Combined Effects of Ketogenic Diet and Aerobic Exercise on Skeletal Muscle Fiber Remodeling and Metabolic Adaptation in Simulated Microgravity Mice
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals and Diets
2.2. Hindlimb Unloading Model
2.3. Aerobic Exercise Protocol
2.4. Respiratory Exchange Ratio (RER) Test
2.5. Assessment of Endurance Exercise Performance
2.6. Tissue Sampling
2.7. Plasma Biochemical Assessment
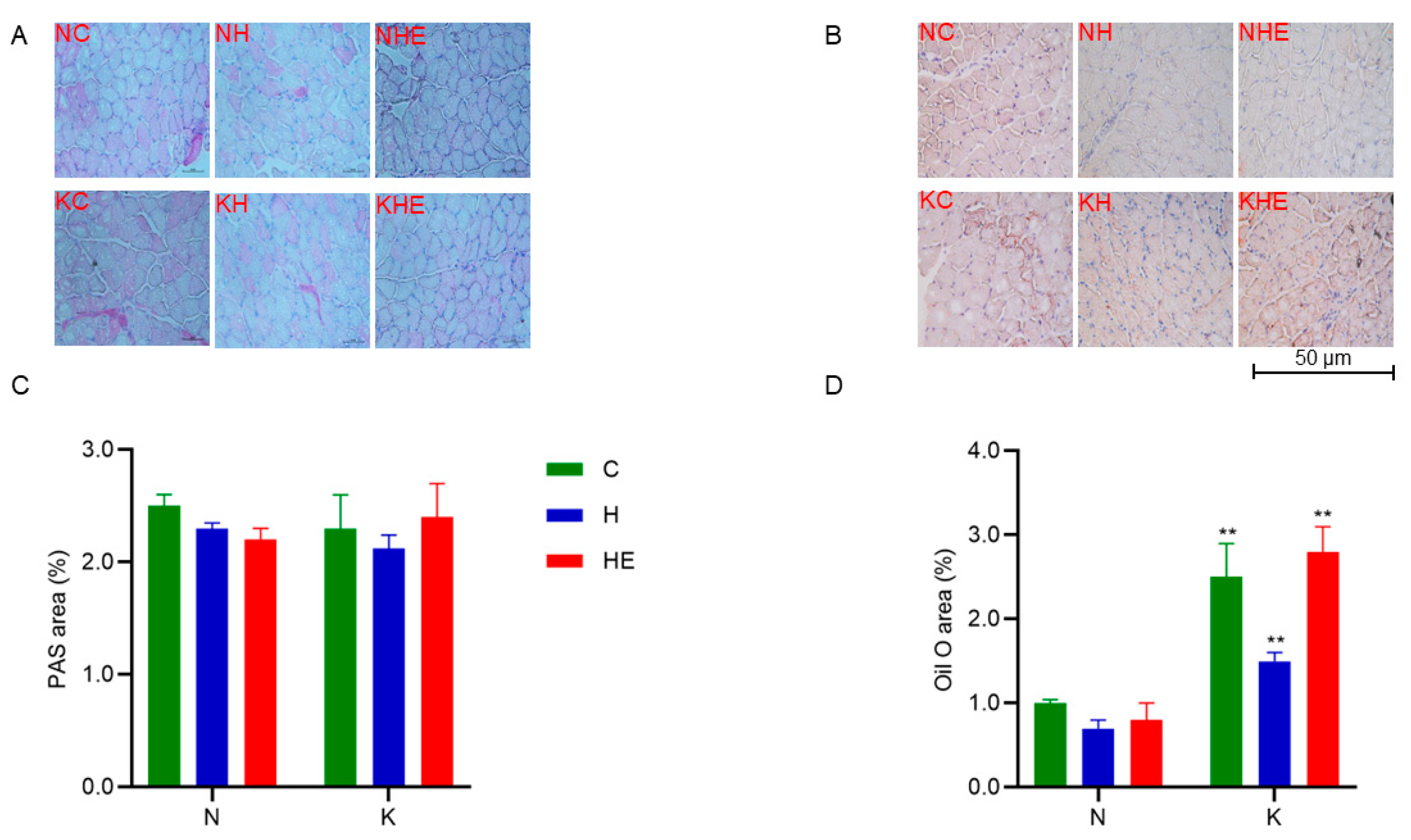
2.8. Oil Red O Staining
2.9. Periodic Acid–Schiff (PAS) Staining
2.10. Quantitative Real-Time Polymerase Chain Reaction
2.11. Histological Staining
2.12. Controlled Variables and Measurement Validation
2.13. Statistical Analysis
3. Results
3.1. Establishment of the Ketogenic Diet Model
3.2. Effects on Body Weight and Skeletal Muscle Mass in Simulated Weightlessness Mice
3.3. Effects on Glucose and Lipid Metabolism in Mice
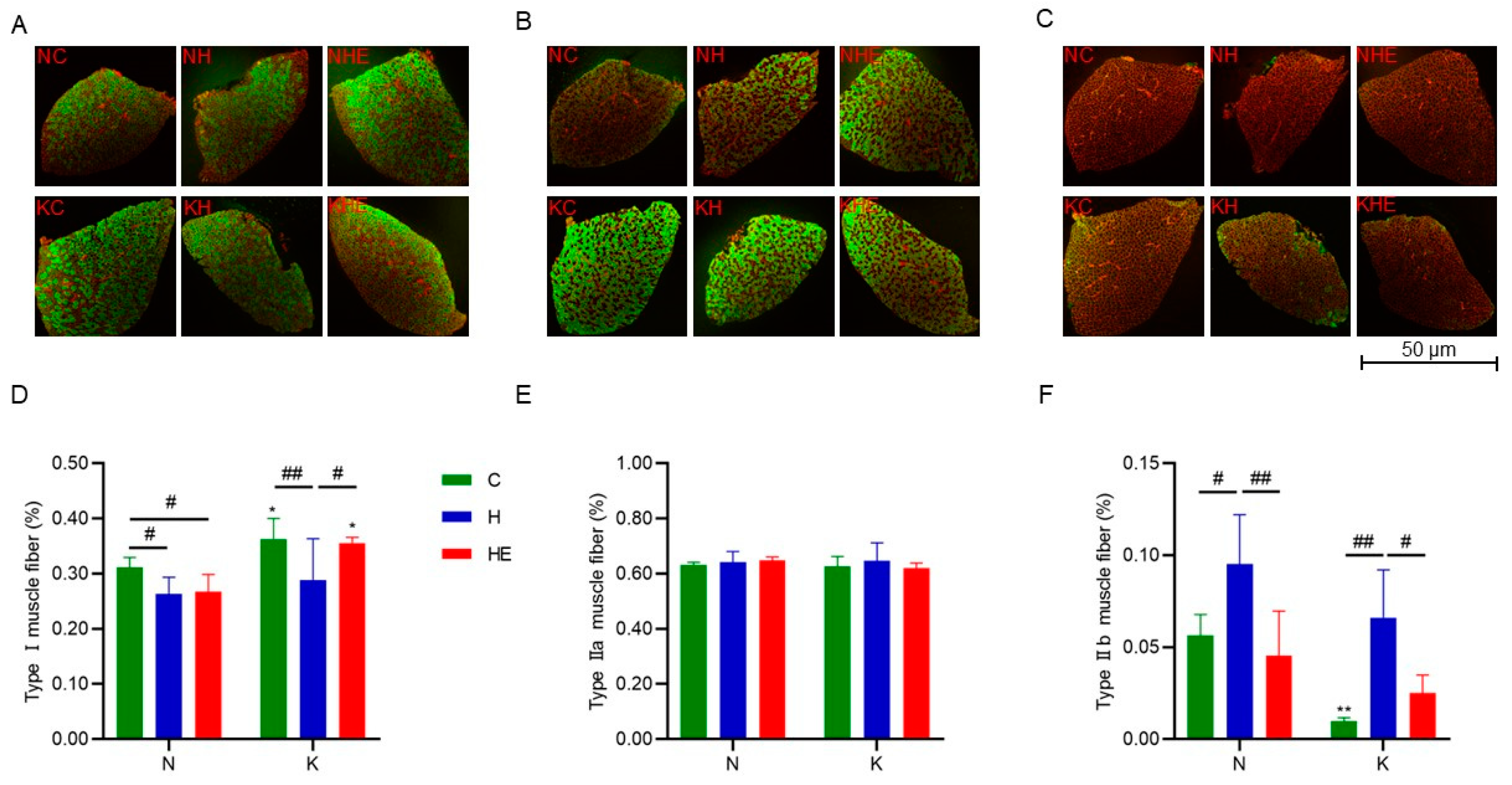
3.4. Effects on Skeletal Muscle Fiber-Type Composition
3.5. Effects on Skeletal Muscle-Related Gene Expression
3.6. Effects on Exercise Capacity in Mice
4. Discussion
4.1. Effects of Ketogenic Diet Combined with Aerobic Exercise on Body Weight and Skeletal Muscle in Simulated Weightlessness Mice
4.2. Effects of Ketogenic Diet Combined with Aerobic Exercise on Skeletal Muscle Fiber Remodeling in Simulated Weightlessness Mice
4.3. Effects of Ketogenic Diet Combined with Aerobic Exercise on Metabolic Adaptations in Skeletal Muscle in Simulated Weightlessness Mice
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Trappe, T.A.; Minchev, K.; Perkins, R.K.; Lavin, K.M.; Jemiolo, B.; Ratchford, S.M.; Claiborne, A.; Lee, G.A.; Finch, W.H.; Ryder, J.W.; et al. NASA SPRINT Exercise Program Efficacy for Vastus Lateralis and Soleus Skeletal Muscle Health during 70 Days of Simulated Microgravity. J. Appl. Physiol. 2024, 136, 1015–1039. [Google Scholar] [CrossRef] [PubMed]
- Li, R.-J.; Liu, Y.; Liu, H.-Q.; Li, J. Ketogenic Diets and Protective Mechanisms in Epilepsy, Metabolic Disorders, Cancer, Neuronal Loss, and Muscle and Nerve Degeneration. J. Food Biochem. 2020, 44, e13140. [Google Scholar] [CrossRef]
- Zhou, Z.; Hagopian, K.; López-Domínguez, J.A.; Kim, K.; Jasoliya, M.; Roberts, M.N.; Cortopassi, G.A.; Showalter, M.R.; Roberts, B.S.; González-Reyes, J.A.; et al. A Ketogenic Diet Impacts Markers of Mitochondrial Mass in a Tissue Specific Manner in Aged Mice. Aging 2021, 13, 7914–7930. [Google Scholar] [CrossRef] [PubMed]
- Fukao, T.; Lopaschuk, G.D.; Mitchell, G.A. Pathways and Control of Ketone Body Metabolism: On the Fringe of Lipid Biochemistry. Prostaglandins Leukot. Essent. Fatty Acids 2004, 70, 243–251. [Google Scholar] [CrossRef] [PubMed]
- McSwiney, F.T.; Wardrop, B.; Hyde, P.N.; Lafountain, R.A.; Volek, J.S.; Doyle, L. Keto-Adaptation Enhances Exercise Performance and Body Composition Responses to Training in Endurance Athletes. Metabolism. 2018, 83, e1–e2. [Google Scholar] [CrossRef]
- Kephart, W.C.; Pledge, C.D.; Roberson, P.A.; Mumford, P.W.; Romero, M.A.; Mobley, C.B.; Martin, J.S.; Young, K.C.; Lowery, R.P.; Wilson, J.M.; et al. The Three-Month Effects of a Ketogenic Diet on Body Composition, Blood Parameters, and Performance Metrics in CrossFit Trainees: A Pilot Study. Sports 2018, 6, 1. [Google Scholar] [CrossRef]
- Bloemberg, D.; Quadrilatero, J. Rapid Determination of Myosin Heavy Chain Expression in Rat, Mouse, and Human Skeletal Muscle Using Multicolor Immunofluorescence Analysis. PLoS ONE 2012, 7, e35273. [Google Scholar] [CrossRef]
- Wallace, M.A.; Aguirre, N.W.; Marcotte, G.R.; Marshall, A.G.; Baehr, L.M.; Hughes, D.C.; Hamilton, K.L.; Roberts, M.N.; Lopez-Dominguez, J.A.; Miller, B.F.; et al. The Ketogenic Diet Preserves Skeletal Muscle with Aging in Mice. Aging Cell 2021, 20, e13322. [Google Scholar] [CrossRef]
- Ogura, Y.; Kakehashi, C.; Yoshihara, T.; Kurosaka, M.; Kakigi, R.; Higashida, K.; Fujiwara, S.-E.; Akema, T.; Funabashi, T. Ketogenic Diet Feeding Improves Aerobic Metabolism Property in Extensor Digitorum Longus Muscle of Sedentary Male Rats. PLoS ONE 2020, 15, e0241382. [Google Scholar] [CrossRef]
- Stein, T.P.; Wade, C.E. Metabolic Consequences of Muscle Disuse Atrophy. J. Nutr. 2005, 135, 1824S–1828S. [Google Scholar] [CrossRef]
- Ciciliot, S.; Rossi, A.C.; Dyar, K.A.; Blaauw, B.; Schiaffino, S. Muscle Type and Fiber Type Specificity in Muscle Wasting. Int. J. Biochem. Cell Biol. 2013, 45, 2191–2199. [Google Scholar] [CrossRef] [PubMed]
- Evans, M.; Cogan, K.E.; Egan, B. Metabolism of Ketone Bodies during Exercise and Training: Physiological Basis for Exogenous Supplementation. J. Physiol. 2017, 595, 2857–2871. [Google Scholar] [CrossRef]
- Miller, W.C.; Bryce, G.R.; Conlee, R.K. Adaptations to a High-Fat Diet That Increase Exercise Endurance in Male Rats. J. Appl. Physiol. 1984, 56, 78–83. [Google Scholar] [CrossRef] [PubMed]
- Ma, S.; Huang, Q.; Yada, K.; Liu, C.; Suzuki, K. An 8-Week Ketogenic Low Carbohydrate, High Fat Diet Enhanced Exhaustive Exercise Capacity in Mice. Nutrients 2018, 10, 673. [Google Scholar] [CrossRef]
- Simi, B.; Sempore, B.; Mayet, M.H.; Favier, R.J. Additive Effects of Training and High-Fat Diet on Energy Metabolism during Exercise. J. Appl. Physiol. 1991, 71, 197–203. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, K.; Saito, H.; Sumi, K.; Sakamoto, Y.; Tachi, Y.; Iida, K. Short-Term and Long-Term Ketogenic Diet Therapy and the Addition of Exercise Have Differential Impacts on Metabolic Gene Expression in the Mouse Energy-Consuming Organs Heart and Skeletal Muscle. Nutr. Res. 2018, 60, 77–86. [Google Scholar] [CrossRef]
- Haba, Y.; Fujimura, T.; Oyama, K.; Kinoshita, J.; Miyashita, T.; Fushida, S.; Harada, S.; Ohta, T. Effect of Oral Branched-Chain Amino Acids and Glutamine Supplementation on Skeletal Muscle Atrophy After Total Gastrectomy in Rat Model. J. Surg. Res. 2019, 243, 281–288. [Google Scholar] [CrossRef]
- Moro, T.; Ebert, S.M.; Adams, C.M.; Rasmussen, B.B. Amino Acid Sensing in Skeletal Muscle. Trends Endocrinol. Metab. 2016, 27, 796–806. [Google Scholar] [CrossRef]
- Pedersen, B.K.; Saltin, B. Exercise as Medicine—Evidence for Prescribing Exercise as Therapy in 26 Different Chronic Diseases. Scand. J. Med. Sci. Sports 2015, 25 (Suppl. S3), 1–72. [Google Scholar] [CrossRef]
- Wang, D.; Li, Z.; Zhang, J.; Liu, S.; Chen, W.; Wang, H.; Wang, Y.; Wang, H.; Wang, L.; Li, Y. A Rat Weightlessness Simulation Treadmill and Exercise Method. Chinese Patent CN103999794B, 24 August 2016. [Google Scholar]
- Dougherty, J.P.; Springer, D.A.; Gershengorn, M.C. The Treadmill Fatigue Test: A Simple, High-Throughput Assay of Fatigue-like Behavior for the Mouse. J. Vis. Exp. JoVE 2016, 111, e54052. [Google Scholar]
- Burch, N.; Arnold, A.-S.; Item, F.; Summermatter, S.; Santos, G.B.S.; Christe, M.; Boutellier, U.; Toigo, M.; Handschin, C. Electric Pulse Stimulation of Cultured Murine Muscle Cells Reproduces Gene Expression Changes of Trained Mouse Muscle. PLoS ONE 2010, 5, e10970. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Nie, C.; Xue, L.; Yan, Y.; Liu, S.; Sun, J.; Fan, M.; Qian, H.; Ying, H.; Wang, L.; et al. Growth Hormone Receptor Disrupts Glucose Homeostasis via Promoting and Stabilizing Retinol Binding Protein 4. Theranostics 2021, 11, 8283–8300. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.-D.; Li, L.-F.; Yan, Y.-J.; Wang, X.-C.; Chen, L.; Dang, K.; Wang, Z.; Wang, H.-P. Metabolic Adaptation Mechanisms of Glycogen Reduction and Lipid Accumulation in Testicular Protection in Daurian Ground Squirrels during Hibernation. Biochimie 2025, 232, 133–141. [Google Scholar] [CrossRef]
- Liu, Q.; Chen, L.; Liang, X.; Cao, Y.; Zhu, X.; Wang, S.; Li, J.; Gao, J.; Xiao, J. Exercise Attenuates angiotensin II-Induced Muscle Atrophy by Targeting PPARγ/miR-29b. J. Sport Health Sci. 2022, 11, 696–707. [Google Scholar] [CrossRef] [PubMed]
- Venturini, C.; Mancinelli, L.; Matacchione, G.; Olivieri, F.; Antonicelli, R. The Cardioprotective Effects of Nutritional Ketosis: Mechanisms and Clinical Implications. Nutrients 2024, 16, 4204. [Google Scholar] [CrossRef]
- Jeukendrup, A.E.; Wallis, G.A. Measurement of Substrate Oxidation during Exercise by Means of Gas Exchange Measurements. Int. J. Sports Med. 2005, 26 (Suppl. S1), S28–S37. [Google Scholar] [CrossRef]
- Fernando, R.; Drescher, C.; Nowotny, K.; Grune, T.; Castro, J.P. Impaired Proteostasis during Skeletal Muscle Aging. Free Radic. Biol. Med. 2019, 132, 58–66. [Google Scholar] [CrossRef]
- Fisher, F.M.; Maratos-Flier, E. Understanding the Physiology of FGF21. Annu. Rev. Physiol. 2016, 78, 223–241. [Google Scholar] [CrossRef]
- Liu, X.; Wang, Y.; Hou, L.; Xiong, Y.; Zhao, S. Fibroblast Growth Factor 21 (FGF21) Promotes Formation of Aerobic Myofibers via the FGF21-SIRT1-AMPK-PGC1α Pathway. J. Cell. Physiol. 2017, 232, 1893–1906. [Google Scholar] [CrossRef]
- Abdurrachim, D.; Woo, C.C.; Teo, X.Q.; Chan, W.X.; Radda, G.K.; Lee, P.T.H. A New Hyperpolarized 13C Ketone Body Probe Reveals an Increase in Acetoacetate Utilization in the Diabetic Rat Heart. Sci. Rep. 2019, 9, 5532. [Google Scholar] [CrossRef]
- Angelini, A.; Saha, P.K.; Jain, A.; Jung, S.Y.; Mynatt, R.L.; Pi, X.; Xie, L. PHDs/CPT1B/VDAC1 Axis Regulates Long-Chain Fatty Acid Oxidation in Cardiomyocytes. Cell Rep. 2021, 37, 109767. [Google Scholar] [CrossRef] [PubMed]
- Dang, Y.; Xu, J.; Zhu, M.; Zhou, W.; Zhang, L.; Ji, G. Gan-Jiang-Ling-Zhu Decoction Alleviates Hepatic Steatosis in Rats by the miR-138-5p/CPT1B Axis. Biomed. Pharmacother. 2020, 127, 110127. [Google Scholar] [CrossRef] [PubMed]
- Maltais, F.; Simard, A.A.; Simard, C.; Jobin, J.; Desgagnés, P.; LeBlanc, P. Oxidative Capacity of the Skeletal Muscle and Lactic Acid Kinetics during Exercise in Normal Subjects and in Patients with COPD. Am. J. Respir. Crit. Care Med. 1996, 153, 288–293. [Google Scholar] [CrossRef]
- Xiang, Y.; Wang, Q.-Q.; Lan, X.-Q.; Zhang, H.-J.; Wei, D.-X. Function and Treatment Strategies of β-Hydroxybutyrate in Aging. Smart Mater. Med. 2023, 4, 160–172. [Google Scholar] [CrossRef]
- Wang, Q.; Lan, X.; Ke, H.; Xu, S.; Huang, C.; Wang, J.; Wang, X.; Huang, T.; Wu, X.; Chen, M.; et al. Histone β-Hydroxybutyrylation Is Critical in Reversal of Sarcopenia. Aging Cell 2024, 23, e14284. [Google Scholar] [CrossRef]
- Beckett, T.L.; Studzinski, C.M.; Keller, J.N.; Paul Murphy, M.; Niedowicz, D.M. A Ketogenic Diet Improves Motor Performance but Does Not Affect β-Amyloid Levels in a Mouse Model of Alzheimer’s Disease. Brain Res. 2013, 1505, 61–67. [Google Scholar] [CrossRef]
- Bielohuby, M.; Menhofer, D.; Kirchner, H.; Stoehr, B.J.M.; Müller, T.D.; Stock, P.; Hempel, M.; Stemmer, K.; Pfluger, P.T.; Kienzle, E.; et al. Induction of Ketosis in Rats Fed Low-Carbohydrate, High-Fat Diets Depends on the Relative Abundance of Dietary Fat and Protein. Am. J. Physiol. Endocrinol. Metab. 2011, 300, E65–E76. [Google Scholar] [CrossRef]
- Bielohuby, M.; Sawitzky, M.; Stoehr, B.J.M.; Stock, P.; Menhofer, D.; Ebensing, S.; Bjerre, M.; Frystyk, J.; Binder, G.; Strasburger, C.; et al. Lack of Dietary Carbohydrates Induces Hepatic Growth Hormone (GH) Resistance in Rats. Endocrinology 2011, 152, 1948–1960. [Google Scholar] [CrossRef] [PubMed]
- Ellenbroek, J.H.; van Dijck, L.; Töns, H.A.; Rabelink, T.J.; Carlotti, F.; Ballieux, B.E.P.B.; de Koning, E.J.P. Long-Term Ketogenic Diet Causes Glucose Intolerance and Reduced β- and α-Cell Mass but No Weight Loss in Mice. Am. J. Physiol. Endocrinol. Metab. 2014, 306, E552–E558. [Google Scholar] [CrossRef]
- Fukushima, A.; Ogura, Y.; Furuta, M.; Kakehashi, C.; Funabashi, T.; Akema, T. Ketogenic Diet Does Not Impair Spatial Ability Controlled by the Hippocampus in Male Rats. Brain Res. 2015, 1622, 36–42. [Google Scholar] [CrossRef]
- Parker, B.A.; Walton, C.M.; Carr, S.T.; Andrus, J.L.; Cheung, E.C.K.; Duplisea, M.J.; Wilson, E.K.; Draney, C.; Lathen, D.R.; Kenner, K.B.; et al. β-Hydroxybutyrate Elicits Favorable Mitochondrial Changes in Skeletal Muscle. Int. J. Mol. Sci. 2018, 19, 2247. [Google Scholar] [CrossRef] [PubMed]
- Parry, H.A.; Kephart, W.C.; Mumford, P.W.; Romero, M.A.; Mobley, C.B.; Zhang, Y.; Roberts, M.D.; Kavazis, A.N. Ketogenic Diet Increases Mitochondria Volume in the Liver and Skeletal Muscle without Altering Oxidative Stress Markers in Rats. Heliyon 2018, 4, e00975. [Google Scholar] [CrossRef]
- Roberts, M.N.; Wallace, M.A.; Tomilov, A.A.; Zhou, Z.; Marcotte, G.R.; Tran, D.; Perez, G.; Gutierrez-Casado, E.; Koike, S.; Knotts, T.A.; et al. A Ketogenic Diet Extends Longevity and Healthspan in Adult Mice. Cell Metab. 2018, 27, 1156. [Google Scholar] [CrossRef] [PubMed]
- Martín-Moraleda, E.; Delisle, C.; Collado Mateo, D.; Aznar-Lain, S. Weight loss and body composition changes through ketogenic diet and physical activity: A methodological and systematic review. Nutr. Hosp. 2019, 36, 1196–1204. [Google Scholar]
- Sahagun, E.; Ward, L.M.; Kinzig, K.P. Attenuation of Stress-Induced Weight Loss with a Ketogenic Diet. Physiol. Behav. 2019, 212, 112654. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Chen, B.; Zou, K. Effect of Ketogenic Diet on Exercise Tolerance and Transcriptome of Gastrocnemius in Mice. Open Life Sci. 2023, 18, 20220570. [Google Scholar] [CrossRef]
- Zhou, Z.; Vidales, J.; González-Reyes, J.A.; Shibata, B.; Baar, K.; Rutkowsky, J.M.; Ramsey, J.J. A 1-Month Ketogenic Diet Increased Mitochondrial Mass in Red Gastrocnemius Muscle, but Not in the Brain or Liver of Middle-Aged Mice. Nutrients 2021, 13, 2533. [Google Scholar] [CrossRef]
- Bilodeau, P.A.; Coyne, E.S.; Wing, S.S. The Ubiquitin Proteasome System in Atrophying Skeletal Muscle: Roles and Regulation. Am. J. Physiol. Cell Physiol. 2016, 311, C392–C403. [Google Scholar] [CrossRef]
- Sandonà, D.; Desaphy, J.-F.; Camerino, G.M.; Bianchini, E.; Ciciliot, S.; Danieli-Betto, D.; Dobrowolny, G.; Furlan, S.; Germinario, E.; Goto, K.; et al. Adaptation of Mouse Skeletal Muscle to Long-Term Microgravity in the MDS Mission. PLoS ONE 2012, 7, e33232. [Google Scholar] [CrossRef]
- Gundersen, K. Excitation-Transcription Coupling in Skeletal Muscle: The Molecular Pathways of Exercise. Biol. Rev. 2011, 86, 564–600. [Google Scholar] [CrossRef]
- Roberts, B.M.; Deemer, S.E.; Smith, D.L.; Mobley, J.A.; Musi, N.; Plaisance, E.P. Effects of an Exogenous Ketone Ester Using Multi-Omics in Skeletal Muscle of Aging C57BL/6J Male Mice. Front. Nutr. 2022, 9, 1041026. [Google Scholar] [CrossRef] [PubMed]
- Aare, S.; Spendiff, S.; Vuda, M.; Elkrief, D.; Perez, A.; Wu, Q.; Mayaki, D.; Hussain, S.N.A.; Hettwer, S.; Hepple, R.T. Failed Reinnervation in Aging Skeletal Muscle. Skelet. Muscle 2016, 6, 29. [Google Scholar] [CrossRef]
- Rowan, S.L.; Rygiel, K.; Purves-Smith, F.M.; Solbak, N.M.; Turnbull, D.M.; Hepple, R.T. Denervation Causes Fiber Atrophy and Myosin Heavy Chain Co-Expression in Senescent Skeletal Muscle. PLoS ONE 2012, 7, e29082. [Google Scholar] [CrossRef]
- Demirel, H.A.; Powers, S.K.; Naito, H.; Hughes, M.; Coombes, J.S. Exercise-Induced Alterations in Skeletal Muscle Myosin Heavy Chain Phenotype: Dose-Response Relationship. J. Appl. Physiol. 1999, 86, 1002–1008. [Google Scholar] [CrossRef] [PubMed]
- Ogura, Y.; Naito, H.; Kakigi, R.; Akema, T.; Sugiura, T.; Katamoto, S.; Aoki, J. Different Adaptations of Alpha-Actinin Isoforms to Exercise Training in Rat Skeletal Muscles. Acta Physiol. 2009, 196, 341–349. [Google Scholar] [CrossRef]
- Ogura, Y.; Naito, H.; Kakigi, R.; Ichinoseki-Sekine, N.; Kurosaka, M.; Katamoto, S. Alpha-Actinin-3 Levels Increase Concomitantly with Fast Fibers in Rat Soleus Muscle. Biochem. Biophys. Res. Commun. 2008, 372, 584–588. [Google Scholar] [CrossRef]
- Huang, T.-Y.; Linden, M.A.; Fuller, S.E.; Goldsmith, F.R.; Simon, J.; Batdorf, H.M.; Scott, M.C.; Essajee, N.M.; Brown, J.M.; Noland, R.C. Combined Effects of a Ketogenic Diet and Exercise Training Alter Mitochondrial and Peroxisomal Substrate Oxidative Capacity in Skeletal Muscle. Am. J. Physiol. Endocrinol. Metab. 2021, 320, E1053–E1067. [Google Scholar] [CrossRef]
- Nakao, R.; Abe, T.; Yamamoto, S.; Oishi, K. Ketogenic Diet Induces Skeletal Muscle Atrophy via Reducing Muscle Protein Synthesis and Possibly Activating Proteolysis in Mice. Sci. Rep. 2019, 9, 19652. [Google Scholar] [CrossRef] [PubMed]
- Burke, L.M.; Ross, M.L.; Garvican-Lewis, L.A.; Welvaert, M.; Heikura, I.A.; Forbes, S.G.; Mirtschin, J.G.; Cato, L.E.; Strobel, N.; Sharma, A.P.; et al. Low Carbohydrate, High Fat Diet Impairs Exercise Economy and Negates the Performance Benefit from Intensified Training in Elite Race Walkers. J. Physiol. 2017, 595, 2785–2807. [Google Scholar] [CrossRef]
- Ji, S.; You, Y.; Kerner, J.; Hoppel, C.L.; Schoeb, T.R.; Chick, W.S.H.; Hamm, D.A.; Sharer, J.D.; Wood, P.A. Homozygous Carnitine Palmitoyltransferase 1b (Muscle Isoform) Deficiency Is Lethal in the Mouse. Mol. Genet. Metab. 2008, 93, 314–322. [Google Scholar] [CrossRef]
- Ma, S.; Huang, Q.; Tominaga, T.; Liu, C.; Suzuki, K. An 8-Week Ketogenic Diet Alternated Interleukin-6, Ketolytic and Lipolytic Gene Expression, and Enhanced Exercise Capacity in Mice. Nutrients 2018, 10, 1696. [Google Scholar] [CrossRef] [PubMed]
- Fritzen, A.M.; Lundsgaard, A.-M.; Kiens, B. Tuning Fatty Acid Oxidation in Skeletal Muscle with Dietary Fat and Exercise. Nat. Rev. Endocrinol. 2020, 16, 683–696. [Google Scholar] [CrossRef]
- Espinoza, D.O.; Boros, L.G.; Crunkhorn, S.; Gami, H.; Patti, M.-E. Dual Modulation of Both Lipid Oxidation and Synthesis by Peroxisome Proliferator-Activated Receptor-Gamma Coactivator-1alpha and -1beta in Cultured Myotubes. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2010, 24, 1003–1014. [Google Scholar]
- Wong, K.E.; Mikus, C.R.; Slentz, D.H.; Seiler, S.E.; DeBalsi, K.L.; Ilkayeva, O.R.; Crain, K.I.; Kinter, M.T.; Kien, C.L.; Stevens, R.D.; et al. Muscle-Specific Overexpression of PGC-1α Does Not Augment Metabolic Improvements in Response to Exercise and Caloric Restriction. Diabetes 2015, 64, 1532–1543. [Google Scholar] [CrossRef]
- Miller, V.J.; LaFountain, R.A.; Barnhart, E.; Sapper, T.S.; Short, J.; Arnold, W.D.; Hyde, P.N.; Crabtree, C.D.; Kackley, M.L.; Kraemer, W.J.; et al. A Ketogenic Diet Combined with Exercise Alters Mitochondrial Function in Human Skeletal Muscle While Improving Metabolic Health. Am. J. Physiol. Endocrinol. Metab. 2020, 319, E995–E1007. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Li, Z.; Zhao, M.; Nie, Y.; Liu, P.; Zhu, Y.; Zhang, X. Skeletal Muscle Lipid Droplets and the Athlete’s Paradox. Cells 2019, 8, 249. [Google Scholar] [CrossRef] [PubMed]
- Cox, P.J.; Clarke, K. Acute Nutritional Ketosis: Implications for Exercise Performance and Metabolism. Extreme Physiol. Med. 2014, 3, 17. [Google Scholar] [CrossRef]
- Sasai, H.; Aoyama, Y.; Otsuka, H.; Abdelkreem, E.; Naiki, Y.; Kubota, M.; Sekine, Y.; Itoh, M.; Nakama, M.; Ohnishi, H.; et al. Heterozygous Carriers of Succinyl-CoA:3-Oxoacid CoA Transferase Deficiency Can Develop Severe Ketoacidosis. J. Inherit. Metab. Dis. 2017, 40, 845–852. [Google Scholar] [CrossRef]
- Puchalska, P.; Crawford, P.A. Multi-Dimensional Roles of Ketone Bodies in Fuel Metabolism, Signaling, and Therapeutics. Cell Metab. 2017, 25, 262–284. [Google Scholar] [CrossRef]




| Diet | Intervention | Group | n |
|---|---|---|---|
| Normal diet (ND) | Control | NC | 8 |
| Hindlimb unloading (HU) | NH | 12 | |
| HU + exercise | NHE | 14 | |
| Ketogenic diet (KD) | Control | KC | 8 |
| Hindlimb unloading (HU) | KH | 12 | |
| HU + exercise | KHE | 14 |
| Gene Name | Forward Primer | Reverse Primer |
|---|---|---|
| Atrogin1 | 5′-TCAGCAGCCTGAACTACGAC-3′ | 5′-GCGCTCCTTCGTACTTCCTT-3′ |
| MURF-1 | 5′-GTGTGAGGTGCCTACTTGCT-3′ | 5′-GACTTTTCCAGCTGCTCCCT-3′ |
| PGC-1α | 5′-AGCCGTGACCACTGACAACGAG-3′ | 5′-GCTGCATGGTTCTGAGTGCTAAG-3′ |
| SIRT1 | 5′-TACCTTGGAGCAGGTTGCAG-3′ | 5′-GCACCGAGGAACTACCTGAT-3′ |
| FGF21 | 5′-GCATACCCCATCCCTGACTC-3′ | 5′-GGATCAAAGTGAGGCGATCC-3′ |
| CPT-1b | 5′-TTCAACACTACACGCATCCC-3′ | 5′-GCCCTCATAGAGCCAGACC-3′ |
| HADH | 5′-ACACCTTCATTCGCCATATTGC-3′ | 5′-TCGGTGAATTTTCTGTAGACCAC-3′ |
| OXCT | 5′-CCCATACCCACTGAAAGACGAA-3′ | 5′-CTGGAGAAGAAAGAGGCTCCTG-3′ |
| 18s | 5′-GGGAGCCTGAGAAACGGC-3′ | 5′-GGGTCGGGAGTGGGTAATTT-3′ |
| Variable | Measurement Instruments | Validation Reference |
|---|---|---|
| Body weight | Electronic Balance | Manufacturer’s protocol |
| Blood ketone/glucose | FreeStyle Optium Neo Blood β-Ketone/Glucose Test Strips | Abbott technical manual [23] |
| RER | Indirect Calorimetry | Manufacturer’s protocol |
| TG | The reagent kits | Manufacturer’s protocol |
| TC | The reagent kits | Manufacturer’s protocol |
| HDL-C | The reagent kits | Manufacturer’s protocol |
| LDL-C | The reagent kits | Manufacturer’s protocol |
| Insulin | The reagent kits | Manufacturer’s protocol |
| Glycogen content | The Periodic Acid–Schiff Staining Kit | Solarbio technical manual [24] |
| Lipid deposition | The Oil Red O Staining Kit | Solarbio technical manual [24] |
| LD | The reagent kits | Manufacturer’s protocol |
| UREA | The reagent kits | Manufacturer’s protocol |
| CK | The reagent kits | Manufacturer’s protocol |
| LDH | The reagent kits | Manufacturer’s protocol |
| Muscle fiber types | Immunofluorescence (MyHC Antibodies) | Manufacturer’s protocol [25] |
| TC (mmol/L) | TG (mmol/L) | HDL-C (mmol/L) | LDL-C (mmol/L) | UREA (mmol/L) | Insulin (ng/mL) | |
|---|---|---|---|---|---|---|
| NC | 1.35 ± 0.15 | 0.38 ± 0.07 | 1.18 ± 0.09 | 0.17 ± 0.03 | 9.57 ± 0.37 | 0.97 ± 0.21 |
| NH | 1.29 ± 0.23 | 0.58 ± 0.18 | 1.12 ± 0.13 | 0.18 ± 0.06 | 9.28 ± 0.34 | 1.72 ± 0.58 ## |
| NHE | 1.24 ± 0.21 | 0.59 ± 0.29 | 1.09 ± 0.21 | 0.20 ± 0.06 | 8.33 ± 0.30 | 1.44 ± 0.56 ## |
| KC | 2.16 ± 0.20 ** | 0.63 ± 0.20 ** | 1.90 ± 0.12 ** | 0.27 ± 0.04 ** | 9.31 ± 0.80 ** | 1.51 ± 0.59 * |
| KH | 1.96 ± 0.23 #** | 0.99 ± 0.16 ##** | 1.64 ± 0.27 ##** | 0.25 ± 0.03 ** | 9.53 ± 0.85 ** | 1.38 ± 0.51 ** |
| KHE | 1.79± 0.36 ##** | 0.74 ± 0.29 # | 1.55 ± 0.38 ##** | 0.23 ± 0.03 ## | 9.25 ± 0.42 ** | 1.65 ± 0.71 ** |
| LA (mmol/L) | UREA (mmol/L) | CK (U/L) | LDH (U/L) | Time (min) | |
|---|---|---|---|---|---|
| NC | 1.81 ± 0.26 | 9.57 ± 0.37 | 850 ± 310 | 513 ± 166 | 136 ± 15 |
| NH | 2.41 ± 0.53 ## | 9.27 ± 0.34 | 866 ± 260 | 655 ± 97 | 83 ± 24 ## |
| NHE | 2.32 ± 0.34 ## | 8.33 ± 0.30 | 749 ± 275 | 547 ± 113 | 84 ± 30 ## |
| KC | 2.43 ± 0.39 ** | 9.20 ± 0.71 | 981 ± 233 | 521 ± 180 | 152 ± 18 |
| KH | 2.23 ± 0.46 | 9.26 ± 0.61 | 863 ± 315 | 615 ± 272 | 81 ± 36 ## |
| KHE | 2.03 ± 0.34 ## | 9.26 ± 0.49 | 955 ± 288 | 486 ± 286 | 136 ± 26 ##* |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, J.; Li, W.; Yu, L.; Zhang, B.; Li, Z.; Zou, P.; Ding, B.; Dai, X.; Wang, Q. Combined Effects of Ketogenic Diet and Aerobic Exercise on Skeletal Muscle Fiber Remodeling and Metabolic Adaptation in Simulated Microgravity Mice. Metabolites 2025, 15, 270. https://doi.org/10.3390/metabo15040270
Chen J, Li W, Yu L, Zhang B, Li Z, Zou P, Ding B, Dai X, Wang Q. Combined Effects of Ketogenic Diet and Aerobic Exercise on Skeletal Muscle Fiber Remodeling and Metabolic Adaptation in Simulated Microgravity Mice. Metabolites. 2025; 15(4):270. https://doi.org/10.3390/metabo15040270
Chicago/Turabian StyleChen, Jun, Wenjiong Li, Liang Yu, Bowei Zhang, Zhili Li, Peng Zou, Bai Ding, Xiaoqian Dai, and Qirong Wang. 2025. "Combined Effects of Ketogenic Diet and Aerobic Exercise on Skeletal Muscle Fiber Remodeling and Metabolic Adaptation in Simulated Microgravity Mice" Metabolites 15, no. 4: 270. https://doi.org/10.3390/metabo15040270
APA StyleChen, J., Li, W., Yu, L., Zhang, B., Li, Z., Zou, P., Ding, B., Dai, X., & Wang, Q. (2025). Combined Effects of Ketogenic Diet and Aerobic Exercise on Skeletal Muscle Fiber Remodeling and Metabolic Adaptation in Simulated Microgravity Mice. Metabolites, 15(4), 270. https://doi.org/10.3390/metabo15040270







