The Potential Role of Intestinal Microbiota on the Intestine-Protective and Lipid-Lowering Effects of Berberine in Zebrafish (Danio rerio) Under High-Lipid Stress
Abstract
:1. Introduction
2. Materials and Methods
2.1. Diet Preparation
2.2. Feeding Trial
2.3. Sampling
2.4. Enzyme, Biochemistry, and Histological Analysis
2.5. Quantitative RT-PCR
2.6. DNA Extraction and 16S rDNA Gene Sequencing
2.7. Bioinformatic Analysis
2.8. Data Analysis
3. Results
3.1. Growth Performance, Morphological Parameters, and Feed Intake
3.2. Intestinal BSH Activity and Hepatic TC and TG Contents
3.3. Related Gene Expression
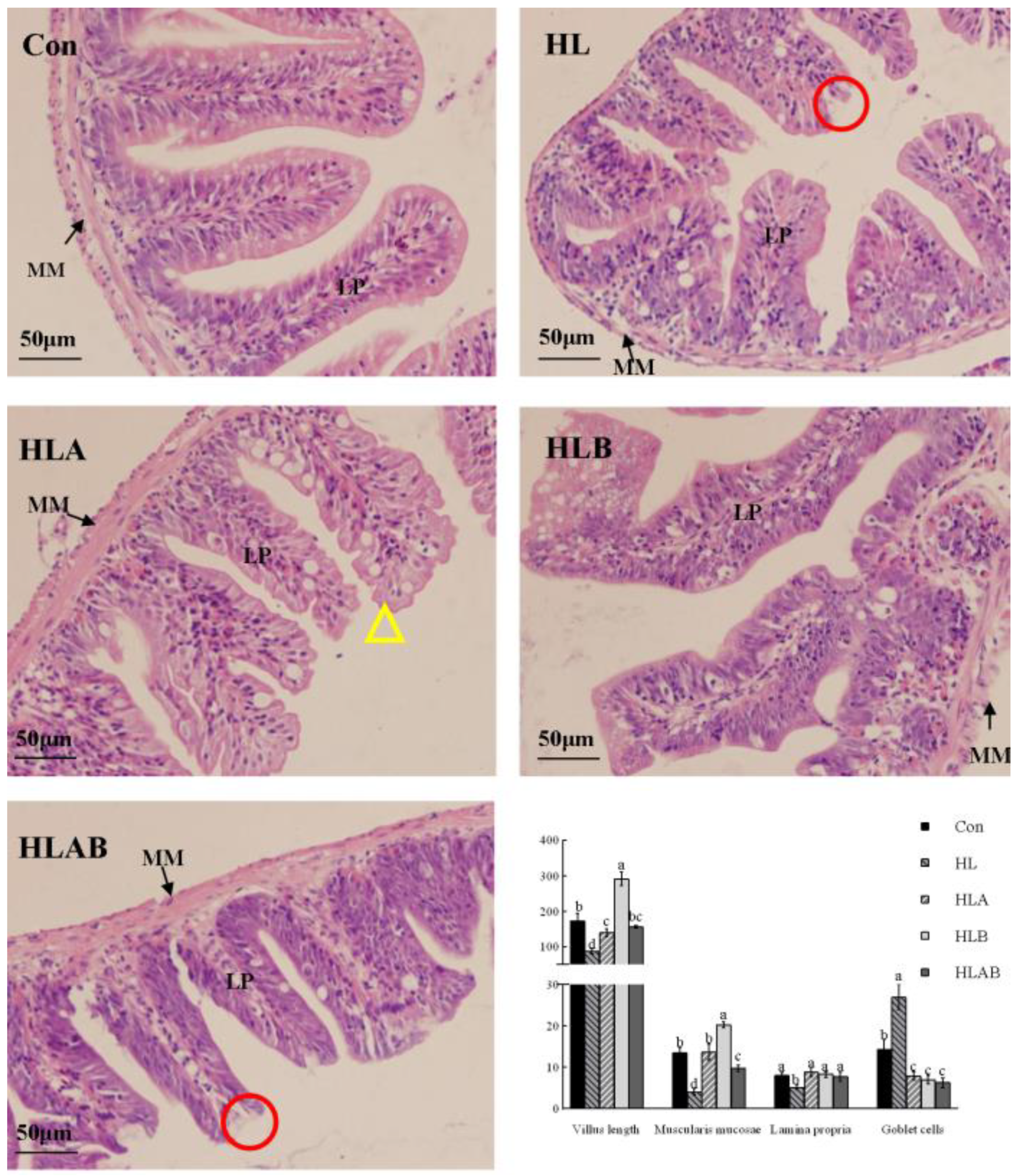
3.4. Intestinal Histological Parameters
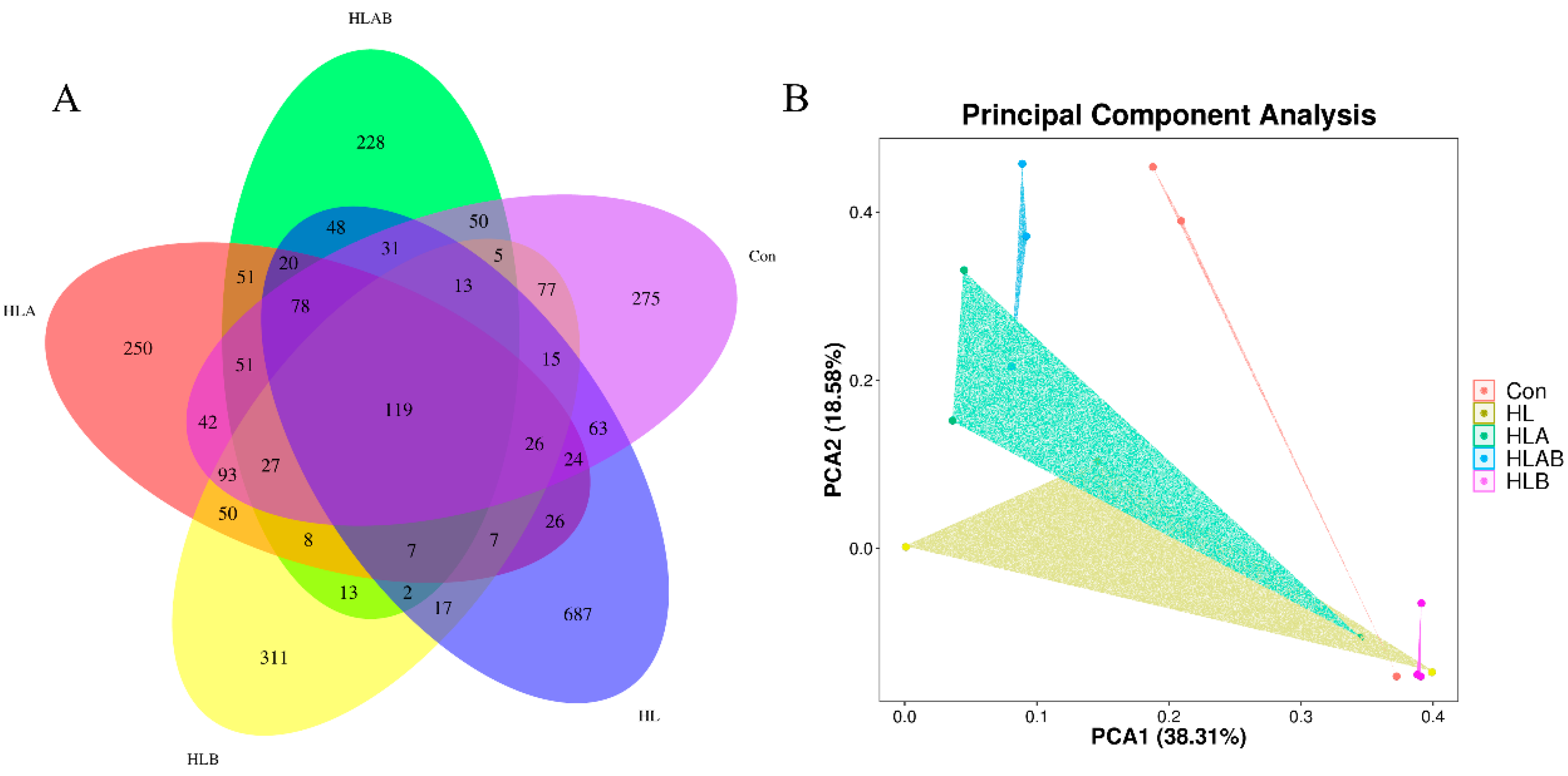
3.5. Alpha and Beta Diversities of the Intestinal Microbiota
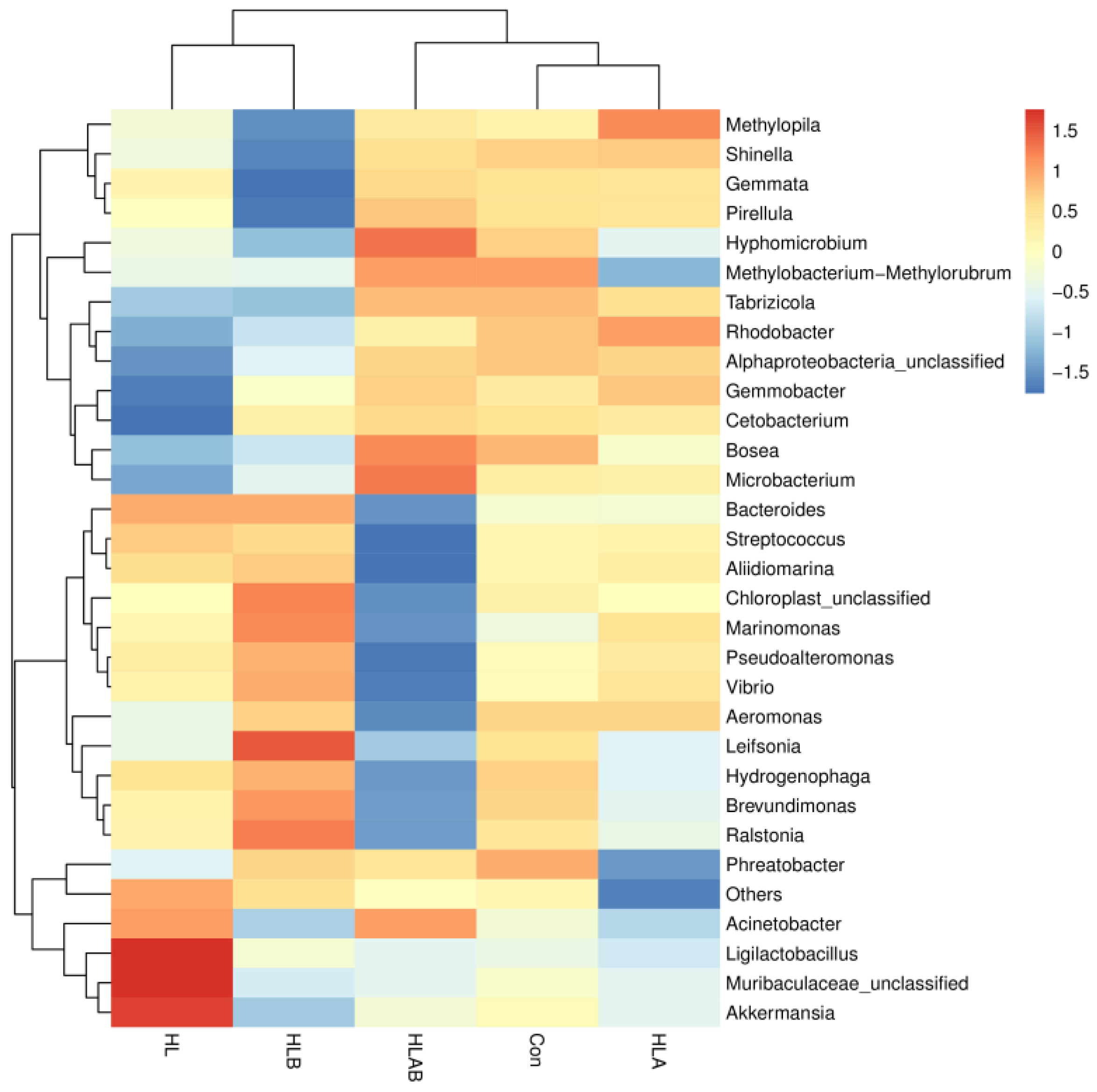
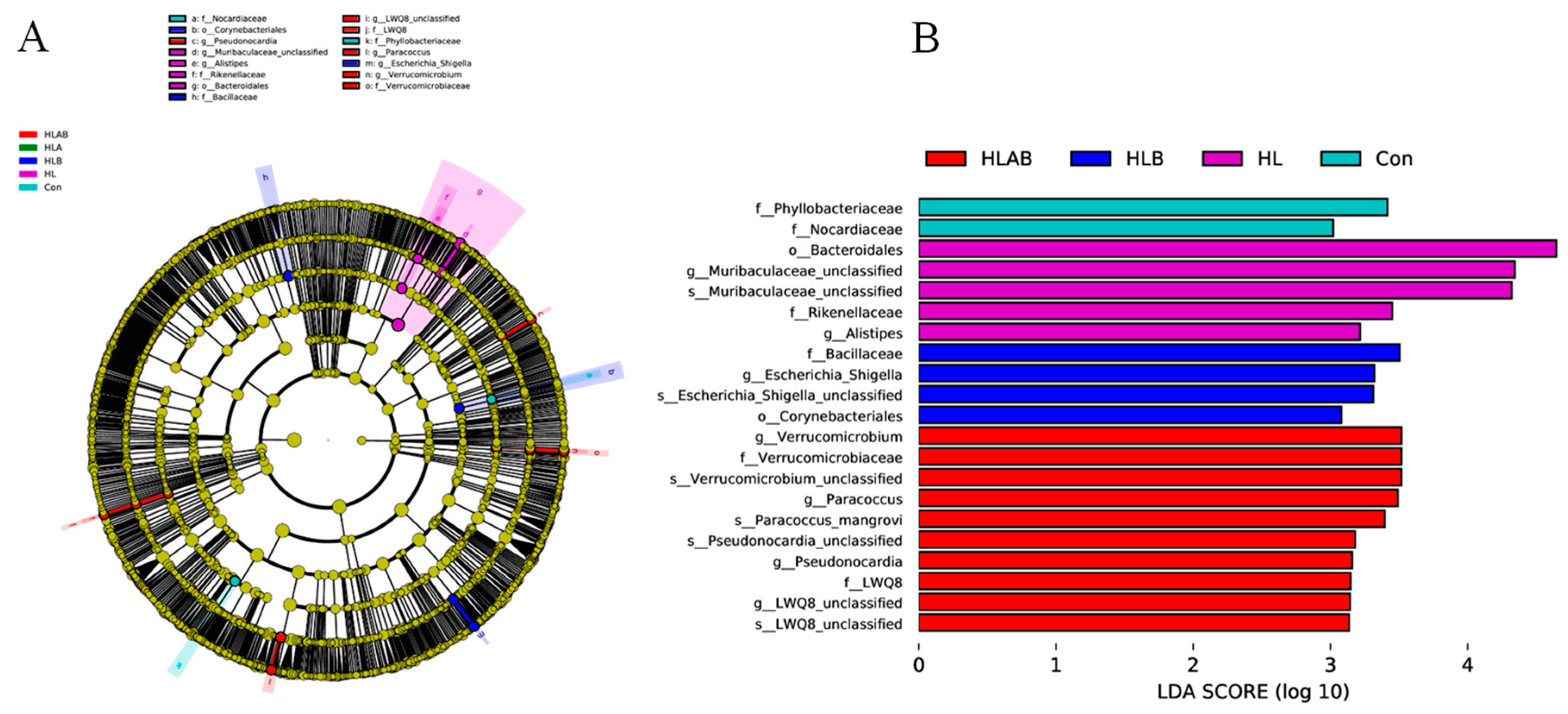
3.6. Taxonomic Composition Analysis
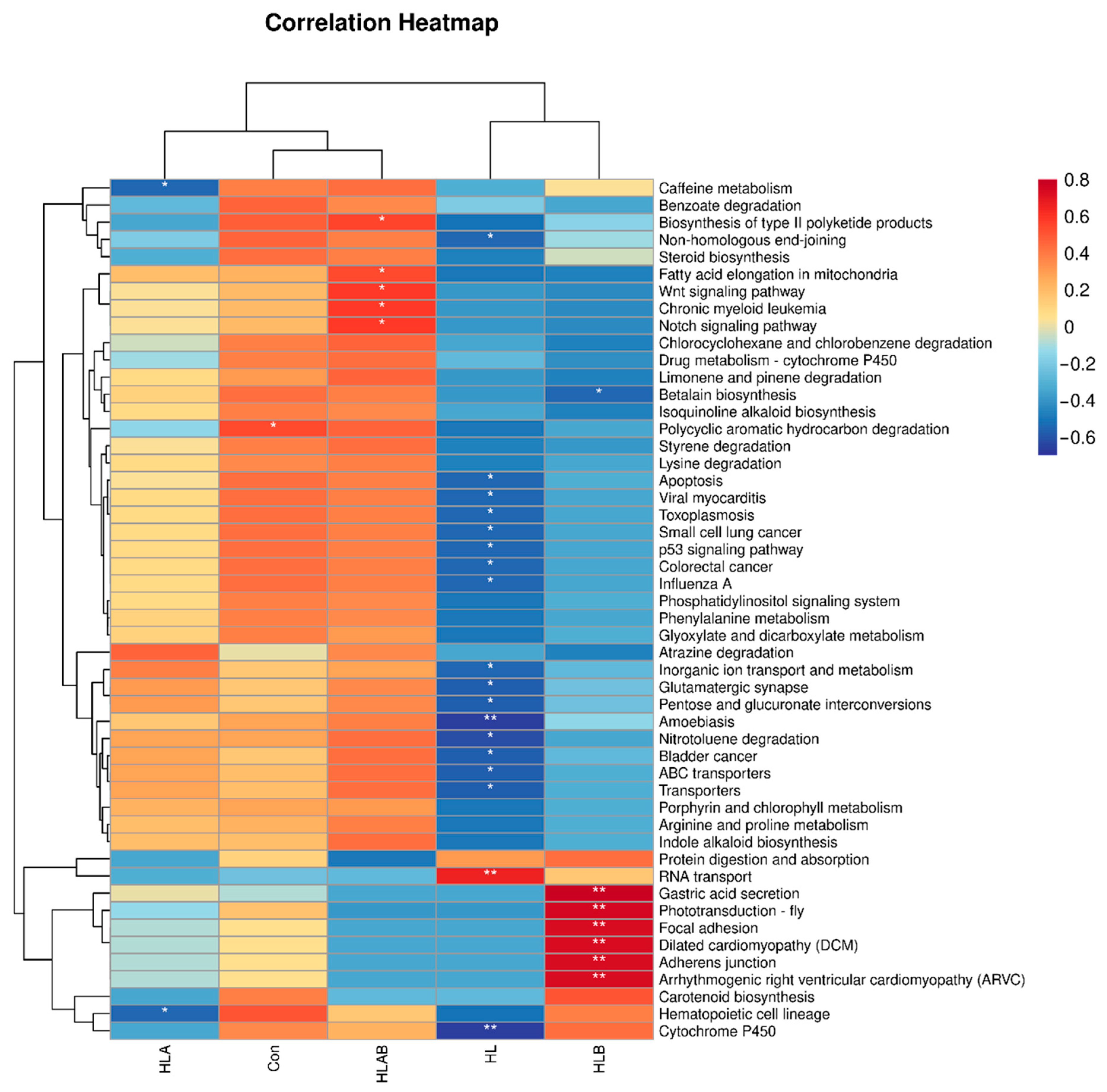
3.7. Functional Prediction Analysis of Intestinal Microbiota
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, L.; Sagada, G.; Wang, C.; Liu, R.; Li, Q.; Zhang, C.; Yan, Y. Exogenous bile acids regulate energy metabolism and improve the health condition of farmed fish. Aquaculture 2023, 562, 738852. [Google Scholar] [CrossRef]
- Wang, L.; Xu, B.; Sagada, G.; Ng, W.K.; Chen, K.; Zhang, J.; Shao, Q. Dietary berberine regulates lipid metabolism in muscle and liver of black sea bream (Acanthopagrus schlegelii) fed normal or high-lipid diets. Br. J. Nutr. 2021, 125, 481–493. [Google Scholar] [CrossRef]
- Naiel, M.A.E.; Negm, S.S.; Ghazanfar, S.; Shukry, M.; Abdelnour, S.A. The risk assessment of high-fat diet in farmed fish and its mitigation approaches: A review. J. Anim. Physiol. Anim. Nutr. 2022, 107, 948–969. [Google Scholar] [CrossRef] [PubMed]
- Arias-Jayo, N.; Abecia, L.; Alonso-Sáez, L.; Ramirez-Garcia, A.; Rodriguez, A.; Pardo, M.A. High-fat diet consumption induces microbiota dysbiosis and intestinal inflammation in zebrafish. Microb. Ecol. 2018, 76, 1089–1101. [Google Scholar] [CrossRef] [PubMed]
- Chiang, J.Y.L.; Pathak, P.; Liu, H.; Donepudi, A.; Ferrell, J.; Boehme, S. Intestinal farnesoid X receptor and takeda G protein couple receptor 5 signaling in metabolic regulation. Dig. Dis. 2017, 35, 241–245. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, M.; Houten, S.M.; Wang, L.; Moschetta, A.; Mangelsdorf, D.J.; Heyman, R.A.; Moore, D.D.; Auwerx, J. Bile acids lower triglyceride levels via a pathway involving FXR, SHP, and SREBP-1c. J. Clin. Investig. 2004, 113, 1408–1418. [Google Scholar] [CrossRef] [PubMed]
- Pineda Torra, I.s.; Claudel, T.; Duval, C.; Kosykh, V.; Fruchart, J.-C.; Staels, B. Bile acids induce the expression of the human peroxisome proliferator-activated receptor α gene via activation of the farnesoid X receptor. Mol. Endocrinol. 2003, 17, 259–272. [Google Scholar] [CrossRef]
- Chiang, J.Y.L. Bile acid metabolism and signaling. Compr. Physiol. 2013, 3, 1191–1212. [Google Scholar]
- Wahlström, A.; Sayin, S.I.; Marschall, H.U.; Bäckhed, F. Intestinal crosstalk between bile acids and microbiota and its impact on host metabolism. Cell Metab. 2016, 24, 41–50. [Google Scholar] [CrossRef]
- Biagioli, M.; Carino, A. Bile Acids and Their Receptors; Handbook of Experimental Pharmacology; Springer: Berlin/Heidelberg, Germany, 2019; Volume 256, pp. 95–108. [Google Scholar]
- Wang, Y.-D.; Chen, W.-D.; Yu, D.; Forman, B.M.; Huang, W. The G-Protein-coupled bile acid receptor, Gpbar1 (TGR5), negatively regulates hepatic inflammatory response through antagonizing nuclear factor kappa light-chain enhancer of activated B cells (NF-κB) in mice. Hepatology 2011, 54, 1421–1432. [Google Scholar] [CrossRef]
- Luo, Y.; Li, M.; Wang, T.; Zhou, N.-N.; Qiao, F.; Du, Z.-Y.; Zhang, M.-L. Bacillus cereus alters bile acid composition and alleviates high-carbohydrate diet-induced hepatic lipid accumulation in Nile tilapia (Oreochromis niloticus). J. Agric. Food Chem. 2023, 71, 4825–4836. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Xie, S.; Chi, S.; Zhang, S.; Cao, J.; Tan, B. Protective effects of taurocholic acid on excessive hepatic lipid accumulation via regulation of bile acid metabolism in grouper. Food Funct. 2022, 13, 3050–3062. [Google Scholar] [CrossRef]
- Kumar, A.; Ekavali; Chopra, K.; Mukherjee, M.; Pottabathini, R.; Dhull, D.K. Current knowledge and pharmacological profile of berberine: An update. Eur. J. Pharmacol. 2015, 761, 288–297. [Google Scholar] [CrossRef]
- Chen, B.; Zheng, Y.-M.; Zhang, M.-Q.; Han, Y.; Zhang, J.-P.; Hu, C.-Q. Microarray expression profiling and raman spectroscopy reveal anti-fatty liver action of berberine in a diet-induced larval zebrafish model. Front. Pharmacol. 2020, 10, 1504. [Google Scholar] [CrossRef]
- Wang, L.; Gao, C.; Yang, L.; Wang, C.; Wang, B.; Wang, H.; Shu, Y.; Yan, Y. The growth-promoting and lipid-lowering effects of berberine are associated with the regulation of intestinal bacteria and bile acid profiles in yellow catfish (Pelteobagrus fulvidraco). Aquacult. Rep. 2023, 33, 101848. [Google Scholar] [CrossRef]
- Wang, L.; Sagada, G.; Wang, B.; Wang, C.; Shao, Q. Berberine in fish nutrition: Impact on hepatoenteric health, antioxidative and immune status. Front. Mar. Sci. 2022, 9, 967748. [Google Scholar] [CrossRef]
- Zhou, W.; Rahimnejad, S.; Lu, K.; Wang, L.; Liu, W. Effects of berberine on growth, liver histology, and expression of lipid-related genes in blunt snout bream (Megalobrama amblycephala) fed high-fat diets. Fish Physiol. Biochem. 2019, 45, 83–91. [Google Scholar] [CrossRef]
- Yu, C.; Zhang, J.; Qin, Q.; Liu, J.; Xu, J.; Xu, W. Berberine improved intestinal barrier function by modulating the intestinal microbiota in blunt snout bream (Megalobrama amblycephala) under dietary high-fat and high-carbohydrate stress. Fish Shellfish Immunol. 2020, 102, 336–349. [Google Scholar] [CrossRef]
- Liu, C.; Zheng, Y.; Zhang, Y.; Long, X. Research progress on berberine with a special focus on its oral bioavailability. Fitoterapia 2016, 109, 274–282. [Google Scholar] [CrossRef] [PubMed]
- Sun, R.; Yang, N.; Kong, B.; Cao, B.; Feng, D.; Yu, X.; Ge, C.; Huang, J.; Shen, J.; Wang, P.; et al. Orally administered berberine modulates hepatic lipid metabolism by altering microbial bile acid metabolism and the intestinal FXR signaling pathway. Mol. Pharmacol. 2017, 91, 110–122. [Google Scholar] [CrossRef]
- Tian, Y.; Cai, J.; Gui, W.; Nichols, R.G.; Koo, I.; Zhang, J.; Anitha, M.; Patterson, A.D. Berberine directly affects the gut microbiota to promote intestinal farnesoid X receptor activation. Drug Metab. Dispos. 2019, 47, 86–93. [Google Scholar] [CrossRef]
- Sun, X.; Zhang, Y.; Cheng, G.; Zhu, T.; Zhang, Z.; Xiong, L.; Hu, H.; Liu, H. Berberine improves DSS-induced colitis in mice by modulating the fecal-bacteria-related bile acid metabolism. Biomed. Pharmacother. 2023, 167, 115430. [Google Scholar] [CrossRef]
- Wang, A.; Meng, D.; Hao, Q.; Xia, R.; Zhang, Q.; Ran, C.; Yang, Y.; Li, D.; Liu, W.; Zhang, Z.; et al. Effect of supplementation of solid-state fermentation product of Bacillus subtilis HGcc-1 to high-fat diet on growth, hepatic lipid metabolism, epidermal mucus, gut and liver health and gut microbiota of zebrafish. Aquaculture 2022, 560, 738542. [Google Scholar] [CrossRef]
- Liang, H.; Xie, Y.; Li, Y.; Xie, M.; Li, M.; Zhou, W.; Chen, J.; Zhang, Z.; Yang, Y.; Ran, C.; et al. Dietary supplementation of yeast mannan enhances antiviral immunity of zebrafish (Danio rerio). Aquaculture 2023, 563, 739003. [Google Scholar] [CrossRef]
- Tian, J.; Jin, Y.; Yu, E.; Sun, J.; Xia, Y.; Zhang, K.; Li, Z.; Gong, W.; Wang, G.; Xie, J. Farnesoid X receptor is an effective target for modulating lipid accumulation in grass carp, Ctenopharyngodon idella. Aquaculture 2021, 534, 736248. [Google Scholar] [CrossRef]
- Lu, K.-L.; Xu, W.-N.; Liu, W.-B.; Wang, L.-N.; Zhang, C.-N.; Li, X.-F. Association of mitochondrial dysfunction with oxidative stress and immune suppression in blunt snout bream Megalobrama amblycephala fed a high-fat diet. J. Aquat. Anim. Health 2014, 26, 100–112. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Yu, H.; Li, P.; Wang, C.; Liu, G.; Zhang, X.; Zhang, C.; Qi, M.; Ji, H. Dietary nano-selenium alleviated intestinal damage of juvenile grass carp (Ctenopharyngodon idella) induced by high-fat diet: Insight from intestinal morphology, tight junction, inflammation, anti-oxidization and intestinal microbiota. Anim. Nutr. 2021, 8, 235–248. [Google Scholar] [CrossRef] [PubMed]
- Limbu, S.M.; Ma, Q.; Zhang, M.-L.; Du, Z.-Y. High fat diet worsens the adverse effects of antibiotic on intestinal health in juvenile Nile tilapia (Oreochromis niloticus). Sci. Total Environ. 2019, 680, 169–180. [Google Scholar] [CrossRef]
- Zhou, L.; Limbu, S.M.; Qiao, F.; Du, Z.-Y.; Zhang, M. Influence of long-yerm feeding antibiotics on the gut health of zebrafish. Zebrafish 2018, 15, 340–348. [Google Scholar] [CrossRef]
- Limbu, S.M.; Chen, L.Q.; Zhang, M.L.; Du, Z.Y. A global analysis on the systemic effects of antibiotics in cultured fish and their potential human health risk: A review. Rev. Aquac. 2021, 13, 1015–1059. [Google Scholar] [CrossRef]
- Xu, W.-N.; Chen, D.-H.; Chen, Q.-Q.; Liu, W.-B. Growth performance, innate immune responses and disease resistance of fingerling blunt snout bream, Megalobrama amblycephala adapted to different berberine-dietary feeding modes. Fish Shellfish Immunol. 2017, 68, 458–465. [Google Scholar] [CrossRef] [PubMed]
- Xia, Y.; Wang, G.; Yu, E.; Tian, J.; Li, Z.; Zhang, K.; Gong, W.; Xie, J. Addition of berberine to formulated feed changes the glucose utilisation, intestinal microbiota and serum metabolites of largemouth bass (Micropterus salmoides). Aquacult. Rep. 2022, 23, 101018. [Google Scholar] [CrossRef]
- Xia, Y.; Yang, H.-C.; Zhang, K.; Tian, J.-J.; Li, Z.-F.; Yu, E.-M.; Li, H.-Y.; Gong, W.-B.; Xie, W.-P.; Wang, G.-J.; et al. Berberine regulates glucose metabolism in largemouth bass by modulating intestinal microbiota. Front. Physiol. 2023, 14, 1147001. [Google Scholar] [CrossRef]
- Doan, H.V.; Hoseinifar, S.H.; Jaturasitha, S.; Dawood, M.A.O.; Harikrishnan, R. The effects of berberine powder supplementation on growth performance, skin mucus immune response, serum immunity, and disease resistance of Nile tilapia (Oreochromis niloticus) fingerlings. Aquaculture 2020, 520, 734927. [Google Scholar] [CrossRef]
- Maselli, K.M.; Gee, K.; Isani, M.; Fode, A.; Schall, K.A.; Grikscheit, T.C. Broad-spectrum antibiotics alter the microbiome, increase intestinal fxr, and decrease hepatic steatosis in zebrafish short bowel syndrome. Am. J. Physiol. Gastrointest. Liver Physiol. 2020, 319, G212–G226. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, W.; Gladstone, S.; Ng, W.-K.; Zhang, J.; Shao, Q. Effects of isoenergetic diets with varying protein and lipid levels on the growth, feed utilization, metabolic enzymes activities, antioxidative status and serum biochemical parameters of black sea bream (Acanthopagrus schlegelii). Aquaculture 2019, 513, 734397. [Google Scholar] [CrossRef]
- Lei, S.; Liu, L.; Ding, L.; Zhang, Y.; Zeng, H. Lotus seed resistant starch affects the conversion of sodium taurocholate by regulating the intestinal microbiota. Int. J. Biol. Macromol. 2021, 186, 227–236. [Google Scholar] [CrossRef]
- Tan, P.; Wang, L.; Chen, R.; Xu, D. Berberine chloride supplementation ameliorates excessive hepatic lipid deposition and proinflammatory gene upregulation in the soybean-oil-based diet of juvenile yellow drum (Nibea albiflora). Aquacult. Nutr. 2022, 2022, 8690138. [Google Scholar] [CrossRef]
- Lu, K.-L.; Zhang, D.-D.; Wang, L.-N.; Xu, W.-N.; Liu, W.-B. Molecular characterization of carnitine palmitoyltransferase IA in Megalobrama amblycephala and effects on its expression of feeding status and dietary lipid and berberine. Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 2016, 191, 20–25. [Google Scholar] [CrossRef]
- Han, B.; Kou, S.-M.; Chen, B.; Peng, Y.-Z.; Wang, Y.; Han, Y.-L.; Ye, X.-L.; Li, X.-G. Efficient and rapid liquid reduction animal model. China J. Chin. Mater. Med. 2015, 40, 4446–4451. [Google Scholar]
- Xu, J.; Xie, S.; Chi, S.; Zhang, S.; Cao, J.; Tan, B. Short-term dietary antibiotics altered the intestinal microbiota and improved the lipid metabolism in hybrid grouper fed medium and high-lipid diets. Aquaculture 2022, 547, 737453. [Google Scholar] [CrossRef]
- Almeida, A.R.; Tacão, M.; Machado, A.L.; Golovko, O.; Zlabek, V.; Domingues, I.; Henriques, I. Long-term effects of oxytetracycline exposure in zebrafish: A multi-level perspective. Chemosphere 2019, 222, 333–344. [Google Scholar] [CrossRef]
- Zhou, X.; Peng, Y.; Li, L.; He, K.; Huang, T.; Mou, S.; Feng, M.; Han, B.; Ye, X.; Li, X. Effects of dietary supplementations with the fibrous root of Rhizoma Coptidis and its main alkaloids on non-specific immunity and disease resistance of common carp. Vet. Immunol. Immunopathol. 2016, 173, 34–38. [Google Scholar] [CrossRef] [PubMed]
- Bustos, A.Y.; Font de Valdez, G.; Fadda, S.; Taranto, M.P. New insights into bacterial bile resistance mechanisms: The role of bile salt hydrolase and its impact on human health. Food Res. Int. 2018, 112, 250–262. [Google Scholar] [CrossRef]
- Zhang, H.; Ran, C.; Teame, T.; Ding, Q.; Hoseinifar, S.H.; Xie, M.; Zhang, Z.; Yang, Y.; Olsen, R.E.; Gatlin, D.M.; et al. Research progress on gut health of farmers teleost fish: A viewpoint concerning the intestinal mucosal barrier and the impact of its damage. Rev. Fish Biol. Fish. 2020, 30, 569–586. [Google Scholar] [CrossRef]
- Zhao, Y.; Hao, Q.; Zhang, Q.; Yang, Y.; Ran, C.; Xu, Q.; Wu, C.; Liu, W.; Li, S.; Zhang, Z.; et al. Nuclease treatment enhanced the ameliorative effect of yeast culture on epidermal mucus, hepatic lipid metabolism, inflammation response and gut microbiota in high-fat diet-fed zebrafish. Fish Shellfish Immunol. 2022, 131, 1182–1191. [Google Scholar] [CrossRef] [PubMed]
- Navarrete, P.; Mardones, P.; Opazo, R.; Espejo, R.; Romero, J. Oxytetracycline treatment reduces bacterial diversity of intestinal microbiota of Atlantic salmon. J. Aquat. Anim. Health 2008, 20, 177–183. [Google Scholar] [CrossRef]
- Yang, L.; Liu, M.; Zhao, M.; Zhi, S.; Zhang, W.; Qu, L.; Xiong, J.; Yan, X.; Qin, C.; Nie, G. Dietary bile acid supplementation could regulate the glucose, lipid metabolism, and microbiota of common carp (Cyprinus carpio L.) fed with a high-lipid diet. Aquacult. Nutr. 2023, 2023, 9953927. [Google Scholar] [CrossRef]
- Das, S.; Ward, L.R.; Burke, C. Prospects of using marine actinobacteria as probiotics in aquaculture. Appl. Microbiol. Biotechnol. 2008, 81, 419–429. [Google Scholar] [CrossRef]
- Fjellheim, A.J.; Klinkenberg, G.; Skjermo, J.; Aasen, I.M.; Vadstein, O. Selection of candidate probionts by two different screening strategies from Atlantic cod (Gadus morhua L.) larvae. Vet. Microbiol. 2010, 144, 153–159. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Zhou, W.-W.; Shi, D.-D.; Pan, F.-F.; Sun, W.-W.; Yang, P.-L.; Li, X.-M. The interaction between oxidative stress biomarkers and gut microbiota in the antioxidant effects of extracts from Sonchus brachyotus DC. in oxazolone-induced intestinal oxidative stress in adult zebrafish. Antioxidants 2023, 12, 192. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Qi, S.; Mu, X.; Yuan, L.; Li, Y.; Qiu, J. Bisphenol F induces liver-gut alteration in zebrafish. Sci. Total Environ. 2022, 851, 157974. [Google Scholar] [CrossRef] [PubMed]
- Banna, G.L.; Torino, F.; Marletta, F.; Santagati, M.; Salemi, R.; Cannarozzo, E.; Falzone, L.; Ferraù, F.; Libra, M. Lactobacillus rhamnosus GG: An overview to explore the rationale of its use in cancer. Front. Pharmacol. 2017, 8, 603. [Google Scholar] [CrossRef]
- Li, Y.; Yan, H.; Zhang, Y.; Li, Q.; Yu, L.; Li, Q.; Liu, C.; Xie, Y.; Chen, K.; Ye, F.; et al. Alterations of the gut microbiome composition and lipid metabolic profile in radiation enteritis. Front. Cell. Infect. Microbiol. 2020, 10, 541178. [Google Scholar] [CrossRef] [PubMed]
- Chattopadhyay, I.; Gundamaraju, R.; Jha, N.K.; Gupta, P.K.; Dey, A.; Mandal, C.C.; Ford, B.M. Interplay between dysbiosis of gut microbiome, lipid metabolism, and tumorigenesis: Can gut dysbiosis stand as a prognostic marker in cancer? Dis. Markers 2022, 2022, 2941248. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Zhao, L.; He, Q.; Adam, A.A.; He, K.; Li, L.; Zhang, X.; Luo, J.; Luo, W.; Li, Z.; et al. Intermittent hypoxia exposure alleviates 2,4,6-trinitrobenzene sulfonic acid-induced enteritis by enhancing the intestinal barrier and inhibiting endoplasmic reticulum stress in juvenile largemouth bass. Aquaculture 2023, 563, 738951. [Google Scholar] [CrossRef]
- Guo, H.; Fu, X.; Lin, Q.; Liu, L.; Liang, H.; Huang, Z.; Li, N.; Su, J. Mandarin fish p53: Genomic structure, alternatively spliced variant and its mRNA expression after virus challenge. Fish Shellfish Immunol. 2017, 70, 536–544. [Google Scholar] [CrossRef]
- Xue, M.; Xu, P.; Wen, H.; Chen, J.; Wang, Q.; He, J.; He, C.; Kong, C.; Li, X.; Li, H.; et al. A high-fat-diet-induced microbiota imbalance correlates with oxidative stress and the inflammatory response in the gut of freshwater drum (Aplodinotus grunniens). Antioxidants 2024, 13, 363. [Google Scholar] [CrossRef]
- Fei, S.; Xia, Y.; Chen, Z.; Liu, C.; Liu, H.; Han, D.; Jin, J.; Yang, Y.; Zhu, X.; Xie, S. A high-fat diet alters lipid accumulation and oxidative stress and reduces the disease resistance of overwintering hybrid yellow catfish (Pelteobagrus fulvidraco♀×P. vachelli♂). Aquacult. Rep. 2022, 23, 101043. [Google Scholar] [CrossRef]
- Wang, N.; Westerterp, M. ABC transporters, cholesterol efflux, and implications for cardiovascular diseases. In Lipid Transfer in Lipoprotein Metabolism and Cardiovascular Disease; Jiang, X.-C., Ed.; Springer: Berlin/Heidelberg, Germany, 2020; Volume 1276, pp. 67–83. [Google Scholar]
- Xu, J.H.; Liu, X.Z.; Pan, W.; Zou, D.J. Berberine protects against diet-induced obesity through regulating metabolic endotoxemia and gut hormone levels. Mol. Med. Rep. 2017, 15, 2765–2787. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Shou, J.W.; Li, X.Y.; Zhao, Z.X.; Fu, J.; He, C.Y.; Feng, R.; Ma, C.; Wen, B.Y.; Guo, F.; et al. Berberine-induced bioactive metabolites of the gut microbiota improve energy metabolism. Metab. Clin. Exp. 2017, 70, 72–84. [Google Scholar] [CrossRef] [PubMed]
- Varrone, C.; Heggeset, T.M.B.; Le, S.B.; Haugen, T.; Markussen, S.; Skiadas, I.V.; Gavala, H.N. Comparison of different strategies for selection/adaptation of mixed microbial cultures able to ferment crude glycerol derived from second-generation biodiesel. BioMed Res. Int. 2015, 2015, 932934. [Google Scholar] [CrossRef]
- Ruiz, A.; Andree, K.B.; Furones, D.; Holhorea, P.G.; Calduch-Giner, J.; Viñas, M.; Pérez-Sánchez, J.; Gisbert, E. Modulation of gut microbiota and intestinal immune response in gilthead seabream (Sparus aurata) by dietary bile salt supplementation. Front. Microbiol. 2023, 14, 1123716. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.H. Endothelial focal adhesions and barrier function. J. Physiol. 2005, 569, 359–366. [Google Scholar] [CrossRef]
- Kong, W.-G.; Li, S.-S.; Chen, X.-X.; Huang, Y.-Q.; Tang, Y.; Wu, Z.-X. A study of the damage of the intestinal mucosa barrier structure and function of Ctenopharyngodon idella with Aeromonas hydrophila. Fish Physiol. Biochem. 2017, 43, 1223–1235. [Google Scholar] [CrossRef] [PubMed]
- Tran, N.T.; Li, Z.; Ma, H.; Zhang, Y.; Zheng, H.; Gong, Y.; Li, S. Clostridium butyricum: A promising probiotic confers positive health benefits in aquatic animals. Rev. Aquac. 2020, 12, 2573–2589. [Google Scholar] [CrossRef]
- Xia, R.; Zhang, Q.; Xia, D.; Hao, Q.; Ding, Q.; Ran, C.; Yang, Y.; Cao, A.; Zhang, Z.; Zhou, Z. The direct and gut microbiota-mediated effects of dietary bile acids on the improvement of gut barriers in largemouth bass (Micropterus salmoides). Anim. Nutr. 2023, 14, 32–42. [Google Scholar] [CrossRef]
- Vargas-Albores, F.; Martínez-Córdova, L.R.; Hernández-Mendoza, A.; Cicala, F.; Lago-Lestón, A.; Martínez-Porchas, M. Therapeutic modulation of fish gut microbiota, a feasible strategy for aquaculture? Aquaculture 2021, 544, 737050. [Google Scholar] [CrossRef]
- Kim, D.-G.; Lee, S.-J.; Lee, J.M.; Lee, E.-W.; Jang, W.J. Changes in the gut microbiota composition of juvenile olive flounder (Paralichthys olivaceus) caused by pathogenic bacterial infection. Fishes 2023, 8, 294. [Google Scholar] [CrossRef]
- Wang, S.; Li, X.; Zhang, M.; Li, M. Effects of dietary sodium acetate on growth, intestinal microbiota composition, and ammonia tolerance of juvenile yellow catfish Pelteobagrus fulvidraco. Aquaculture 2024, 581, 740480. [Google Scholar] [CrossRef]
- Wang, L.; Hu, C.; Wang, B.; Wang, H.; Wang, C.; Shu, Y.; Gao, C.; Yan, Y. Chronic environmentally relevant concentration of copper exposure induces intestinal oxidative stress, inflammation, and microbiota disturbance in freshwater grouper (Acrossocheilus fasciatus). Aquat. Toxicol. 2023, 263, 106702. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Ji, T.; Jiang, Y.; Zheng, C.; Yang, H.; Liu, Q. Long-term effects of three compound probiotics on water quality, growth performances, microbiota distributions and resistance to Aeromonas veronii in crucian carp Carassius auratus gibelio. Fish Shellfish Immunol. 2022, 120, 233–241. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.; Huang, J.; Rao, L.; Zhu, W.; Yu, Y.; Xiao, F.; Chen, X.; Yu, H.; Wu, Y.; Xu, K.; et al. Resistance and resilience of fish gut microbiota to silver nanoparticles. mSystems 2021, 6, e0063021. [Google Scholar] [CrossRef] [PubMed]
- Amoah, K.; Huang, Q.-C.; Tan, B.-P.; Zhang, S.; Chi, S.-Y.; Yang, Q.-H.; Liu, H.-Y.; Dong, X.-H. Dietary supplementation of probiotic Bacillus coagulans ATCC 7050, improves the growth performance, intestinal morphology, microflora, immune response, and disease confrontation of Pacific white shrimp, Litopenaeus vannamei. Fish Shellfish Immunol. 2019, 87, 796–808. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Long, M.; Ji, C.; Shen, Z.; Gatesoupe, F.-J.; Zhang, X.; Zhang, Q.; Zhang, L.; Zhao, Y.; Liu, X.; et al. Alterations of the gut microbiome of largemouth bronze gudgeon (Coreius guichenoti) suffering from furunculosis. Sci. Rep. 2016, 6, 30606. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Bao, X.; Yue, Y.; Yang, K.; Liu, H.; Yang, Y.; Yu, H.; Yu, Y.; Duan, N. Combined effects of cadmium and nanoplastics on oxidative stress, histopathology, and intestinal microbiota in largemouth bass (Micropterus salmoides). Aquaculture 2023, 569, 739363. [Google Scholar] [CrossRef]
- Tan, P.; Wu, X.; Zhu, W.; Lou, B.; Chen, R.; Wang, L. Effect of tributyrin supplementation in high-soya bean meal diet on growth performance, body composition, intestine morphology and microbiota of juvenile yellow drum (Nibea albiflora). Aquacult. Res. 2020, 51, 2004–2019. [Google Scholar] [CrossRef]
- Xu, X.; Yi, H.; Wu, J.; Kuang, T.; Zhang, J.; Li, Q.; Du, H.; Xu, T.; Jiang, G.; Fan, G. Therapeutic effect of berberine on metabolic diseases: Both pharmacological data and clinical evidence. Biomed. Pharmacother. 2021, 133, 110984. [Google Scholar] [CrossRef] [PubMed]
- Medina-Félix, D.; Garibay-Valdez, E.; Vargas-Albores, F.; Martínez-Porchas, M. Fish disease and intestinal microbiota: A close and indivisible relationship. Rev. Aquac. 2022, 15, 820–839. [Google Scholar] [CrossRef]
- Wang, E.; Yuan, Z.; Wang, K.; Gao, D.; Liu, Z.; Liles, M.R. Consumption of florfenicol-medicated feed alters the composition of the channel catfish intestinal microbiota including enriching the relative abundance of opportunistic pathogens. Aquaculture 2019, 501, 111–118. [Google Scholar] [CrossRef]
- Yang, H.-T.; Zou, S.-S.; Zhai, L.-J.; Wang, Y.; Zhang, F.-M.; An, L.-G.; Yang, G.-W. Pathogen invasion changes the intestinal microbiota composition and induces innate immune responses in the zebrafish intestine. Fish Shellfish Immunol. 2017, 71, 35–42. [Google Scholar] [CrossRef]
- Wang, L.; Wang, B.; Hu, C.; Wang, C.; Gao, C.; Jiang, H.; Yan, Y. Influences of chronic copper exposure on intestinal histology, antioxidative and immune status, and transcriptomic response in freshwater grouper (Acrossocheilus fasciatus). Fish Shellfish Immunol. 2023, 139, 108861. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Wang, C.; Huang, C.; Gao, C.; Wang, B.; He, J.; Yan, Y. Dietary berberine against intestinal oxidative stress, inflammation response, and microbiota disturbance caused by chronic copper exposure in freshwater grouper (Acrossocheilus fasciatus). Fish Shellfish Immunol. 2023, 139, 108910. [Google Scholar] [CrossRef] [PubMed]
- Yan, W.; Hamid, N.; Deng, S.; Jia, P.-P.; Pei, D.-S. Individual and combined toxicogenetic effects of microplastics and heavy metals (Cd, Pb, and Zn) perturb gut microbiota homeostasis and gonadal development in marine medaka (Oryzias melastigma). J. Hazard. Mater. 2020, 397, 122795. [Google Scholar] [CrossRef]
- Li, Z.; Yan, L.; Junaid, M.; Chen, X.; Liao, H.; Gao, D.; Wang, Q.; Zhang, Y.; Wang, J. Impacts of polystyrene nanoplastics at the environmentally relevant and sub-lethal concentrations on the oxidative stress, immune responses, and gut microbiota to grass carp (Ctenopharyngodon idella). J. Hazard. Mater. 2023, 441, 129995. [Google Scholar] [CrossRef]
- Wu, Y.; Liu, W.; Li, Q.; Li, Y.; Yan, Y.; Huang, F.; Wu, X.; Zhou, Q.; Shu, X.; Ruan, Z. Dietary chlorogenic acid regulates gut microbiota, serum-free amino acids and colonic serotonin levels in growing pigs. Int. J. Food Sci. Nutr. 2018, 69, 566–573. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhang, P.; Yu, P.; Shang, X.; Lu, Y.; Li, Y. Transcriptome analysis reveals the mechanism of common carp brain injury after exposure to lead. Sci. Total Environ. 2020, 743, 140796. [Google Scholar] [CrossRef]
- Cheesman, S.E.; Neal, J.T.; Mittge, E.; Seredick, B.M.; Guillemin, K. Epithelial cell proliferation in the developing zebrafish intestine is regulated by the Wnt pathway and microbial signaling via Myd88. Proc. Natl. Acad. Sci. USA 2011, 108, 4570–4577. [Google Scholar] [CrossRef] [PubMed]
- Min, X.-H.; Yu, T.; Qing, Q.; Yuan, Y.-H.; Zhong, W.; Chen, G.-C.; Zhao, L.-N.; Deng, N.; Zhang, L.-F.; Chen, Q.-K. Abnormal differentiation of intestinal epithelium and intestinal barrier dysfunction in diabetic mice associated with depressed Notch/NICD transduction in Notch/Hes1 signal pathway. Cell Biol. Int. 2014, 38, 1194–1204. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, I.; Chandrakesan, P.; Tawfik, O.; Xia, L.; Anant, S.; Umar, S. Critical roles of Notch and Wnt/β-Catenin pathways in the regulation of hyperplasia and/or colitis in response to bacterial infection. Infect. Immun. 2012, 80, 3107–3121. [Google Scholar] [CrossRef] [PubMed]




| Ingredients (g/kg) | Con | HL | HLA | HLB | HLAB |
|---|---|---|---|---|---|
| Fishmeal | 300 | 300 | 300 | 300 | 300 |
| Fermented soybean meal | 250 | 250 | 250 | 250 | 250 |
| Corn meal | 180 | 180 | 180 | 180 | 180 |
| Corn gluten meal | 80 | 80 | 80 | 80 | 80 |
| Corn oil | 10 | 30 | 30 | 30 | 30 |
| Fish oil | 10 | 30 | 30 | 30 | 30 |
| Soybean lecithin | 20 | 20 | 20 | 20 | 20 |
| Vitamin premix 2 | 15 | 15 | 15 | 15 | 15 |
| Mineral premix 3 | 15 | 15 | 15 | 15 | 15 |
| Lysine | 10 | 10 | 10 | 10 | 10 |
| Methionine | 10 | 10 | 10 | 10 | 10 |
| Berberine 4 | 0 | 0 | 0 | 0.4 | 0.4 |
| Antibiotic cocktail 5 | 0 | 0 | 2.43 | 0.00 | 2.43 |
| Sodium alginate | 15 | 15 | 15 | 15 | 15 |
| Carboxymethylcellulose | 59.5 | 19.5 | 19.1 | 18.29 | 18.69 |
| CaHPO4·2H2O | 20 | 20 | 20 | 20 | 20 |
| Betaine | 5 | 5 | 5 | 5 | 5 |
| Antioxidants | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 |
| Total | 1000 | 1000 | 1000 | 1000 | 1000 |
| Approximate analyzed composition | |||||
| Moisture | 52.9 | 54.6 | 60.2 | 61.6 | 59.3 |
| Crude protein | 422.3 | 415.5 | 416.8 | 411.1 | 420.1 |
| Crude lipid | 81.7 | 128.0 | 130.5 | 126.7 | 127.3 |
| Ash | 96.4 | 90.5 | 92.3 | 92.1 | 93.5 |
| Gene | Primer Sequences (5′to·3′) | Accession Number |
|---|---|---|
| accα | F: AGGAGGACAGCAAGAGCATT | NM_001271308.1 |
| R: TGATCTGTCGGTCTTTGTGC | ||
| cpt1 | F: GTCCCGATCAGTAGGTACA | NM_001044854.1 |
| R: TCCCATTGAGCAGAACAGAG | ||
| cyp7a1 | F: ACCTTCAACGAGCTGAGCAA | NM_201173.2 |
| R: TGTCCAACTGCTCCCTTGTC | ||
| fas | F: CAGATAAAGTGCAGACTGAGGAAGC | XM_685355.7 |
| R: GTATGACCTACAGTACGACTGCTCA | ||
| fgf19 | F: AGCTCGGACAGTAAGTTTGAT | NM_001012246.2 |
| R: TTGTAGCCGTCTGGAAGGATG | ||
| fxr | F: ACATCGTGCATGATCCGTC | XM_005166733.4 |
| R: GCACTTCTGTAAGCAGACACTC | ||
| hsl | F: GCAATCCATCTACGTTGGTACT | XM_005159495.4 |
| R: CGTCTCATATGCATTGCCAGT | ||
| il1β | F: CTGGAGATGTGGACTTCGCA | NM_212844.2 |
| R: CGTTCACTTCACGCTCTTGG | ||
| lxr | F: TCTTCTCAGCAGACCGACCA | YP_006568.1 |
| R: CGTAGGCTGACCAGCTTCAT | ||
| nfκb | F: CTAACTACAGCGGACACACG | NM_213184.2 |
| R: CAGGTCTACGGCCAAATGGA | ||
| pparα | F: CTGCGGGACATCTCTCAGTC | NM_001102567.1 |
| R: CTCGACATCTCGTTCTCCCG | ||
| shp | F: TTAGCGACATCTCGCCACTC | NM_001256191.1 |
| R: CCATTGCACTTGCACCTTCC | ||
| srebp1 | F: CAGCCGCAGTTCATTAAGGC | NM_001105129.1 |
| R: ACGTCCACTTCCATGGTCAC | ||
| tgr5 | F: CTGGAGCGCCTGCTCTT | XM_017357898.2 |
| R: CAGCGAGTCCACGAGTATCC | ||
| tnfα | F: AGCAGCATGGTGAGATACGA | NM_001024447.1 |
| R: CCTTCTTCGTTTGGCTTCATCA | ||
| zo1 | F: CCTCTCCCCTACCTCACACA | XM_009303250.3 |
| R: GTACCATGCCGCTAGGACC | ||
| β-actin | F: GGACTCTGGTGATGGTGTGA | EU161066 |
| R: CTGTAGCCTCTCTCGGTCAG |
| Index 2 | Con | HL | HLA | HLB | HLAB |
|---|---|---|---|---|---|
| SR | 91.11 ± 2.22 a | 73.33 ± 3.84 b | 82.22 ± 3.85 ab | 82.22 ± 3.85 ab | 84.44 ± 4.44 ab |
| FW | 0.30 ± 0.06 ab | 0.29 ± 0.06 ab | 0.27 ± 0.04 b | 0.47 ± 0.08 a | 0.45 ± 0.15 ab |
| WG | 206 ± 37 ab | 193 ± 35 ab | 173 ± 27 b | 370 ± 50 a | 350 ± 90 ab |
| SGR | 3.06 ± 0.83 abc | 3.22 ± 0.67 ab | 3.31 ± 0.34 a | 5.11 ± 0.37 c | 4.88 ± 0.63 bc |
| CF | 4.35 ± 0.13 a | 4.31 ± 0.19 a | 4.80 ± 0.09 a | 3.53 ± 0.30 b | 2.33 ± 0.21 c |
| FI | 9.30 ± 0.14 c | 11.00 ± 0.28 bc | 11.20 ± 1.09 bc | 16.28 ± 0.12 a | 12.07 ± 0.61 b |
| Index | Con | HL | HLA | HLB | HLAB |
|---|---|---|---|---|---|
| Observed OTUs | 443 ± 21 | 484 ± 175 | 380 ± 50 | 409 ± 18 | 396 ± 40 |
| Shannon | 5.42 ± 0.53 | 5.05 ± 0.46 | 4.17 ± 0.98 | 5.20 ± 0.42 | 4.51 ± 0.83 |
| Simpson | 0.91 ± 0.04 | 0.89 ± 0.04 | 0.80 ± 0.13 | 0.91 ± 0.03 | 0.81 ± 0.13 |
| Pielou-E | 0.62 ± 0.06 | 0.57 ± 0.05 | 0.49 ± 0.10 | 0.60 ±0.05 | 0.52 ± 0.10 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, C.; Wang, H.; Xue, X.; Qi, L.; Lin, Y.; Wang, L. The Potential Role of Intestinal Microbiota on the Intestine-Protective and Lipid-Lowering Effects of Berberine in Zebrafish (Danio rerio) Under High-Lipid Stress. Metabolites 2025, 15, 118. https://doi.org/10.3390/metabo15020118
Gao C, Wang H, Xue X, Qi L, Lin Y, Wang L. The Potential Role of Intestinal Microbiota on the Intestine-Protective and Lipid-Lowering Effects of Berberine in Zebrafish (Danio rerio) Under High-Lipid Stress. Metabolites. 2025; 15(2):118. https://doi.org/10.3390/metabo15020118
Chicago/Turabian StyleGao, Chang, Heng Wang, Xuan Xue, Lishun Qi, Yanfeng Lin, and Lei Wang. 2025. "The Potential Role of Intestinal Microbiota on the Intestine-Protective and Lipid-Lowering Effects of Berberine in Zebrafish (Danio rerio) Under High-Lipid Stress" Metabolites 15, no. 2: 118. https://doi.org/10.3390/metabo15020118
APA StyleGao, C., Wang, H., Xue, X., Qi, L., Lin, Y., & Wang, L. (2025). The Potential Role of Intestinal Microbiota on the Intestine-Protective and Lipid-Lowering Effects of Berberine in Zebrafish (Danio rerio) Under High-Lipid Stress. Metabolites, 15(2), 118. https://doi.org/10.3390/metabo15020118






