Wang, H.; Tian, K.; Jin, P.; Liu, X.; McHunu, N.P.; Permaul, K.; Singh, S.; Niu, D.; et al. Tandem mass tag-based quantitative proteomics analyses reveal the response of Bacillus licheniformis to high growth temperatures. Ann. Microbiol. 2017, 67, 501–510. [Google Scholar] [CrossRef]
- Mordukhova, E.A.; Lee, H.S.; Pan, J.G. Improved thermostability and acetic acid tolerance of Escherichia coli via directed evolution of homoserine o-succinyltransferase. Appl. Environ. Microbiol. 2008, 74, 7660–7668. [Google Scholar] [CrossRef] [PubMed]
- Rudolph, B.; Gebendorfer, K.M.; Buchner, J.; Winter, J. Evolution of Escherichia coli for growth at high temperatures. J. Biol. Chem. 2010, 285, 19029–19034. [Google Scholar] [CrossRef] [PubMed]
- Van Derlinden, E.; Bernaerts, K.; Van Impe, J.F. Dynamics of Escherichia coli at elevated temperatures: Effect of temperature history and medium. J. Appl. Microbiol. 2008, 104, 438–453. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Fandos, E.; Vazquez de Castro, M.; Martinez-Laorden, A.; Perez-Arnedo, I. Behavior of Listeria monocytogenes and Other Microorganisms in Sliced Riojano Chorizo (Spanish Dry-Cured Sausage) during Storage under Modified Atmospheres. Microorganisms 2021, 9, 1384. [Google Scholar] [CrossRef]
- Zhan, Y.; Xu, H.; Tan, H.T.; Ho, Y.S.; Yang, D.; Chen, S.; Ow, D.S.; Lv, X.; Wei, F.; Bi, X.; et al. Systematic Adaptation of Bacillus licheniformis to 2-Phenylethanol Stress. Appl. Environ. Microbiol. 2023, 89, e0156822. [Google Scholar] [CrossRef]
- Song, C.W.; Chelladurai, R.; Park, J.M.; Song, H. Engineering a newly isolated Bacillus licheniformis strain for the production of (2R,3R)-butanediol. J. Ind. Microbiol. Biotechnol. 2020, 47, 97–108. [Google Scholar] [CrossRef]
- Inan, K.; Sal, F.A.; Rahman, A.; Putman, R.J.; Agblevor, F.A.; Miller, C.D. Microbubble assisted polyhydroxybutyrate production in Escherichia coli. BMC Res. Notes 2016, 9, 338. [Google Scholar] [CrossRef]
- Soini, J.; Ukkonen, K.; Neubauer, P. High cell density media for Escherichia coli are generally designed for aerobic cultivations—Consequences for large-scale bioprocesses and shake flask cultures. Microb. Cell Fact. 2008, 7, 26. [Google Scholar] [CrossRef]
- Hauck, N.; Seixas, N.; Centeno, S.P.; Schlüßler, R.; Cojoc, G.; Müller, P.; Guck, J.; Wöll, D.; Wessjohann, L.A.; Thiele, J. Droplet-Assisted Microfluidic Fabrication and Characterization of Multifunctional Polysaccharide Microgels Formed by Multicomponent Reactions. Polymers 2018, 10, 1055. [Google Scholar] [CrossRef]
- Ibrahim, D.; Zhu, H.L.; Yusof, N.; Isnaeni; Hong, L.S. Bacillus licheniformis BT5.9 Isolated from Changar Hot Spring, Malang, Indonesia, as a Potential Producer of Thermostable α-amylase. Trop. Life Sci. Res. 2013, 24, 71–84. [Google Scholar]
- Wiegand, S.; Voigt, B.; Albrecht, D.; Bongaerts, J.; Evers, S.; Hecker, M.; Daniel, R.; Liesegang, H. Fermentation stage-dependent adaptations of Bacillus licheniformis during enzyme production. Microb. Cell Fact. 2013, 12, 120. [Google Scholar] [CrossRef] [PubMed]
- Manzoni, M.; Bergomi, S.; Molinari, F.; Cavazzoni, V. Production and purification of an extracellularly produced K4 polysaccharide from Escherichia coli. Biotechnol. Lett. 1996, 18, 383–386. [Google Scholar] [CrossRef]
- Wu, J.; Huang, M.; Liu, H.; Wu, Y.; Hu, X.; Wang, J.; Wang, X. Engineering Escherichia coli to Efficiently Produce Colanic Acid with Low Molecular Mass and Viscosity. J. Agric. Food Chem. 2024, 72, 15811–15822. [Google Scholar] [CrossRef] [PubMed]
- Doan, C.T.; Tran, T.N.; Nguyen, T.T.; Tran, T.P.H.; Nguyen, V.B.; Tran, T.D.; Nguyen, A.D.; Wang, S.L. Production of Sucrolytic Enzyme by Bacillus licheniformis by the Bioconversion of Pomelo Albedo as a Carbon Source. Polymers 2021, 13, 1959. [Google Scholar] [CrossRef]
- Fritschie, K.J.; Olden, J.D. Disentangling the influences of mean body size and size structure on ecosystem functioning: An example of nutrient recycling by a non-native crayfish. Ecol. Evol. 2016, 6, 159–169. [Google Scholar] [CrossRef]
- Lee, A.J.; Cadelis, M.M.; Kim, S.H.; Swift, S.; Copp, B.R.; Villas-Boas, S.G. Epipyrone A, a Broad-Spectrum Antifungal Compound Produced by Epicoccum nigrum ICMP 19927. Molecules 2020, 25, 5997. [Google Scholar] [CrossRef]
- Hochstrasser, T.; Rühling, S.; Hecher, K.; Fabisch, K.H.; Chrzanowski, U.; Brendel, M.; Eckenweber, F.; Sacher, C.; Schmitz, C.; Kipp, M. Stereological Investigation of Regional Brain Volumes after Acute and Chronic Cuprizone-Induced Demyelination. Cells 2019, 8, 1024. [Google Scholar] [CrossRef]
- Reynolds, J.E.; Long, X.; Paniukov, D.; Bagshawe, M.; Lebel, C. Calgary Preschool magnetic resonance imaging (MRI) dataset. Data Brief. 2020, 29, 105224. [Google Scholar] [CrossRef]
- Bainter, T.; Selya, A.S.; Oancea, S.C. A key indicator of nicotine dependence is associated with greater depression symptoms, after accounting for smoking behavior. PLoS ONE 2020, 15, e0233656. [Google Scholar] [CrossRef]
- Morris, D.R.; Bounds, S.E.; Liu, H.; Ding, W.-Q.; Chen, Y.; Liu, Y.; Cai, J. Exosomal MiRNA Transfer between Retinal Microglia and RPE. Int. J. Mol. Sci. 2020, 21, 3541. [Google Scholar] [CrossRef]
- Gudiña, E.J.; Teixeira, J.A. Bacillus licheniformis: The unexplored alternative for the anaerobic production of lipopeptide biosurfactants? Biotechnol. Adv. 2022, 60, 108013. [Google Scholar] [CrossRef] [PubMed]
- Heyman, B.; Tulke, H.; Putri, S.P.; Fukusaki, E.; Büchs, J. Online monitoring of the respiratory quotient reveals metabolic phases during microaerobic 2,3-butanediol production with Bacillus licheniformis. Eng. Life Sci. 2020, 20, 133–144. [Google Scholar] [CrossRef] [PubMed]
- García, Á.C.; Hauptmann, P.; Neubauer, P. Glucose-Limited Fed-Batch Cultivation Strategy to Mimic Large-Scale Effects in Escherichia coli Linked to Accumulation of Non-Canonical Branched-Chain Amino Acids by Combination of Pyruvate Pulses and Dissolved Oxygen Limitation. Microorganisms 2021, 9, 1110. [Google Scholar] [CrossRef]
- Okaro, U.; George, S.; Anderson, B. What Is in a Cat Scratch? Growth of Bartonella henselae in a Biofilm. Microorganisms 2021, 9, 835. [Google Scholar] [CrossRef]
- Bajaj, B.K.; Manhas, K. Production and characterization of xylanase from Bacillus licheniformis P11(C) with potential for fruit juice and bakery industry. Biocatal. Agric. Biotechnol. 2012, 1, 330–337. [Google Scholar] [CrossRef]
- Deepika, P.; MubarakAli, D. Production and assessment of microalgal liquid fertilizer for the enhanced growth of four crop plants. Biocatal. Agric. Biotechnol. 2020, 28, 101701. [Google Scholar] [CrossRef]
- Yang, G.; Zhang, G.; Wang, H. Current state of sludge production, management, treatment and disposal in China. Water Res. 2015, 78, 60–73. [Google Scholar] [CrossRef]


| Names | Description | Sources |
|---|---|---|
| Plasmids | ||
| pHY300-PLK | Expression vetor, TetR | Lab stock |
| pHY-P2-EanA | pHY300-PLK containing EanA | This work |
| pHY-P2-EanB | pHY300-PLK containing EanB | This work |
| pHY-P2-EanAN | pHY300-PLK containing EanAN | This work |
| pHY-P2-EanAN | pHY300-PLK containing EanBN | This work |
| pHY-P2-EanAB | pHY300-PLK containing EanA, EanB | This work |
| pHY-P2-EanANBN | pHY300-PLK containing EanAN, EanBN | This work |
| Strains | ||
| B. licheniformis | Wild-type (ATCC 9945a) | Lab stock |
| E. coli JM109 | E. coli Wild-type | Lab stock |
| B. licheniformis-PHY | B. licheniformis (ATCC 9945a) harboring pHY300-PLK | This work |
| E. coli-EanAB | E. coli JM109 harboring pHY-P2-EanAB | This work |
| E. coli-EanANBN | E. coli JM109 harboring pHY-P2-EanANBN | This work |
| EanAB | B. licheniformis (ATCC 9945a) harboring pHY-P2-EanAB | This work |
| EanANBN | B. licheniformis (ATCC 9945a) harboring pHY-P2-EanANBN | This work |
| Final Amino Acid Concentration | ||||||
|---|---|---|---|---|---|---|
| Transformation Buffer | No Added Amino Acids | Histidine, Methionine, Cysteine | Histidine, Cysteine, SAM | Histidine, Methionine, Cysteine, SAM | ||
| Constituencies | A1, A2 | B1, B2 | C1, C2 | D1, D2 | E1, E2 | F1, F2 |
| Concentration | 0 g/L | 2.5 g/L | 5 g/L | 10 g/L | 10 g/L, SAM 5 g/L | 10 g/L, SAM 5 g/L |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Z.; Xiao, F.; Zhang, Y.; Lu, J.; Li, Y.; Shi, G. Heterologous and High Production of Ergothioneine in Bacillus licheniformis by Using Genes from Anaerobic Bacteria. Metabolites 2025, 15, 45. https://doi.org/10.3390/metabo15010045
Liu Z, Xiao F, Zhang Y, Lu J, Li Y, Shi G. Heterologous and High Production of Ergothioneine in Bacillus licheniformis by Using Genes from Anaerobic Bacteria. Metabolites. 2025; 15(1):45. https://doi.org/10.3390/metabo15010045
Chicago/Turabian StyleLiu, Zhe, Fengxu Xiao, Yupeng Zhang, Jiawei Lu, Youran Li, and Guiyang Shi. 2025. "Heterologous and High Production of Ergothioneine in Bacillus licheniformis by Using Genes from Anaerobic Bacteria" Metabolites 15, no. 1: 45. https://doi.org/10.3390/metabo15010045
APA StyleLiu, Z., Xiao, F., Zhang, Y., Lu, J., Li, Y., & Shi, G. (2025). Heterologous and High Production of Ergothioneine in Bacillus licheniformis by Using Genes from Anaerobic Bacteria. Metabolites, 15(1), 45. https://doi.org/10.3390/metabo15010045






