Effects of Acorns on Subcutaneous Fat Deposition in Yuxi Black Pigs by Transcriptomic Analysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Design and Diets
2.2. Slaughter and Sample Collection
2.3. Backfat Thickness
2.4. Determination of Lipase Activity
2.5. RNA Extraction, Sequencing, and Transcriptome Data Analysis
2.6. Differential Expression and Functional Analysis
2.7. The qRT-PCR Validation
2.8. Statistical Analysis
3. Results
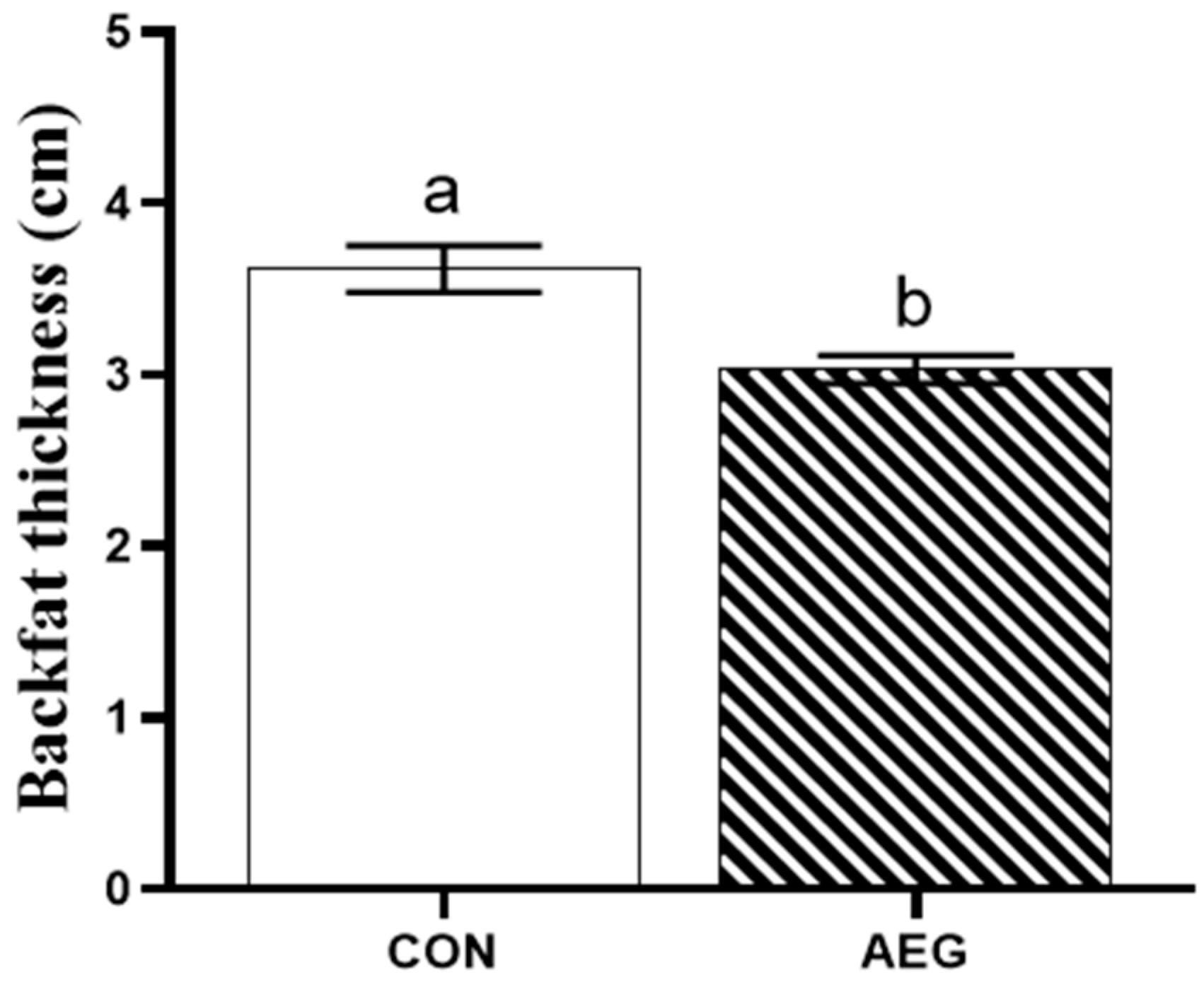
3.1. Backfat Thickness
3.2. Lipase Activity
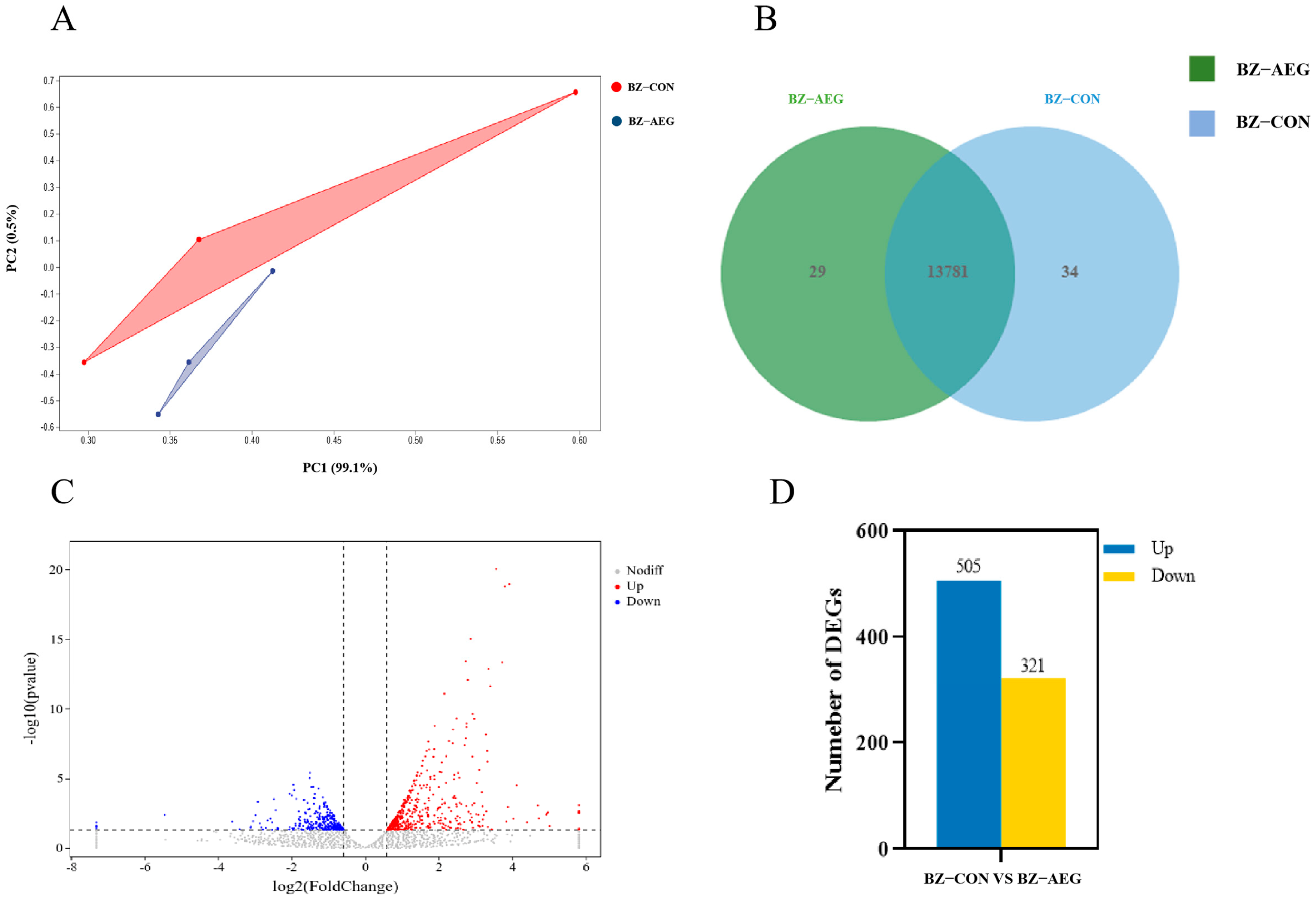
3.3. Overview of Sequencing Data
3.4. Screening DEGs
3.5. GO Enrichment Analysis of DEGs
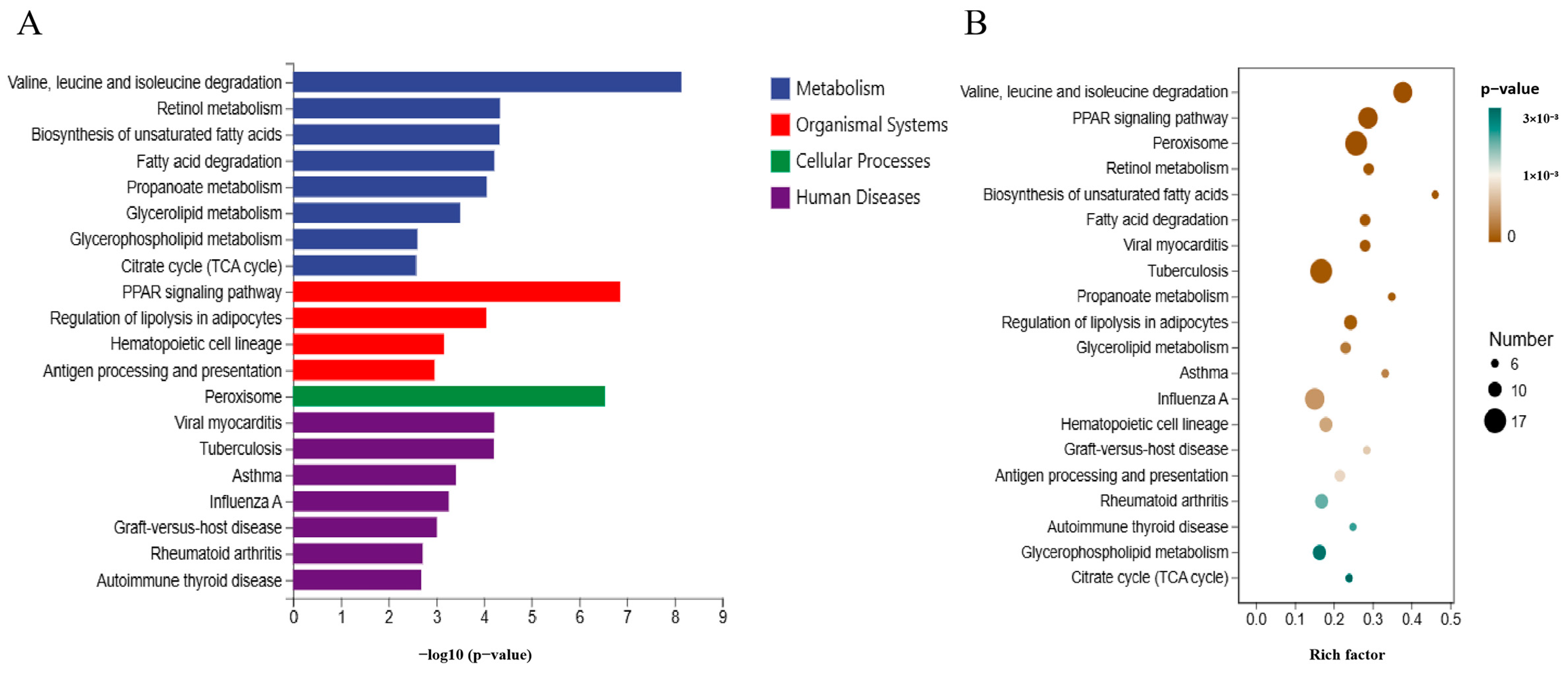
3.6. KEGG Pathway Analysis of DEGs
3.7. The qRT-PCR Validation of Transcriptome Data Results
4. Discussion
4.1. Backfat Thickness
4.2. Lipase Activity
4.3. Analysis of Transcriptome Data Results
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tous, N.; Lizardo, R.; Vilà, B.; Gispert, M.; Font-I-Furnols, M.; Esteve-Garcia, E. Effect of a High Dose of CLA in Finishing Pig Diets on Fat Deposition and Fatty Acid Composition in Intramuscular Fat and Other Fat Depots. Meat Sci. 2013, 93, 517–524. [Google Scholar] [CrossRef] [PubMed]
- Scollan, N.D.; Price, E.M.; Morgan, S.A.; Huws, S.A.; Shingfield, K.J. Can We Improve the Nutritional Quality of Meat? Proc. Nutr. Soc. 2017, 76, 603–618. [Google Scholar] [CrossRef]
- Xing, K.; Zhao, X.; Liu, Y.; Zhang, F.; Tan, Z.; Qi, X.; Wang, X.; Ni, H.; Guo, Y.; Sheng, X.; et al. Identification of Differentially Expressed MicroRNAs and Their Potential Target Genes in Adipose Tissue from Pigs with Highly Divergent Backfat Thickness. Animals 2020, 10, 624. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, J.; Ji, Y.; Lin, X.; Zhao, Y. Effect of Betaine Diet on Growth Performance, Carcass Quality and Fat Deposition in Finishing Ningxiang Pigs. Animals 2021, 11, 3408. [Google Scholar] [CrossRef] [PubMed]
- Wiegand, B.R.; Parrish, F.C.; E Swan, J.; Larsen, S.T.; Baas, T.J. Conjugated Linoleic Acid Improves Feed Efficiency, Decreases Subcutaneous Fat, and Improves Certain Aspects of Meat Quality in Stress-Genotype Pigs. J. Anim. Sci. 2001, 79, 2187–2195. [Google Scholar] [CrossRef] [PubMed]
- Pasqualone, A.; Makhlouf, F.Z.; Barkat, M.; Difonzo, G.; Summo, C.; Squeo, G.; Caponio, F. Effect of Acorn Flour on the Physico-Chemical and Sensory Properties of Biscuits. Heliyon 2019, 5, e02242. [Google Scholar] [CrossRef]
- Szyndler-Nędza, M.; Świątkiewicz, M.; Migdał, Ł.; Migdał, W. The Quality and Health-Promoting Value of Meat from Pigs of the Native Breed as the Effect of Extensive Feeding with Acorns. Animals 2021, 11, 789. [Google Scholar] [CrossRef]
- Tejeda, J.F.; Hernández-Matamoros, A.; Paniagua, M.; González, E. Effect of Free-Range and Low-Protein Concentrated Diets on Growth Performance, Carcass Traits, and Meat Composition of Iberian Pig. Animals 2020, 10, 273. [Google Scholar] [CrossRef]
- Sun, Z.; Liu, D.; An, S.; Wu, X.; Zhang, J.; Miao, Z. Effects of Acorns on Fatty Acid Composition and Lipid Metabolism in Adipose Tissue of Yuxi Black Pigs. Animals 2024, 14, 3271. [Google Scholar] [CrossRef]
- Qiao, R.; Li, X.; Han, X.; Wang, K.; Lv, G.; Ren, G. Population Structure and Genetic Diversity of Four Henan Pig Populations. Anim. Genet. 2019, 50, 262–265. [Google Scholar] [CrossRef] [PubMed]
- Xing, K.; Wang, K.; Ao, H.; Chen, S.; Tan, Z.; Wang, Y.; Xitong, Z.; Yang, T.; Zhang, F.; Liu, Y.; et al. Comparative Adipose Transcriptome Analysis Digs out Genes Related to Fat Deposition in Two Pig Breeds. Sci. Rep. 2019, 9, 12925. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Hu, H.; Lin, H.; Wang, C.; Wang, Y.; Wang, J. Muscle Transcriptome Analysis Reveals Potential Candidate Genes and Pathways Affecting Intramuscular Fat Content in Pigs. Front. Genet. 2020, 11, 877. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Chang, Y.; Huang, L.; An, S.; Liu, D.; Zhang, J.; Miao, Z. Effects of Acorns on Meat Quality and Lipid Metabolism-Related Gene Expression in Muscle Tissues of Yuxi Black Pigs. Metabolites 2024, 14, 578. [Google Scholar] [CrossRef] [PubMed]
- Pan, H.; Huang, T.; Yu, L.; Wang, P.; Su, S.; Wu, T.; Bai, Y.; Teng, Y.; Wei, Y.; Zhou, L.; et al. Transcriptome Analysis of the Adipose Tissue of Luchuan and Duroc Pigs. Animals 2022, 12, 2258. [Google Scholar] [CrossRef] [PubMed]
- Hoa, V.; Seo, H.; Seong, P.; Cho, S.; Kang, S.; Kim, Y.; Moon, S.; Choi, Y.; Kim, J.; Seol, K. Back-Fat Thickness as a Primary Index Reflecting the Yield and Overall Acceptance of Pork Meat. Anim. Sci. J. = Nihon Chikusan Gakkaiho 2021, 92, e13515. [Google Scholar] [CrossRef]
- He, W.; A Posey, E.; Steele, C.C.; Savell, J.W.; Bazer, F.W.; Wu, G. Dietary Glycine Supplementation Enhances Postweaning Growth and Meat Quality of Pigs with Intrauterine Growth Restriction. J. Anim. Sci. 2023, 101, skad354. [Google Scholar] [CrossRef]
- Oanh, N.C.; Thu, C.T.T.; Hong, N.T.; Giang, N.T.P.; Hornick, J.-L.; Dang, P.K. Growth Performance, Meat Quality, and Blood Characteristics of Finisher Crossbred Pigs Fed Diets Supplemented with Different Levels of Green Tea (Camellia Sinensis) by-Products. Vet. World 2023, 16, 27–34. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Tian, X.; Yang, G.; Yu, T. The impact of phenolic compounds on pig fat deposition. Sheng Wu Gong Cheng Xue Bao = Chin. J. Biotechnol. 2024, 40, 1486–1497. [Google Scholar]
- Tejeda, J.F.; Hernández-Matamoros, A.; González, E. Free-Range and Low-Protein Concentrated Diets in Iberian Pigs: Effect on Plasma Insulin and Leptin Concentration, Lipogenic Enzyme Activity, and Fatty Acid Composition of Adipose Tissue. Animals 2020, 10, 1917. [Google Scholar] [CrossRef] [PubMed]
- Catlin, N.R.; Bowman, C.J.; Campion, S.N.; Davenport, S.D.; Esler, W.P.; Kumpf, S.W.; Lewis, E.M.; Nowland, W.S.; Ross, T.T.; Stedman, D.S.; et al. Inhibition of Acetyl-CoA Carboxylase Causes Malformations in Rats and Rabbits: Comparison of Mammalian Findings and Alternative Assays. Toxicol. Sci. Off. J. Soc. Toxicol. 2021, 179, 183–194. [Google Scholar] [CrossRef] [PubMed]
- Griffith, D.A.; Dow, R.L.; Huard, K.; Edmonds, D.J.; Bagley, S.W.; Polivkova, J.; Zeng, D.; Garcia-Irizarry, C.N.; Southers, J.A.; Esler, W.; et al. Spirolactam-Based Acetyl-CoA Carboxylase Inhibitors: Toward Improved Metabolic Stability of a Chromanone Lead Structure. J. Med. Chem. 2013, 56, 7110–7119. [Google Scholar] [CrossRef]
- Sun, S.; Meng, Q.; Luo, Z.; Shi, B.; Bi, C.; Shan, A. Effects of Dietary Resveratrol Supplementation during Gestation and Lactation of Sows on Milk Composition of Sows and Fat Metabolism of Sucking Piglets. J. Anim. Physiol. Anim. Nutr. 2019, 103, 813–821. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.; Youm, J.; Lee, W.J.; Kang, S.; Kim, Y.J. Polyphenol-Rich Apple Extract Inhibits Dexamethasone-Induced Sebaceous Lipids Production by Regulating SREBP1 Expression. Exp. Dermatol. 2017, 26, 958–960. [Google Scholar] [CrossRef] [PubMed]
- Selinski, J.; Scheibe, R. Malate Valves: Old Shuttles with New Perspectives. Plant Biol. 2019, 21 (Suppl. S1), 21–30. [Google Scholar] [CrossRef]
- Peterson, C.N.; Cornely, K.; Parente, A.D.; Springer, A.L.; Provost, J.J. Uncovering Malate Dehydrogenase: Structure, Function and Role in Disease. Essays Biochem. 2024, 68, 53–55. [Google Scholar] [PubMed]
- Lee, Y.B.; Kauffman, R.G. Cellularity and Lipogenic Enzyme Activities of Porcine Intramuscular Adipose Tissue. J. Anim. Sci. 1974, 38, 538–544. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Eckel, R.H. Lipoprotein Lipase: From Gene to Obesity. American Journal of Physiology. Endocrinol. Metab. 2009, 297, E271–E288. [Google Scholar]
- Chang, Q.; Lu, Z.; He, M.; Gao, R.; Bai, H.; Shi, B.; Shan, A. Effects of Dietary Supplementation of Fulvic Acid on Lipid Metabolism of Finishing Pigs. J. Anim. Sci. 2014, 92, 4921–4926. [Google Scholar] [CrossRef]
- Chen, X.; Chen, L.; Qin, Y.; Mao, Z.; Huang, Z.; Jia, G.; Zhao, H.; Liu, G. Dietary L-Theanine Supplementation Improves Lipid Metabolism and Antioxidant Capacity in Weaning Piglets. Anim. Biotechnol. 2022, 33, 1407–1415. [Google Scholar] [CrossRef]
- Xing, K.; Zhao, X.; Ao, H.; Chen, S.; Yang, T.; Tan, Z.; Wang, Y.; Zhang, F.; Liu, Y.; Ni, H.; et al. Transcriptome Analysis of miRNA and mRNA in the Livers of Pigs with Highly Diverged Backfat Thickness. Sci. Rep. 2019, 9, 16740. [Google Scholar] [CrossRef] [PubMed]
- Gong, X.; Zheng, M.; Zhang, J.; Ye, Y.; Duan, M.; Chamba, Y.; Wang, Z.; Shang, P. Transcriptomics-Based Study of Differentially Expressed Genes Related to Fat Deposition in Tibetan and Yorkshire Pigs. Front. Vet. Sci. 2022, 9, 919904. [Google Scholar] [CrossRef] [PubMed]
- Obradovic, M.; Sudar-Milovanovic, E.; Soskic, S.; Essack, M.; Arya, S.; Stewart, A.J.; Gojobori, T.; Isenovic, E.R. Leptin and Obesity: Role and Clinical Implication. Front. Endocrinol. 2021, 12, 585887. [Google Scholar] [CrossRef]
- Minokoshi, Y.; Kim, Y.-B.; Peroni, O.D.; Fryer, L.G.D.; Müller, C.; Carling, D.; Kahn, B.B. Leptin Stimulates Fatty-Acid Oxidation by Activating AMP-Activated Protein Kinase. Nature 2002, 415, 339–343. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, C.M.; Mancuso, D.J.; Yan, W.; Sims, H.F.; Gibson, B.; Gross, R.W. Identification, Cloning, Expression, and Purification of Three Novel Human Calcium-Independent Phospholipase A2 Family Members Possessing Triacylglycerol Lipase and Acylglycerol Transacylase Activities. J. Biol. Chem. 2004, 279, 48968–48975. [Google Scholar] [CrossRef]
- O’Rourke, L.; Yeaman, S.J.; Shepherd, P.R. Insulin and Leptin Acutely Regulate Cholesterol Ester Metabolism in Macrophages by Novel Signaling Pathways. Diabetes 2001, 50, 955–961. [Google Scholar] [CrossRef] [PubMed]
- Ramsay, T.G. Porcine Leptin Alters Insulin Inhibition of Lipolysis in Porcine Adipocytes in Vitro. J. Anim. Sci. 2001, 79, 653–657. [Google Scholar] [CrossRef] [PubMed]
- Siegrist-Kaiser, C.A.; Pauli, V.; Juge-Aubry, C.E.; Boss, O.; Pernin, A.; Chin, W.W.; Cusin, I.; Rohner-Jeanrenaud, F.; Burger, A.G.; Zapf, J.; et al. Direct Effects of Leptin on Brown and White Adipose Tissue. J. Clin. Investig. 1997, 100, 2858–2864. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Zhou, M. Structure of a Eukaryotic Cholinephosphotransferase-1 Reveals Mechanisms of Substrate Recognition and Catalysis. Nat. Commun. 2023, 14, 2753. [Google Scholar] [CrossRef] [PubMed]
- Chouchani, E.T.; Kazak, L.; Spiegelman, B.M. New Advances in Adaptive Thermogenesis: UCP1 and Beyond. Cell Metab. 2019, 29, 27–37. [Google Scholar] [CrossRef]
- Brun, S.; Carmona, M.C.; Mampel, T.; Viñas, O.; Giralt, M.; Iglesias, R.; Villarroya, F. Activators of Peroxisome Proliferator-Activated Receptor-Alpha Induce the Expression of the Uncoupling Protein-3 Gene in Skeletal Muscle: A Potential Mechanism for the Lipid Intake-Dependent Activation of Uncoupling Protein-3 Gene Expression at Birth. Diabetes 1999, 48, 1217–1222. [Google Scholar] [CrossRef] [PubMed]
- Duan, G.; Zheng, C.; Yu, J.; Zhang, P.; Wan, M.; Zheng, J.; Duan, Y. β-Hydroxy-β-Methyl Butyrate Regulates the Lipid Metabolism, Mitochondrial Function, and Fat Browning of Adipocytes. Nutrients 2023, 15, 2550. [Google Scholar] [CrossRef] [PubMed]
- Griffin, E.N.; Ackerman, S.L. Lipid Metabolism and Axon Degeneration: An ACOX1 Balancing Act. Neuron 2020, 106, 551–553. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.-H.; Liu, B.; Meng, Q.; Zhang, D.; Yang, H.; Li, G.; Wang, Y.; Liu, M.; Liu, N.; Yu, J.; et al. ACOX1 Deficiency-Induced Lipid Metabolic Disorder Facilitates Chronic Interstitial Fibrosis Development in Renal Allografts. Pharmacol. Res. 2024, 201, 107105. [Google Scholar] [CrossRef] [PubMed]
- Chi, Y.; Wu, Z.; Du, C.; Zhang, M.; Wang, X.; Xie, A.; Wang, P.; Li, R. Regulatory Effects Mediated by Ulvan Oligosaccharide and Its Zinc Complex on Lipid Metabolism in High-Fat Diet-Fed Mice. Carbohydr. Polym. 2023, 300, 120249. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Zou, X.; Chang, Q.; Zhang, Y.; Li, Y.; Zhang, L.; Huang, J.; Liang, B. The Evolutionary Pattern and the Regulation of Stearoyl-CoA Desaturase Genes. BioMed Res. Int. 2013, 2013, 856521. [Google Scholar] [CrossRef] [PubMed]
- Pinterić, M.; Podgorski, I.I.; Hadžija, M.P.; Bujak, I.T.; Tadijan, A.; Balog, T.; Sobočanec, S. Chronic High Fat Diet Intake Impairs Hepatic Metabolic Parameters in Ovariectomized Sirt3 KO Mice. Int. J. Mol. Sci. 2021, 22, 4277. [Google Scholar] [CrossRef]
- Strable, M.S.; Ntambi, J.M. Genetic Control of de Novo Lipogenesis: Role in Diet-Induced Obesity. Crit. Rev. Biochem. Mol. Biol. 2010, 45, 199–214. [Google Scholar] [CrossRef] [PubMed]
- Wanders, R.J.A.; Vreken, P.; Ferdinandusse, S.; Jansen, G.A.; Waterham, H.R.; Van Roermund, C.W.T.; Van Grunsven, E.G. Peroxisomal Fatty Acid Alpha- and Beta-Oxidation in Humans: Enzymology, Peroxisomal Metabolite Transporters and Peroxisomal Diseases. Biochem. Soc. Trans. 2001, 29 Pt 2, 250–267. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Liu, X.; Niu, L.; Li, Q. Proteomics Analysis Reveals an Important Role for the PPAR Signaling Pathway in DBDCT-Induced Hepatotoxicity Mechanisms. Molecules 2017, 22, 1113. [Google Scholar] [CrossRef]
- Wang, Y.; Li, X.; Cao, Y.; Xiao, C.; Liu, Y.; Jin, H. Effect of the ACAA1 Gene on Preadipocyte Differentiation in Sheep. Front. Genet. 2021, 12, 649140. [Google Scholar] [CrossRef] [PubMed]



| Item | Content | |
|---|---|---|
| CON | AEG | |
| Ingredient, % | ||
| Corn | 65.5 | 44.9 |
| Soybean meal | 3.0 | 5.9 |
| Wheat bran | 22.2 | 7.5 |
| Walnut dregs | 5.3 | 7.7 |
| Acorn | 0 | 30.0 |
| Premix | 4.0 | 4.0 |
| Total | 100 | 100 |
| Nutrient levels | ||
| ME, MJ·kg−1 | 11.8 | 11.4 |
| CP, % | 11.9 | 11.5 |
| Lys, % | 0.5 | 0.5 |
| Cys, % | 0.3 | 0.2 |
| Met + Cys, % | 0.5 | 0.4 |
| Protein energy ratio, g·MJ−1 | 10.1 | 10.1 |
| Gene | Accession Number | Primer Sequences, 5′-3′ |
|---|---|---|
| ACAA1 | XM_003132103.4 | F: ACGATGACAAGGGCAATGAGAAGAG R: ACAGGGATGGCATAGGCAGGTC |
| ACADL | NM_213897.1 | F: TTACAAATCGTGAAGCTCGCTCTCC R: CACCGTCTGTATATGTGCCACTGTC |
| ADIPOQ | EF601160.1 | F: TGTCTACCGTTCAGCATTCAGTGTG R: GAAGGAAGCCTGTGAAGATGGAGTC |
| SCD5 | NM_001114278.1 | F: ATCCTCCGCTACACCGTCTCAC R: CAGCCAGCACATGAAGTCAATGAAC |
| GAPDH | NM_001206359.1 | F: CAAGGCTGTGGGCAAGGTCATC R: AAGTGGTCGTTGAGGGCAATGC |
| ACOX1 | NM_001101028.1 | F: GTATGCCCAGGTGAAGCCAGATG R: ATTAGCCGTCCAGGATGTGAAAGC |
| UCP3 | NM_214049.1 | F: CCATCGCCAGGGAGGAAGGG R: TGTCCGTGAGCAGGTGGTAGTC |
| Sample | Clean Reads | Clean Bases | Useful Reads% | Useful Bases% | GC Content% | Q30 |
|---|---|---|---|---|---|---|
| BZ_CON1 | 44,888,886 | 6,737,128,958 | 98.01 | 97.42 | 46.48 | 94.94 |
| BZ_CON2 | 45,363,754 | 6,816,335,257 | 98.06 | 97.58 | 47.17 | 95.09 |
| BZ_CON3 | 46,014,420 | 6,884,658,949 | 98.16 | 97.27 | 45.83 | 95.31 |
| BZ_AEG1 | 41,436,780 | 6,209,061,499 | 97.97 | 97.22 | 46.02 | 95.06 |
| BZ_AEG2 | 47,571,278 | 7,129,574,508 | 98.14 | 97.41 | 47.16 | 95.01 |
| BZ_AEG3 | 53,530,398 | 8,042,326,168 | 98.22 | 97.72 | 47.04 | 95.23 |
| Sample | Total Reads | Mapped Reads | Multiple Mapped | Uniquely Mapped | Exon Mapped | Intergene Mapped |
|---|---|---|---|---|---|---|
| BZ_CON1 | 44,888,886 | 43,826,402 (97.63%) | 1,043,665 (2.38%) | 42,782,737 (97.62%) | 34,555,175 (84.95%) | 2,107,313 (4.93%) |
| BZ_CON2 | 45,363,754 | 44,421,001 (97.92%) | 1,088,487 (2.45%) | 43,332,514 (97.55%) | 36,293,893 (87.42%) | 1,813,785 (4.19%) |
| BZ_CON3 | 46,014,420 | 44,936,991 (97.66%) | 1,177,070 (2.62%) | 43,759,921 (97.38%) | 35,889,451 (86.09%) | 2,072,345 (4.74%) |
| BZ_AEG1 | 41,436,780 | 40,508,660 (97.76%) | 972,344 (2.40%) | 39,536,316 (97.60%) | 33,464,234 (88.57%) | 1,755,463 (4.44%) |
| BZ_AEG2 | 47,571,278 | 46,502,896 (97.75%) | 1,159,622 (2.49%) | 45,343,274 (97.51%) | 37,684,542 (86.75%) | 1,900,984 (4.19%) |
| BZ_AEG3 | 53,530,398 | 52,438,782 (97.96%) | 1,331,336 (2.54%) | 51,107,446 (97.46%) | 43,247,481 (88.23%) | 2,088,170 (4.09%) |
| Gene | Gene Name | Gene Description | Log2FC | p-Value |
|---|---|---|---|---|
| ENSSSCG00000040464 | LEP | leptin | 2.146658349 | 0.037113913 |
| ENSSSCG00000000864 | CHPT1 | choline phosphotransferase 1 | 1.10832771 | 0.000429612 |
| ENSSSCG00000014834 | UCP3 | uncoupling protein 3 | 3.804562568 | 1.73 × 10−19 |
| ENSSSCG00000017198 | ACOX1 | acyl-CoA oxidase 1 | 1.274871269 | 3.53 × 10−5 |
| ENSSSCG00000009245 | SCD5 | stearoyl-CoA desaturase 5 | −1.55683637 | 0.013021827 |
| ENSSSCG00000011250 | ACAA1 | acetyl-CoA acyltransferase 1 | 2.387704936 | 3.05 × 10−8 |
| ENSSSCG00000039103 | ADIPOQ | adiponectin | 2.767298331 | 1.20 × 10−9 |
| ENSSSCG00000016156 | ACADL | acyl-CoA dehydrogenase long chain | 1.175895382 | 0.000321714 |
| ENSSSCG00000015784 | ACSL1 | acyl-CoA synthetase long chain family member 1 | 1.851766159 | 0.000145487 |
| ENSSSCG00000002349 | ACOT4 | acyl-CoA thioesterase 4 | 0.746617108 | 0.014478849 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, Z.; Liu, D.; An, S.; Zhang, J.; Lei, L.; Miao, Z. Effects of Acorns on Subcutaneous Fat Deposition in Yuxi Black Pigs by Transcriptomic Analysis. Metabolites 2025, 15, 71. https://doi.org/10.3390/metabo15020071
Sun Z, Liu D, An S, Zhang J, Lei L, Miao Z. Effects of Acorns on Subcutaneous Fat Deposition in Yuxi Black Pigs by Transcriptomic Analysis. Metabolites. 2025; 15(2):71. https://doi.org/10.3390/metabo15020071
Chicago/Turabian StyleSun, Zhe, Dongyang Liu, Siyuan An, Jinzhou Zhang, Lei Lei, and Zhiguo Miao. 2025. "Effects of Acorns on Subcutaneous Fat Deposition in Yuxi Black Pigs by Transcriptomic Analysis" Metabolites 15, no. 2: 71. https://doi.org/10.3390/metabo15020071
APA StyleSun, Z., Liu, D., An, S., Zhang, J., Lei, L., & Miao, Z. (2025). Effects of Acorns on Subcutaneous Fat Deposition in Yuxi Black Pigs by Transcriptomic Analysis. Metabolites, 15(2), 71. https://doi.org/10.3390/metabo15020071








