Construction of a Plasmid-Free Escherichia coli Strain with Enhanced Heme Supply to Produce Active Hemoglobins
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. General Procedures
2.3. The Construction of Plasmids and Strains
2.4. Culture Conditions and the Preparation for the Samples
2.5. The Expression of Hemoglobins
2.6. Discovery Studio Analysis
2.7. Analytical Methods
3. Results and Discussion
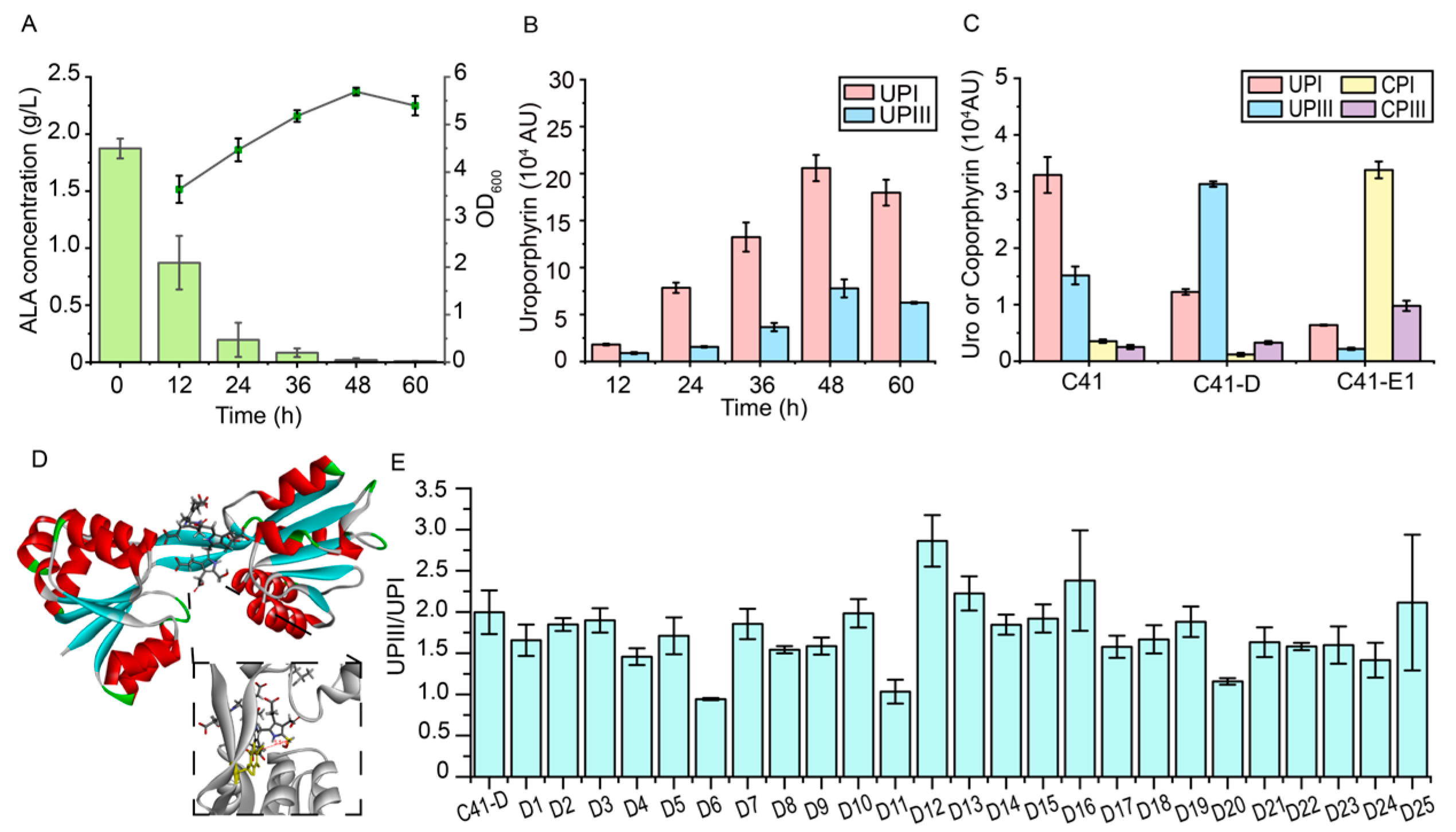
3.1. Identification of the Rate-Limiting Steps in the Downstream Heme Biosynthetic Pathway
3.2. The Engineering of UROS (HemD) to Reduce the Formation of By-Product UPGI
3.3. The Assembly of Rate-Limiting Enzymes HemC and HemD to Enhance the Synthesis of Intermediate UPGIII
3.4. Regulation of the Expressions of hemC and hemD Genes in the Genome to Enhance the Synthesis of Intermediate UPGIII
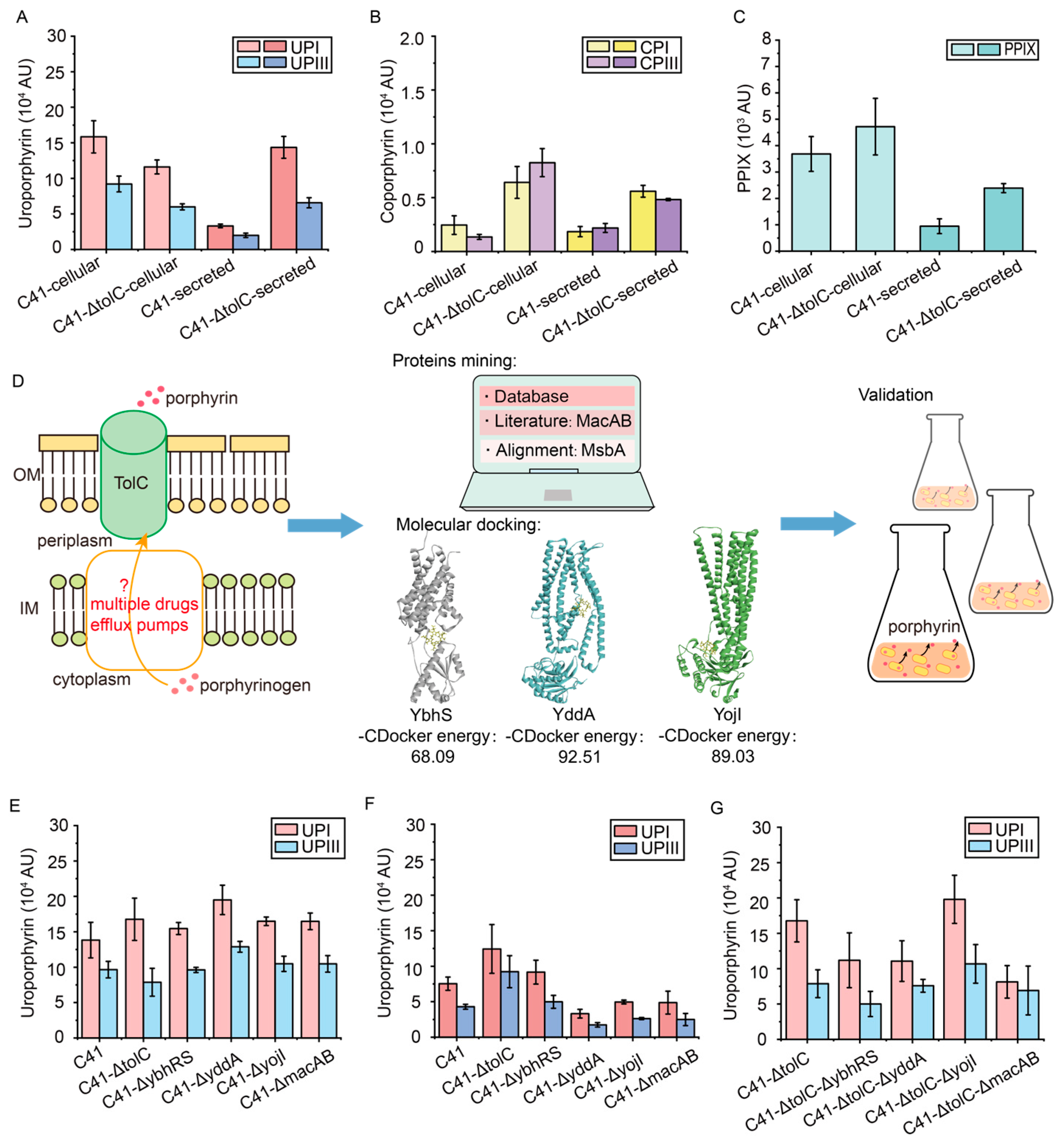
3.5. Reduce the Efflux of Porphyrin Intermediates by Transporter Engineering
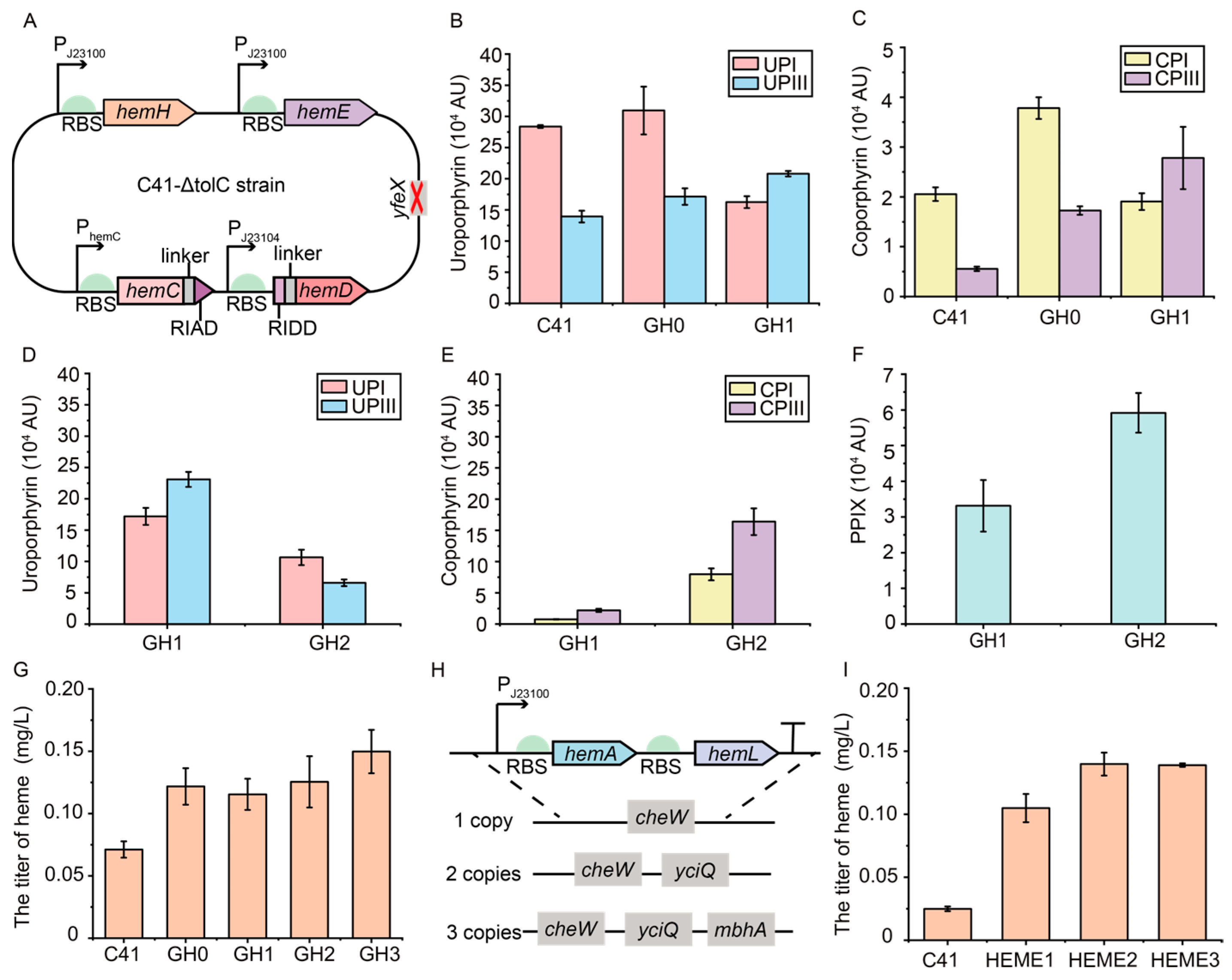
3.6. Construction of a Plasmid-Free Escherichia coli Strain with Enhanced Heme Supply
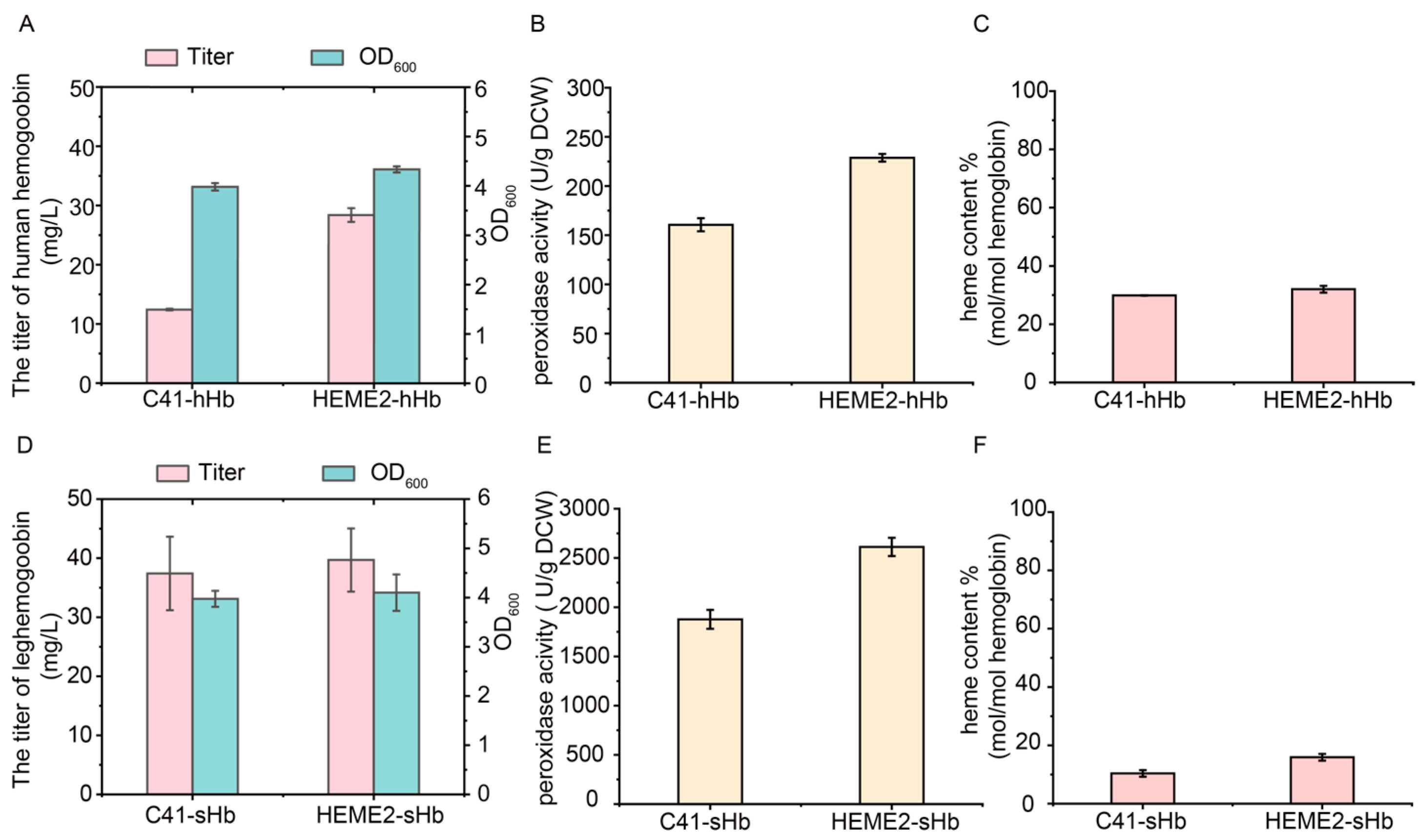
3.7. Efficient Synthesis of High-Active Hemoglobins Using the Strain HEME2 with Stable and Enhanced Heme Supply
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhang, J.; Cui, Z.; Zhu, Y.; Zhu, Z.; Qi, Q.; Wang, Q. Recent advances in microbial production of high-value compounds in the tetrapyrrole biosynthesis pathway. Biotechnol. Adv. 2022, 55, 107904. [Google Scholar] [CrossRef] [PubMed]
- Poulos, T.L. Heme enzyme structure and function. Chem. Rev. 2014, 114, 3919–3962. [Google Scholar] [CrossRef]
- Seligman, P.A.; Moore, G.M.; Schleicher, R.B. Clinical studies of hip: An oral heme-iron product. Nutr. Res. 2000, 20, 1279–1286. [Google Scholar] [CrossRef]
- Waltz, E. Appetite grows for biotech foods with health benefits. Nat. Biotechnol. 2019, 37, 573–575. [Google Scholar] [CrossRef] [PubMed]
- Kumar, N.; He, J.; Rusling, J.F. Electrochemical transformations catalyzed by cytochrome P450s and peroxidases. Chem. Soc. Rev. 2023, 52, 5135–5171. [Google Scholar] [CrossRef] [PubMed]
- Fischer, H.; Zeile, K. Synthese des haematoporphyrins, protoporphyrins und haemins. Eur. J. Org. Chem. 1929, 468, 98–116. [Google Scholar]
- In, M.-J.; Kim, D.C.; Chae, H.J.; Oh, N.-S. Effects of degree of hydrolysis and pH on the solubility of heme-iron enriched peptide in hemoglobin hydrolysate. Biosci. Biotechnol. Biochem. 2003, 67, 365–367. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, G.C.; Gong, J. 5-Aminolevulinate synthase and the first step of heme biosynthesis. J. Bioenerg. Biomembr. 1995, 27, 151–159. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.R.; Choi, K.R.; Lee, S.Y. Metabolic engineering of Escherichia coli for secretory production of free haem. Nat. Catal. 2018, 1, 720–728. [Google Scholar] [CrossRef]
- Su, H.; Chen, X.; Chen, S.; Guo, M.; Liu, H. Applications of the whole-cell system in the efficient biosynthesis of heme. Int. J. Mol. Sci. 2023, 24, 8384. [Google Scholar] [CrossRef] [PubMed]
- Belot, A.; Puy, H.; Hamza, I.; Bonkovsky, H.L. Update on heme biosynthesis, tissue-specific regulation, heme transport, relation to iron metabolism and cellular energy. Liver Int. 2024, 44, 2235–2250. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Wang, Y.; Wang, W.; Cao, T.; Zhang, L.; Wang, Z.; Chi, X.; Shi, T.; Wang, H.; He, X.; et al. High-yield porphyrin production through metabolic engineering and biocatalysis. Nat. Biotechnol. 2024, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Ishchuk, O.P.; Domenzain, I.; Sánchez, B.J.; Muñiz-Paredes, F.; Martínez, J.L.; Nielsen, J.; Petranovic, D. Genome-scale modeling drives 70-fold improvement of intracellular heme production in Saccharomyces cerevisiae. Proc. Natl. Acad. Sci. USA 2022, 119, e2108245119. [Google Scholar] [CrossRef] [PubMed]
- Ko, Y.J.; Kim, M.; You, S.K.; Shin, S.K.; Chang, J.; Choi, H.J.; Jeong, W.Y.; Lee, M.E.; Hwang, D.H.; Han, S.O. Animal-free heme production for artificial meat in Corynebacterium glutamicum via systems metabolic and membrane engineering. Metab. Eng. 2021, 66, 217–228. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.J. Effect of gene amplifications in porphyrin pathway on heme biosynthesis in a recombinant Escherichia coli. J. Microbiol. Biotechnol. 2013, 23, 668–673. [Google Scholar] [CrossRef] [PubMed]
- Geng, Z.; Ge, J.; Cui, W.; Zhou, H.; Deng, J.; Xu, B. Efficient de novo biosynthesis of heme by membrane engineering in Escherichia coli. Int. J. Mol. Sci. 2022, 23, 15524. [Google Scholar] [CrossRef] [PubMed]
- Hu, B.D.; Yu, H.B.; Zhou, J.W.; Li, J.H.; Chen, J.; Du, G.C.; Lee, S.Y.; Zhao, X.R. Whole-cell P450 biocatalysis Using engineered Escherichia coli with fine-tuned heme biosynthesis. Adv. Sci. 2022, 10, e2205580. [Google Scholar] [CrossRef] [PubMed]
- Ge, J.; Wang, X.; Bai, Y.; Wang, Y.; Wang, Y.; Tu, T.; Qin, X.; Su, X.; Luo, H.; Yao, B.; et al. Engineering Escherichia coli for efficient assembly of heme proteins. Microb. Cell Fact. 2023, 22, 59. [Google Scholar] [CrossRef]
- Zhang, J.; Li, Q.; Wang, Q.; Zhao, J.; Zhu, Y.; Su, T.; Qi, Q.; Wang, Q. Heme biosensor-guided in vivo pathway optimization and directed evolution for efficient biosynthesis of heme. Biotechnol. Biofuels Bioprod. 2023, 16, 33. [Google Scholar] [CrossRef] [PubMed]
- Arab, B.; Westbrook, A.W.; Moo-Young, M.; Liu, Y.; Perry Chou, C. Bio-based production of uroporphyrin in Escherichia coli. Synth. Biol. Eng. 2024, 2, 10002. [Google Scholar] [CrossRef]
- Arab, B.; Westbrook, A.; Moo-Young, M.; Liu, Y.; Chou, C.P. High-level bio-based production of coproporphyrin in Escherichia coli. Fermentation 2024, 10, 250. [Google Scholar] [CrossRef]
- Xu, J.; Zhu, Y.; Zhou, Z. Systematic engineering of the rate-limiting step of β-alanine biosynthesis in Escherichia coli. Electron. J. Biotechnol. 2021, 51, 88–94. [Google Scholar] [CrossRef]
- Yao, J.; He, Y.; Su, N.; Bharath, S.R.; Tao, Y.; Jin, J.-M.; Chen, W.; Song, H.; Tang, S.-Y. Developing a highly efficient hydroxytyrosol whole-cell catalyst by de-bottlenecking rate-limiting steps. Nat. Commun. 2020, 11, 1515. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Ouyang, X.; Meng, S.; Zhao, B.; Liu, L.; Li, C.; Li, H.; Zheng, H.; Liu, Y.; Shi, T.; et al. Rational multienzyme architecture design with iMARS. Cell 2025, 188, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Hu, M.; Bao, T.; Qin, Z.; Wang, Q.; Zhang, H.; Wang, Y.; You, J.; Xue, Z.; Zhang, R.; Yang, S.-T.; et al. Programmable Scaffold-Mediated Assembly Regulation Tool for Dynamic Control of a Multienzyme Biocatalyst. ACS Catal. 2025, 15, 2236–2249. [Google Scholar] [CrossRef]
- Wang, S.-Z.; Zhang, Y.-H.; Ren, H.; Wang, Y.-L.; Jiang, W.; Fang, B.-S. Strategies and perspectives of assembling multi-enzyme systems. Crit. Rev. Biotechnol. 2017, 37, 1024–1037. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Jiang, Y.; Liu, H.; Yuan, H.; Huang, D.; Wang, T. Research progress of multi-enzyme complexes based on the design of scaffold protein. Bioresour. Bioprocess. 2023, 10, 72. [Google Scholar] [CrossRef] [PubMed]
- Lin, P.; Yang, H.; Nakata, E.; Morii, T. Mechanistic Aspects for the Modulation of Enzyme Reactions on the DNA Scaffold. Molecules 2022, 27, 6309. [Google Scholar] [CrossRef] [PubMed]
- Han, G.H.; Seong, W.; Fu, Y.; Yoon, P.K.; Kim, S.K.; Yeom, S.-J.; Lee, D.-H.; Lee, S.-G. Leucine zipper-mediated targeting of multi-enzyme cascade reactions to inclusion bodies in Escherichia coli for enhanced production of 1-butanol. Metab. Eng. 2017, 40, 41–49. [Google Scholar] [CrossRef]
- Navale, G.R.; Sharma, P.; Said, M.S.; Ramkumar, S.; Dharne, M.S.; Thulasiram, H.V.; Shinde, S.S. Enhancing epi-cedrol production in Escherichia coli by fusion expression of farnesyl pyrophosphate synthase and epi-cedrol synthase. Eng. Life Sci. 2019, 19, 606–616. [Google Scholar] [CrossRef] [PubMed]
- Monterrey, D.T.; Ayuso-Fernández, I.; Oroz-Guinea, I.; García-Junceda, E. Design and biocatalytic applications of genetically fused multifunctional enzymes. Biotechnol. Adv. 2022, 60, 108016. [Google Scholar] [CrossRef] [PubMed]
- Huo, Y.-X.; Ren, H.; Yu, H.; Zhao, L.; Yu, S.; Yan, Y.; Chen, Z. CipA-mediating enzyme self-assembly to enhance the biosynthesis of pyrogallol in Escherichia coli. Appl. Microbiol. Biotechnol. 2018, 102, 10005–10015. [Google Scholar] [CrossRef] [PubMed]
- Park, S.Y.; Eun, H.; Lee, M.H.; Lee, S.Y. Metabolic engineering of Escherichia coli with electron channelling for the production of natural products. Nat. Catal. 2022, 5, 726–737. [Google Scholar] [CrossRef]
- Zhao, W.; Ruan, J.; Wang, Q.; Du, G.; Zhou, J.; Liu, S. Metabolic pathway optimization through fusion with self-assembling amphipathic peptides. Process Biochem. 2021, 100, 117–123. [Google Scholar] [CrossRef]
- Wan, L.; Zhu, Y.; Chen, G.; Luo, G.; Zhang, W.; Mu, W. Efficient production of 2′-fucosyllactose from l-fucose via self-assembling multienzyme complexes in engineered Escherichia coli. ACS Synth. Biol. 2021, 10, 2488–2498. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Zhang, T.; Liu, Y.; Chen, P.; Qin, H.; Yang, J.; Sun, Y. Self-assembled multienzyme complex facilitates synthesis of glucosylglycerol from maltodextrin and glycerol. J. Sci. Food Agric. 2023, 104, 266–272. [Google Scholar] [CrossRef] [PubMed]
- Qu, J.; Cao, S.; Wei, Q.; Zhang, H.; Wang, R.; Kang, W.; Ma, T.; Zhang, L.; Liu, T.; Wing-Ngor Au, S.; et al. Synthetic multienzyme complexes, catalytic nanomachineries for cascade biosynthesis In vivo. ACS Nano 2019, 13, 9895–9906. [Google Scholar] [CrossRef]
- Liu, J.; Wang, X.; Jin, K.; Liu, Y.; Li, J.; Du, G.; Lv, X.; Liu, L. In silico prediction and mining of exporters for secretory bioproduction of terpenoids in Saccharomyces cerevisiae. ACS Synth. Biol. 2023, 12, 863–876. [Google Scholar] [CrossRef]
- Tatsumi, R.; Wachi, M. TolC-dependent exclusion of porphyrins in Escherichia coli. J. Bacteriol. 2008, 190, 6228–6233. [Google Scholar] [CrossRef]
- Feng, Z.; Liu, D.; Wang, L.; Wang, Y.; Zang, Z.; Liu, Z.; Song, B.; Gu, L.; Fan, Z.; Yang, S.; et al. A putative efflux transporter of the ABC family, YbhFSR, in Escherichia coli functions in tetracycline efflux and Na+(Li+)/H+ transport. Front. Microbiol. 2020, 11, 566. [Google Scholar] [CrossRef] [PubMed]
- Turlin, E.; Heuck, G.; Simões Brandão, M.I.; Szili, N.; Mellin, J.R.; Lange, N.; Wandersman, C. Protoporphyrin (PPIX) efflux by the MacAB-TolC pump in Escherichia coli. MicrobiologyOpen 2014, 3, 849–859. [Google Scholar] [CrossRef] [PubMed]
- Wessels, M.R.; Fernandez, A.; Lechardeur, D.; Derré-Bobillot, A.; Couvé, E.; Gaudu, P.; Gruss, A. Two coregulated efflux transporters modulate intracellular heme and protoporphyrin IX availability in Streptococcus agalactiae. PLoS Pathog. 2010, 6, e1000860. [Google Scholar]
- Lee, S.S.; Park, J.G.; Jang, E.; Choi, S.H.; Kim, S.; Kim, J.W.; Jin, M.S. W546 stacking disruption traps the human porphyrin transporter ABCB6 in an outward-facing transient state. Commun. Biol. 2023, 6, 960. [Google Scholar] [CrossRef] [PubMed]
- Qi, Q.; Gu, R.; Zhu, J.; Anderson, K.E.; Ma, X. Roles of the ABCG2 transporter in protoporphyrin IX distribution and toxicity. Drug Metab. Dispos. 2024, 52, 1201–1207. [Google Scholar] [CrossRef] [PubMed]
- Bonifer, C.; Glaubitz, C. MsbA: An ABC transporter paradigm. Biochem. Soc. Trans. 2021, 49, 2917–2927. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Chen, X.Y.; Du, H.T.; Zhang, X.; Ma, Y.M.; Chen, J.C.; Ye, J.W.; Jiang, X.R.; Chen, G.Q. Chromosome engineering of the TCA cycle in Halomonas bluephagenesis for production of copolymers of 3-hydroxybutyrate and 3-hydroxyvalerate (PHBV). Metab. Eng. 2019, 54, 69–82. [Google Scholar] [CrossRef]
- Li, Q.; Sun, B.; Chen, J.; Zhang, Y.; Jiang, Y.; Yang, S. A modified pCas/pTargetF system for CRISPR-Cas9-assisted genome editing in Escherichia coli. Acta Biochim. Biophys. Sin. 2021, 53, 620–627. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Chen, J.; Xu, W.; Zhang, W.; Chen, Y.; Mu, W. Efficient Utilization of Fruit Peels for the Bioproduction of D-Allulose and D-Mannitol. Foods 2022, 11, 3613. [Google Scholar] [CrossRef] [PubMed]
- Tupta, B.; Stuehr, E.; Sumi, M.P.; Sweeny, E.A.; Smith, B.; Stuehr, D.J.; Ghosh, A. GAPDH is involved in the heme-maturation of myoglobin and hemoglobin. FASEB J. 2021, 36, e22099. [Google Scholar] [CrossRef] [PubMed]
- Zhao, P.; Chen, J.; Ma, N.; Chen, J.; Qin, X.; Liu, C.; Yao, F.; Yao, L.; Jin, L.; Cong, Z. Enabling highly (R)-enantioselective epoxidation of styrene by engineering unique non-natural P450 peroxygenases. Chem. Sci. 2021, 12, 6307–6314. [Google Scholar] [CrossRef]
- Sumbalova, L.; Stourac, J.; Martinek, T.; Bednar, D.; Damborsky, J. HotSpot Wizard 3.0: Web server for automated design of mutations and smart libraries based on sequence input information. Nucleic Acids Res. 2018, 46, W356–W362. [Google Scholar] [CrossRef]
- Le, P.H.; Verscheure, L.; Le, T.T.; Verheust, Y.; Raes, K. Implementation of HPLC analysis for γ-aminobutyric acid (GABA) in fermented food matrices. Food Anal. Methods 2020, 13, 1190–1201. [Google Scholar] [CrossRef]
- Quehl, P.; Hollender, J.; Schüürmann, J.; Brossette, T.; Maas, R.; Jose, J. Co-expression of active human cytochrome P450 1A2 and cytochrome P450 reductase on the cell surface of Escherichia coli. Microb. Cell Fact. 2016, 15, 26. [Google Scholar] [CrossRef]
- Ohtake, T.; Kawase, N.; Pontrelli, S.; Nitta, K.; Laviña, W.A.; Shen, C.R.; Putri, S.P.; Liao, J.C.; Fukusaki, E. Metabolomics-driven identification of the rate-limiting steps in 1-propanol production. Front. Microbiol. 2022, 13, 871624. [Google Scholar] [CrossRef] [PubMed]
- Usher, J.J.; Hughes, D.W.; Lewis, M.A.; Chiang, S.-J.D. Determination of the rate-limiting step(s) in the biosynthetic pathways leading to penicillin and cephalosporin. J. Ind. Microbiol. 1992, 10, 157–163. [Google Scholar] [CrossRef] [PubMed]
- Phillips, J.D. Heme biosynthesis and the porphyrias. Mol. Genet. Metab. 2019, 128, 164–177. [Google Scholar] [CrossRef] [PubMed]
- Kim, P.; Lay, B.W.; Choi, S.-I.; Pranawidjaja, S. Analysis of heme biosynthetic pathways in a recombinant Escherichia coli. J. Microbiol. Biotechnol. 2015, 25, 880–886. [Google Scholar]
- Li, F.; Wang, Y.; Gong, K.; Wang, Q.; Liang, Q.; Qi, Q. Constitutive expression of RyhB regulates the heme biosynthesis pathway and increases the 5-aminolevulinic acid accumulation in Escherichia coli. FEMS Microbiol. Lett. 2014, 350, 209–215. [Google Scholar] [CrossRef]
- Schubert, H.L.; Phillips, J.D.; Heroux, A.; Hill, C.P. Structure and mechanistic implications of a uroporphyrinogen III synthase−product complex. Biochemistry 2008, 47, 8648–8655. [Google Scholar] [CrossRef] [PubMed]
- Newbury, S.F.; Smith, N.H.; Higgins, C.F. Differential mRNA stability controls relative gene expression within a polycistronic operon. Cell 1987, 51, 1131–1143. [Google Scholar] [CrossRef] [PubMed]
- Auda, I.G.; Ali Salman, I.M.; Odah, J.G. Efflux pumps of Gram-negative bacteria in brief. Gene Rep. 2020, 20, 100666. [Google Scholar] [CrossRef]
- Elbourne, L.D.H.; Tetu, S.G.; Hassan, K.A.; Paulsen, I.T. TransportDB 2.0: A database for exploring membrane transporters in sequenced genomes from all domains of life. Nucleic Acids Res. 2017, 45, D320–D324. [Google Scholar] [CrossRef] [PubMed]
- Karow, M.; Georgopoulos, C. The essential Escherichia coli msbA gene, a multicopy suppressor of null mutations in the htrB gene, is related to the universally conserved family of ATP-dependent translocators. Mol. Microbiol. 1993, 7, 69–79. [Google Scholar] [CrossRef]
- Wang, X.; Lu, L.; Liu, Q.; Li, J.; Wang, T.; Wang, J.; Sun, X.; Shen, X.; Yuan, Q. Integration site library for efficient construction of plasmid-free microbial cell factories in Escherichia coli. J. Agric. Food Chem. 2024, 72, 24687–24696. [Google Scholar] [CrossRef]
- Gell, D.A. Structure and function of haemoglobins. Blood Cell Mol. Dis. 2018, 70, 13–42. [Google Scholar] [CrossRef] [PubMed]
- Svistunenko, D.A. Reaction of haem containing proteins and enzymes with hydroperoxides: The radical view. Biochim. Biophys. Acta 2005, 1707, 127–155. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Z.; Hu, B.; Zhou, J.; Li, J.; Chen, J.; Du, G.; Zhao, X. Construction of a Plasmid-Free Escherichia coli Strain with Enhanced Heme Supply to Produce Active Hemoglobins. Metabolites 2025, 15, 151. https://doi.org/10.3390/metabo15030151
Zhang Z, Hu B, Zhou J, Li J, Chen J, Du G, Zhao X. Construction of a Plasmid-Free Escherichia coli Strain with Enhanced Heme Supply to Produce Active Hemoglobins. Metabolites. 2025; 15(3):151. https://doi.org/10.3390/metabo15030151
Chicago/Turabian StyleZhang, Zihan, Baodong Hu, Jingwen Zhou, Jianghua Li, Jian Chen, Guocheng Du, and Xinrui Zhao. 2025. "Construction of a Plasmid-Free Escherichia coli Strain with Enhanced Heme Supply to Produce Active Hemoglobins" Metabolites 15, no. 3: 151. https://doi.org/10.3390/metabo15030151
APA StyleZhang, Z., Hu, B., Zhou, J., Li, J., Chen, J., Du, G., & Zhao, X. (2025). Construction of a Plasmid-Free Escherichia coli Strain with Enhanced Heme Supply to Produce Active Hemoglobins. Metabolites, 15(3), 151. https://doi.org/10.3390/metabo15030151





