Cellular Metabolomics Reveals Differences in the Scope of Liver Protection Between Ammonium-Based Glycyrrhizinate and Magnesium Isoglycyrrhizinate
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Pharmacokinetics
2.2.1. Animal Experiments
2.2.2. HPLC Method
2.3. Cell Viability
2.4. Cell Metabolomics
2.4.1. Measurement Conditions
2.4.2. Sample Preparation
2.4.3. Data Reliability Analysis
2.4.4. Metabolomics Data Analysis
2.4.5. Pathway and Enrichment Analysis
2.5. Protein Expression
2.6. Statistical Analysis
3. Results and Discussion
3.1. Pharmacokinetics Analysis
3.2. Cell Viability Analysis
3.3. Data Reliability of Analysis
3.4. Analysis of Differential Metabolites
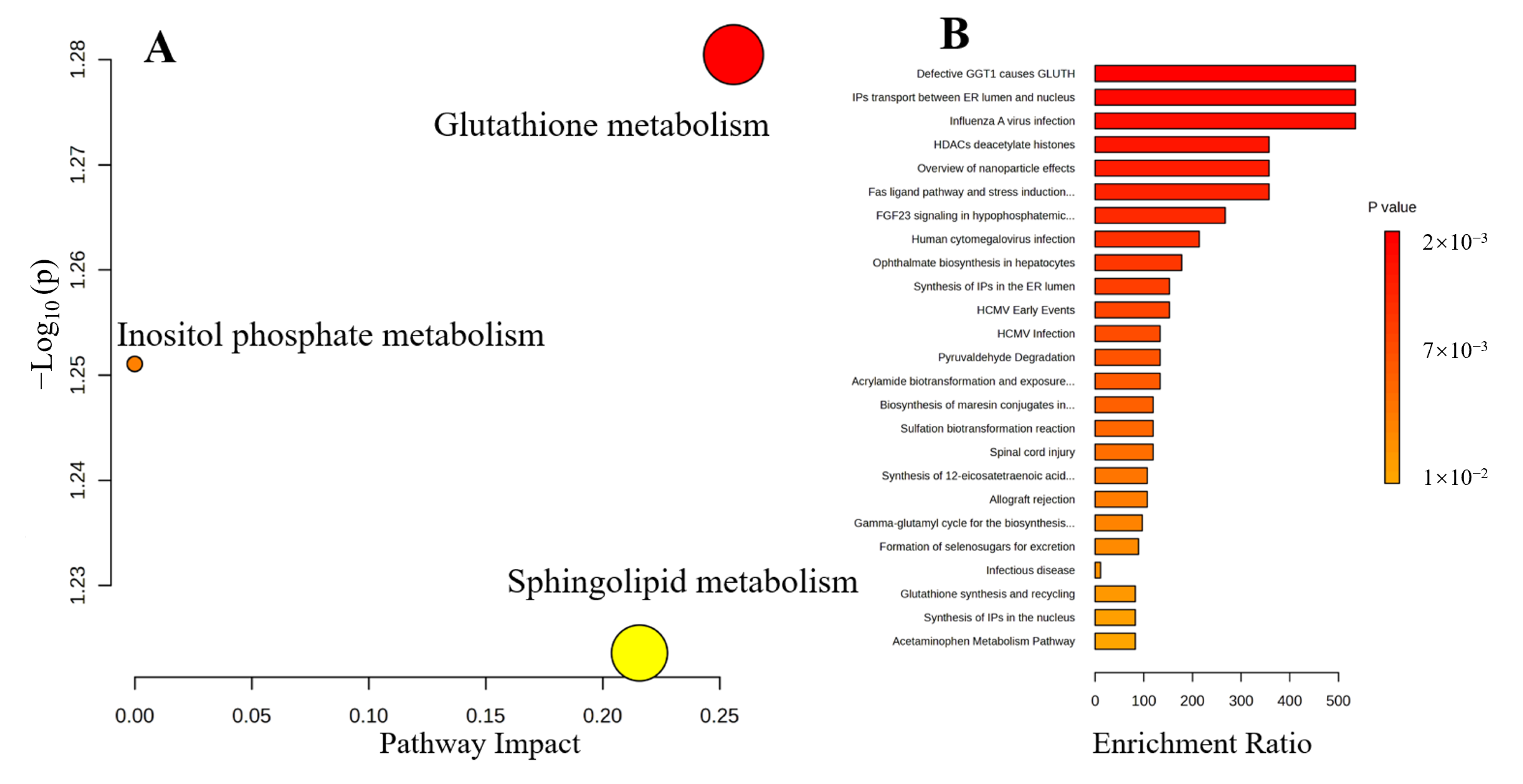
3.5. Metabolic Pathways and Enrichment Analysis
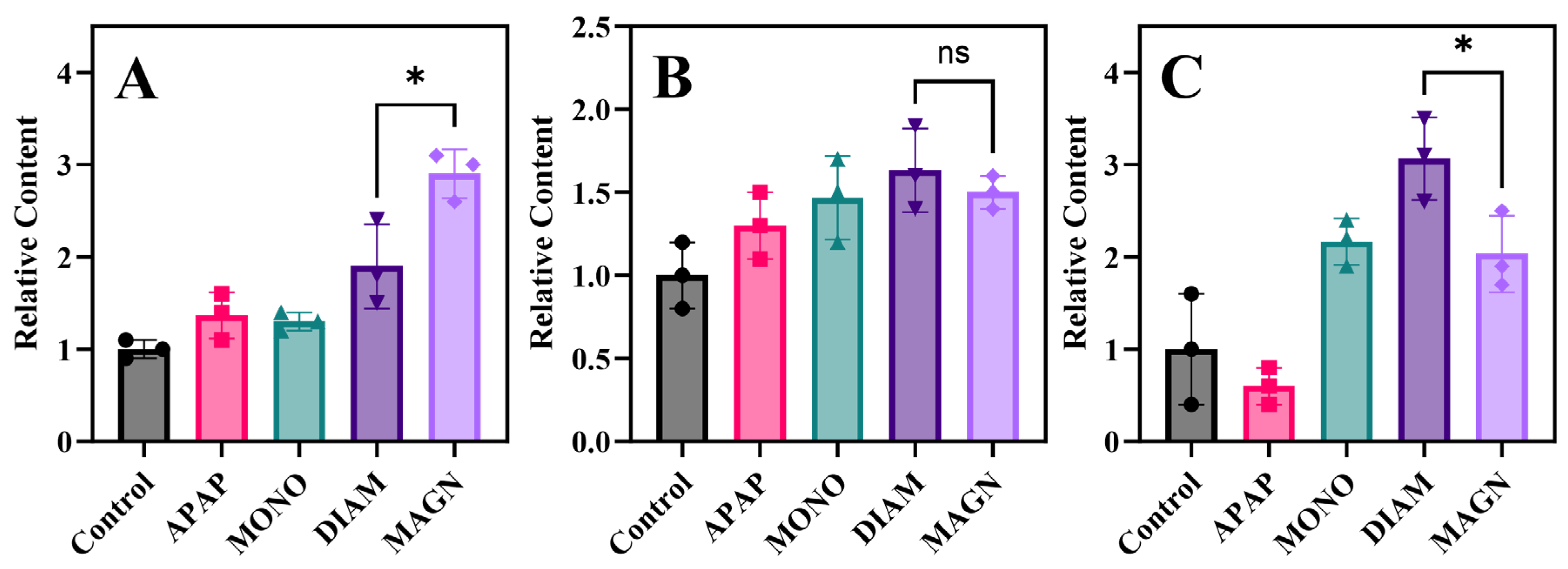
3.5.1. Antioxidant and Anti-Inflammatory Properties
3.5.2. Immune Regulation
3.5.3. Sphingolipids and Ceramides Metabolism
3.6. Protein Expression Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bernal, W.; Auzinger, G.; Dhawan, A.; Wendon, J. Acute liver failure. Lancet 2010, 376, 190–201. [Google Scholar] [CrossRef] [PubMed]
- Hillman, L.; Gottfried, M.; Whitsett, M.; Rakela, J.; Schilsky, M.; Lee, W.M.; Ganger, D. Clinical features and outcomes of complementary and alternative medicine induced acute liver failure and injury. Off. J. Am. Coll. Gastroenterol. ACG 2016, 111, 958–965. [Google Scholar] [CrossRef] [PubMed]
- Rotundo, L.; Pyrsopoulos, N. Liver injury induced by paracetamol and challenges associated with intentional and unintentional use. World J. Hepatol 2020, 12, 125. [Google Scholar] [CrossRef]
- Bunchorntavakul, C.; Reddy, K.R. Acetaminophen (APAP or N-acetyl-p-aminophenol) and acute liver failure. Clin. Liver Dis. 2018, 22, 325–346. [Google Scholar] [CrossRef]
- Yang, R.; Wang, L.-Q.; Yuan, B.-C.; Liu, Y. The Pharmacological Activities of Licorice. Planta Med. 2015, 81, 1654–1669. [Google Scholar] [CrossRef]
- Dang, L.; Jin, Y.; Yuan, Y.; Shao, R.; Wang, Y. Licorice: Comprehensive review of its chemical composition, pharmacodynamics, and medicinal value. Acupunct. Herb. Med. 2024, 4, 136–150. [Google Scholar]
- Baltina, L.A.; Serdyuk, N.G.; Flekhter, O.B.; Krasnova, L.V.; Davydova, V.A.; Ismagilova, A.F.; Zarudii, F.S.; Tolstikov, G.A. Isomerization of glycyrrhizic acid. Antiulcer activity. Pharm. Chem. J. 1996, 30, 613–616. [Google Scholar] [CrossRef]
- Tan, Q.-Y.; Hu, Q.; Zhu, S.-N.; Jia, L.-L.; Xiao, J.; Su, H.-Z.; Huang, S.-Y.; Zhang, J.; Jin, J. Licorice root extract and magnesium isoglycyrrhizinate protect against triptolide-induced hepatotoxicity via up-regulation of the Nrf2 pathway. Drug Deliv. 2018, 25, 1213–1223. [Google Scholar] [CrossRef]
- Zhou, L.; Song, Y.; Zhao, J.; Qin, H.; Zhang, G.; Zhou, Y.; Wu, X.a. Monoammonium glycyrrhizinate protects rifampicin- and isoniazid-induced hepatotoxicity via regulating the expression of transporter Mrp2, Ntcp, and Oatp1a4 in liver. Pharm. Biol. 2016, 54, 931–937. [Google Scholar] [CrossRef]
- Shi, J.-R.; Mao, L.-G.; Jiang, R.-A.; Qian, Y.; Tang, H.-F.; Chen, J.-Q. Monoammonium glycyrrhizinate inhibited the inflammation of LPS-induced acute lung injury in mice. Int. Immunopharmacol. 2010, 10, 1235–1241. [Google Scholar] [CrossRef]
- Wang, X.; Lin, J.; Chen, T.; Zhou, M.; Su, M.; Jia, W. Metabolic profiling reveals the protective effect of diammonium glycyrrhizinate on acute hepatic injury induced by carbon tetrachloride. Metabolomics 2011, 7, 226–236. [Google Scholar] [CrossRef]
- Gao, M.; Li, X.; He, L.; Yang, J.; Ye, X.; Xiao, F.; Wei, H. Diammonium Glycyrrhizinate Mitigates Liver Injury Via Inhibiting Proliferation of NKT Cells and Promoting Proliferation of Tregs. Drug Des. Dev. Ther. 2019, 13, 3579–3589. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Zhang, Y.; Song, T.; Song, Q.; Zhang, Y.; Zhang, X.; Han, X.; Zhang, J.; Chu, L. Magnesium isoglycyrrhizinate ameliorates doxorubicin-induced acute cardiac and hepatic toxicity via anti-oxidant and anti-apoptotic mechanisms in mice. Exp. Ther. Med. 2018, 15, 1005–1012. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Tian, Y.; Zhang, X.; Zhang, X.; Duan, Z.; Ren, F.; Chen, Y. Magnesium isoglycyrrhizinate ameliorates concanavalin A-induced liver injury via the p38 and JNK MAPK pathway. Immunopharmacol. Immunotoxicol. 2020, 42, 445–455. [Google Scholar] [CrossRef]
- Fukuno, S.; Nagai, K.; Fujiike, M.; Sasaki, Y.; Konishi, H. Conflicting alterations in hepatic expression of CYP3A and enzyme kinetics in rats exposed to 5-fluorouracil: Relevance to pharmacokinetics of midazolam. Xenobiotica 2019, 49, 1470–1477. [Google Scholar] [CrossRef]
- Hou, L.; Guan, S.; Jin, Y.; Sun, W.; Wang, Q.; Du, Y.; Zhang, R. Cell metabolomics to study the cytotoxicity of carbon black nanoparticles on A549 cells using UHPLC-Q/TOF-MS and multivariate data analysis. Sci. Total Environ. 2020, 698, 134122. [Google Scholar] [CrossRef]
- Li, B.; He, X.; Jia, W.; Li, H. Novel applications of metabolomics in personalized medicine: A mini-review. Molecules 2017, 22, 1173. [Google Scholar] [CrossRef]
- Seo, S.; Park, S.; Kim, E.; Lee, K.; Na, C.; Son, H. A GC-MS based metabolomics approach to determine the effect of salinity on Kimchi. Food Res. Int. 2018, 105, 492–498. [Google Scholar] [CrossRef]
- Čuperlović-Culf, M.; Barnett, D.A.; Culf, A.S.; Chute, I. Cell culture metabolomics: Applications and future directions. Drug Discov. Today 2010, 15, 610–621. [Google Scholar] [CrossRef]
- Huang, J.-W.; Kuo, C.-H.; Kuo, H.-C.; Shih, J.-Y.; Tsai, T.-W.; Chang, L.-C. Differences in fatty acid oxidation between nab-paclitaxel-and solvent-based paclitaxel-treated A549 cells based on metabolomics. ACS Omega 2021, 6, 5138–5145. [Google Scholar] [CrossRef]
- Kou, F.; Zhu, B.; Zhou, W.; Lv, C.; Cheng, Y.; Wei, H. Targeted metabolomics in the cell culture media reveals increased uptake of branched amino acids by breast cancer cells. Anal. Biochem. 2021, 624, 114192. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Zhou, L.; Hao, H.; Bao, Y.; Yin, Q.; Xie, C. Cocrystals of Propylthiouracil and Nutraceuticals toward Sustained-Release: Design, Structure Analysis, and Solid-State Characterization. Cryst. Growth Des. 2021, 21, 1202–1217. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, H.; Liu, X.; Wang, Q.; Zhao, D.; Su, M.; Jia, Z.; Shen, S. Integrating Metabolomics and Network Pharmacology to Decipher the Hepatoprotective Effect Mechanisms of Magnesium Isoglycyrrhizinate Injection. Curr. Issues Mol. Biol. 2024, 46, 279–298. [Google Scholar] [CrossRef] [PubMed]
- Loguercio, C.; Federico, A. Oxidative stress in viral and alcoholic hepatitis. Free Radical Biol. Med. 2003, 34, 1–10. [Google Scholar] [CrossRef]
- Gong, T.; Liu, L.; Jiang, W.; Zhou, R. DAMP-sensing receptors in sterile inflammation and inflammatory diseases. Nat. Rev. Immunol. 2020, 20, 95–112. [Google Scholar] [CrossRef]
- Jaeschke, H. Reactive oxygen and mechanisms of inflammatory liver injury: Present concepts. J. Gastroenterol. Hepatol. 2011, 26, 173–179. [Google Scholar] [CrossRef]
- Aoun, M.; Feillet-Coudray, C.; Fouret, G.; Chabi, B.; Crouzier, D.; Ferreri, C.; Chatgilialoglu, C.; Wrutniak-Cabello, C.; Cristol, J.P.; Carbonneau, M.-A. Rat liver mitochondrial membrane characteristics and mitochondrial functions are more profoundly altered by dietary lipid quantity than by dietary lipid quality: Effect of different nutritional lipid patterns. Br. J. Nutr. 2012, 107, 647–659. [Google Scholar] [CrossRef]
- Zhou, X.; Fu, Y.; Chen, J.; Liu, P. Progress in clinical and basic research of fuzheng Huayu formula for the treatment of liver fibrosis. J. Ethnopharmacol. 2024, 327, 118018. [Google Scholar] [CrossRef]
- Issaq, H.J.; Waybright, T.J.; Veenstra, T.D. Cancer biomarker discovery: Opportunities and pitfalls in analytical methods. Electrophoresis 2011, 32, 967–975. [Google Scholar] [CrossRef]
- Wang, X.; Gao, Y.; Tian, Y.; Liu, X.; Zhang, G.; Wang, Q.; Xie, W.; Liu, K.; Qian, Q.; Wang, Q. Integrative serum metabolomics and network analysis on mechanisms exploration of Ling-Gui-Zhu-Gan Decoction on doxorubicin-induced heart failure mice. J. Ethnopharmacol. 2020, 250, 112397. [Google Scholar] [CrossRef]
- Zhang, C.-Y.; Liu, S.; Yang, M. Antioxidant and anti-inflammatory agents in chronic liver diseases: Molecular mechanisms and therapy. World J. Hepatol 2023, 15, 180. [Google Scholar] [CrossRef] [PubMed]
- Salyha, N. Effect of glutamic acid and cysteine on oxidative stress markers in rats. Ukr. Biochem. J. 2020, 92, 165–172. [Google Scholar] [CrossRef]
- Jones, D.P. Radical-free biology of oxidative stress. Am. J. Physiol. Cell Physiol 2008, 295, C849–C868. [Google Scholar] [CrossRef]
- Averill-Bates, D.A. The antioxidant glutathione. In Vitamins and Hormones; Elsevier: Amsterdam, The Netherlands, 2023; Volume 121, pp. 109–141. [Google Scholar]
- Chakravorty, S.; Malvi, A.; Chaturvedi, A.; Sonkusare, K.; Dave, N. Glutathione–the master antioxidant. Int. J. Med. Res. Pharm. Sci. 2020, 7, 1–11. [Google Scholar]
- Perazza, F.; Leoni, L.; Colosimo, S.; Musio, A.; Bocedi, G.; D’Avino, M.; Agnelli, G.; Nicastri, A.; Rossetti, C.; Sacilotto, F. Metformin and the Liver: Unlocking the Full Therapeutic Potential. Metabolites 2024, 14, 186. [Google Scholar] [CrossRef]
- Galli, F.; Piroddi, M.; Lannone, A.; Pagliarani, S.; Tomasi, A.; Floridi, A. A comparison between the antioxidant and peroxynitrite-scavenging functions of the vitamin E metabolites alpha- and gamma-carboxyethyl-6-hydroxychromans. Int. J. Vitam. Nutr. Res. 2004, 74, 362–373. [Google Scholar] [CrossRef]
- Krajewska, M.; Witkowska-Sędek, E.; Rumińska, M.; Stelmaszczyk-Emmel, A.; Sobol, M.; Majcher, A.; Pyrżak, B. Vitamin d effects on selected anti-inflammatory and pro-inflammatory markers of obesity-related chronic inflammation. Front. Endocrinol. 2022, 13, 920340. [Google Scholar] [CrossRef]
- Czaja, A.J.; Montano-Loza, A.J. Evolving role of vitamin D in immune-mediated disease and its implications in autoimmune hepatitis. Digest. Dis. Sci. 2019, 64, 324–344. [Google Scholar] [CrossRef]
- Nimer, A.; Mouch, A. Vitamin D improves viral response in hepatitis C genotype 2-3 naïve patients. World J. Gastroenterol. WJG 2012, 18, 800. [Google Scholar] [CrossRef]
- Li, K.; Tang, Y.; Fawcett, J.P.; Gu, J.; Zhong, D. Characterization of the pharmacokinetics of dioscin in rat. Steroids 2005, 70, 525–530. [Google Scholar] [CrossRef]
- Liu, M.; Xu, L.; Yin, L.; Qi, Y.; Xu, Y.; Han, X.; Zhao, Y.; Sun, H.; Yao, J.; Lin, Y. Potent effects of dioscin against obesity in mice. Sci. Rep. 2015, 5, 7973. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Wang, Y.; Wu, C.; Pei, R.; Song, J.; Chen, S.; Chen, X. Dioscin’s antiviral effect in vitro. Virus Res. 2013, 172, 9–14. [Google Scholar] [CrossRef] [PubMed]
- Paquissi, F.C. Immune imbalances in non-alcoholic fatty liver disease: From general biomarkers and neutrophils to interleukin-17 axis activation and new therapeutic targets. Front. Immunol. 2016, 7, 490. [Google Scholar] [CrossRef] [PubMed]
- VanSaun, M.N.; Mendonsa, A.M.; Lee Gorden, D. Hepatocellular proliferation correlates with inflammatory cell and cytokine changes in a murine model of nonalchoholic fatty liver disease. PLoS ONE 2013, 8, e73054. [Google Scholar] [CrossRef]
- Ong, S.C.; Gaston, R.S. Thirty years of tacrolimus in clinical practice. Transplantation 2021, 105, 484–495. [Google Scholar] [CrossRef]
- Hanouneh, M.; Ritchie, M.M.; Ascha, M.; Ascha, M.S.; Chedid, A.; Sanguankeo, A.; Zein, N.N.; Hanouneh, I.A. A review of the utility of tacrolimus in the management of adults with autoimmune hepatitis. Scand. J. Gastroenterol. 2019, 54, 76–80. [Google Scholar] [CrossRef]
- Hulskotte, E.; Gupta, S.; Xuan, F.; van Zutven, M.; O’Mara, E.; Feng, H.P.; Wagner, J.; Butterton, J. Pharmacokinetic interaction between the hepatitis C virus protease inhibitor boceprevir and cyclosporine and tacrolimus in healthy volunteers. Hepatology 2012, 56, 1622–1630. [Google Scholar] [CrossRef]
- Muratori, L.; Lohse, A.W.; Lenzi, M. Diagnosis and management of autoimmune hepatitis. BMJ 2023, 380, e070201. [Google Scholar] [CrossRef]
- Kelly, P.A.; Burckart, G.J.; Venkataramanan, R. Tacrolimus: A new immunosuppressive agent. Am. J. Health-Syst. Pharm. 1995, 52, 1521–1535. [Google Scholar] [CrossRef]
- Miller, W.L. Steroid hormone synthesis in mitochondria. Mol. Cell. Endocrinol. 2013, 379, 62–73. [Google Scholar] [CrossRef]
- Kou, F.; Zhu, B.; Zhou, W.; Lv, C.; Cheng, Y.; Wei, H. Targeted metabolomics reveals dynamic portrayal of amino acids and derivatives in triple-negative breast cancer cells and culture media. Mol. Omics 2021, 17, 142–152. [Google Scholar] [CrossRef] [PubMed]
- Pei, G.; Zyla, J.; He, L.; Moura-Alves, P.; Steinle, H.; Saikali, P.; Lozza, L.; Nieuwenhuizen, N.; Weiner, J.; Mollenkopf, H.J.; et al. Cellular stress promotes NOD1/2-dependent inflammation via the endogenous metabolite sphingosine-1-phosphate. EMBO J. 2021, 40, e106272. [Google Scholar] [CrossRef] [PubMed]
- Xia, J.; Chen, H.; Wang, X.; Chen, W.; Lin, J.; Xu, F.; Nie, Q.; Ye, C.; Zhong, B.; Zhao, M. Sphingosine d18: 1 promotes nonalcoholic steatohepatitis by inhibiting macrophage HIF-2α. Nat. Commun. 2024, 15, 4755. [Google Scholar] [CrossRef] [PubMed]
- Raichur, S. Ceramide Synthases Are Attractive Drug Targets for Treating Metabolic Diseases. Front. Endocrinol. 2020, 11, 483. [Google Scholar] [CrossRef]
- Guerra, S.; Mocciaro, G.; Gastaldelli, A. Adipose tissue insulin resistance and lipidome alterations as the characterizing factors of non-alcoholic steatohepatitis. Eur. J. Clin. Investig. 2022, 52, e13695. [Google Scholar] [CrossRef]
- Zhang, T.; Barclay, L.; Walensky, L.D.; Saghatelian, A. Regulation of mitochondrial ceramide distribution by members of the BCL-2 family [S]. J. Lipid Res. 2015, 56, 1501–1510. [Google Scholar] [CrossRef]
- Pattni, K.; Banting, G. Ins (1, 4, 5) P3 metabolism and the family of IP3-3Kinases. Cell. Signal. 2004, 16, 643–654. [Google Scholar] [CrossRef]
- Lu, S.C. Regulation of glutathione synthesis. Mol. Asp. Med. 2009, 30, 42–59. [Google Scholar] [CrossRef]
- Morgan, S.A.; McCabe, E.L.; Gathercole, L.L.; Hassan-Smith, Z.K.; Larner, D.P.; Bujalska, I.J.; Stewart, P.M.; Tomlinson, J.W.; Lavery, G.G. 11β-HSD1 is the major regulator of the tissue-specific effects of circulating glucocorticoid excess. Proc. Natl. Acad. Sci. USA 2014, 111, E2482–E2491. [Google Scholar] [CrossRef]





| MONO | DIAM | MAGN | |
|---|---|---|---|
| AUC0-t (μg·h·mL−1) | 320.23 ± 78.63 | 358.04 ± 56.36 | 552.31 ± 119.93 * |
| Cmax (μg·mL−1) | 14.99 ± 3.64 | 18.86 ± 2.03 | 23.19 ± 4.18 * |
| Tmax (h) | 8 ± 0 | 6 ± 0 | 9.33 ± 2.31 # |
| t1/2 (h) | 10.82 ± 0.66 | 11.57 ± 1.29 | 23.71 ± 2.44 ***,### |
| R2X | R2Y | Q2 | |
|---|---|---|---|
| Positive | 0.984 | 0.956 | 0.876 |
| Negative | 0.945 | 0.907 | 0.874 |
| NO. | Description | Formular | RT (min) | Ion Mode | Highest | p Value | VIP | FC |
|---|---|---|---|---|---|---|---|---|
| 1 | Floctafenine | C20H17F3N2O4 | 0.72 | ESI− | DIAM | 0.009 | 1.08 | 4.41 |
| 2 | Tacrolimus | C44H71NO13 | 0.77 | ESI− | DIAM | <0.001 | 1.13 | >10 |
| 3 | Onnamide A | C39H63N5O12 | 0.77 | ESI− | DIAM | 0.037 | 1.03 | 2.42 |
| 4 | Sampangine | C15H8N2O | 0.79 | ESI− | DIAM | <0.001 | 1.11 | >10 |
| 5 | Varespladib | C21H20N2O5 | 0.97 | ESI− | DIAM | 0.005 | 1.12 | 1.71 |
| 6 | Ins(3,4,5,6)P4 | C6H16O18P4 | 0.97 | ESI− | DIAM | 0.025 | 1.09 | 1.50 |
| 7 | GSH | C10H17N3O6S | 1.25 | ESI+ | MAGN | 0.041 | 3.06 | 1.24 |
| 8 | GSSG | C20H32N6O12S2 | 1.25 | ESI+ | MAGN | 0.025 | 1.14 | 1.28 |
| 9 | 8-Demethyl meiqx | C10H9N5 | 1.27 | ESI− | DIAM | 0.023 | 1.02 | 1.92 |
| 10 | Tirilazad | C38H52N6O2 | 3.12 | ESI− | DIAM | <0.001 | 1.13 | 1.74 |
| 11 | Butyroin | C8H16O2 | 3.14 | ESI− | DIAM | 0.026 | 1.04 | 1.23 |
| 12 | Dioscin | C45H72O16 | 3.20 | ESI− | DIAM | <0.001 | 1.03 | >10 |
| 13 | Prednisolone sodium tetrahydrophthalic | C29H35NaO8 | 3.70 | ESI− | DIAM | <0.001 | 1.08 | 1.66 |
| 14 | Ergocryptine | C32H41N5O5 | 3.86 | ESI− | DIAM | <0.001 | 1.09 | 1.92 |
| 15 | Gypsogenin | C30H46O4 | 3.90 | ESI− | DIAM | 0.001 | 1.07 | 1.79 |
| 16 | 7-Dehydrocholesterol benzoate | C34H48O2 | 4.41 | ESI− | DIAM | <0.001 | 1.11 | >10 |
| 17 | Sphingosine-1-phosphate (d16:1) | C16H34NO5P | 4.45 | ESI− | DIAM | 0.002 | 1.05 | 3.78 |
| 18 | Argiotoxin 659 | C31H53N11O5 | 4.46 | ESI− | DIAM | 0.047 | 1.03 | 2.98 |
| 19 | Tisocalcitate | C31H48O5 | 4.54 | ESI− | DIAM | 0.001 | 1.08 | 1.39 |
| 20 | Glabric acid | C30H46O5 | 4.60 | ESI+ | DIAM | <0.001 | 3.44 | >10 |
| 21 | Glycyrrhizin | C42H62O16 | 4.84 | ESI+ | DIAM | 0.042 | 5.52 | 1.24 |
| 22 | Propapyriogenin A2 | C30H44O5 | 4.86 | ESI+ | DIAM | 0.000 | 1.63 | 6.06 |
| 23 | 25-Hydroxyvitamin D2 | C28H44O2 | 4.98 | ESI+ | DIAM | <0.001 | 1.79 | 3.85 |
| 24 | Ammonium glycyrrhizate | C42H65NO16 | 4.98 | ESI+ | DIAM | <0.001 | 1.69 | 2.12 |
| 25 | Stanozolol | C21H32N2O | 5.36 | ESI− | DIAM | 0.002 | 1.06 | 1.81 |
| 26 | Glycyrrhetaldehyde | C30H46O3 | 5.57 | ESI+ | DIAM | <0.001 | 1.92 | >10 |
| 27 | Trametenolic acid | C30H48O3 | 5.77 | ESI+ | DIAM | <0.001 | 2.52 | >10 |
| 28 | Cerlapirdine | C22H23N3O3S | 6.33 | ESI+ | DIAM | 0.002 | 1.03 | >10 |
| 29 | Testosterone ketolaurate | C31H48O4 | 6.33 | ESI+ | DIAM | <0.001 | 1.25 | 1.67 |
| 30 | 3-(10Z)-10-Heptadecen-1-ylphenol | C23H38O | 6.86 | ESI− | DIAM | 0.026 | 1.01 | 1.68 |
| 31 | Pristimerin | C30H40O4 | 7.38 | ESI− | DIAM | 0.007 | 1.06 | 1.26 |
| 32 | Euscaphic acid | C30H48O5 | 10.99 | ESI+ | DIAM | <0.001 | 2.39 | 4.79 |
| 33 | Alpha-Cehc | C16H22O4 | 11.14 | ESI+ | MAGN | <0.001 | 1.67 | 1.21 |
| 34 | Pinnatoxin G | C42H63NO7 | 13.29 | ESI+ | DIAM | 0.012 | 1.31 | 1.55 |
| 35 | Glycerol phenylbutyrate | C33H38O6 | 15.79 | ESI+ | DIAM | 0.013 | 1.27 | 1.36 |
| 36 | Solanidine | C27H43NO | 16.82 | ESI+ | MAGN | 0.009 | 1.60 | 1.32 |
| 37 | N-eicosanoylsphinganine | C38H77NO3 | 18.11 | ESI+ | DIAM | <0.001 | 1.67 | 2.07 |
| 38 | C14 Ceramide | C32H63NO3 | 19.27 | ESI+ | DIAM | 0.010 | 1.13 | 1.20 |
| 39 | CETYL BENZOATE | C23H38O2 | 19.67 | ESI+ | DIAM | 0.024 | 1.13 | 1.37 |
| 40 | N-tetracosanoylsphinganine | C42H85NO3 | 20.30 | ESI+ | DIAM | 0.026 | 1.69 | >10 |
| 41 | C16 Lactosylceramide (d18:1/16:0) | C46H87NO13 | 20.96 | ESI+ | DIAM | 0.030 | 3.30 | 1.24 |
| 42 | Ceramide (d18:1/16:0) | C34H67NO3 | 20.98 | ESI+ | DIAM | 0.001 | 2.92 | 1.20 |
| 43 | N-[(15Z)-tetracosenoyl]sphing-4-enine-1-phosphocholine | C47H93N2O6P | 21.99 | ESI+ | MAGN | <0.001 | 1.07 | 2.55 |
| 44 | Menaquinol-8 | C51H74O2 | 22.01 | ESI+ | MAGN | 0.009 | 6.20 | 1.68 |
| 45 | Dioleoylphosphatidylserine | C42H78NO10P | 22.01 | ESI+ | MAGN | 0.005 | 1.18 | 1.92 |
| 46 | Tetrahydrospirilloxanthin | C42H64O2 | 27.84 | ESI− | DIAM | <0.001 | 1.08 | 1.29 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Hao, H.; Li, H.; Duan, Q.; Zheng, X.; Feng, Y.; Yang, K.; Shen, S. Cellular Metabolomics Reveals Differences in the Scope of Liver Protection Between Ammonium-Based Glycyrrhizinate and Magnesium Isoglycyrrhizinate. Metabolites 2025, 15, 263. https://doi.org/10.3390/metabo15040263
Zhang Y, Hao H, Li H, Duan Q, Zheng X, Feng Y, Yang K, Shen S. Cellular Metabolomics Reveals Differences in the Scope of Liver Protection Between Ammonium-Based Glycyrrhizinate and Magnesium Isoglycyrrhizinate. Metabolites. 2025; 15(4):263. https://doi.org/10.3390/metabo15040263
Chicago/Turabian StyleZhang, Yihua, Han Hao, Hui Li, Qiong Duan, Xiaoming Zheng, Yan Feng, Kun Yang, and Shigang Shen. 2025. "Cellular Metabolomics Reveals Differences in the Scope of Liver Protection Between Ammonium-Based Glycyrrhizinate and Magnesium Isoglycyrrhizinate" Metabolites 15, no. 4: 263. https://doi.org/10.3390/metabo15040263
APA StyleZhang, Y., Hao, H., Li, H., Duan, Q., Zheng, X., Feng, Y., Yang, K., & Shen, S. (2025). Cellular Metabolomics Reveals Differences in the Scope of Liver Protection Between Ammonium-Based Glycyrrhizinate and Magnesium Isoglycyrrhizinate. Metabolites, 15(4), 263. https://doi.org/10.3390/metabo15040263







