Plant Secondary Metabolites—Central Regulators Against Abiotic and Biotic Stresses
Abstract
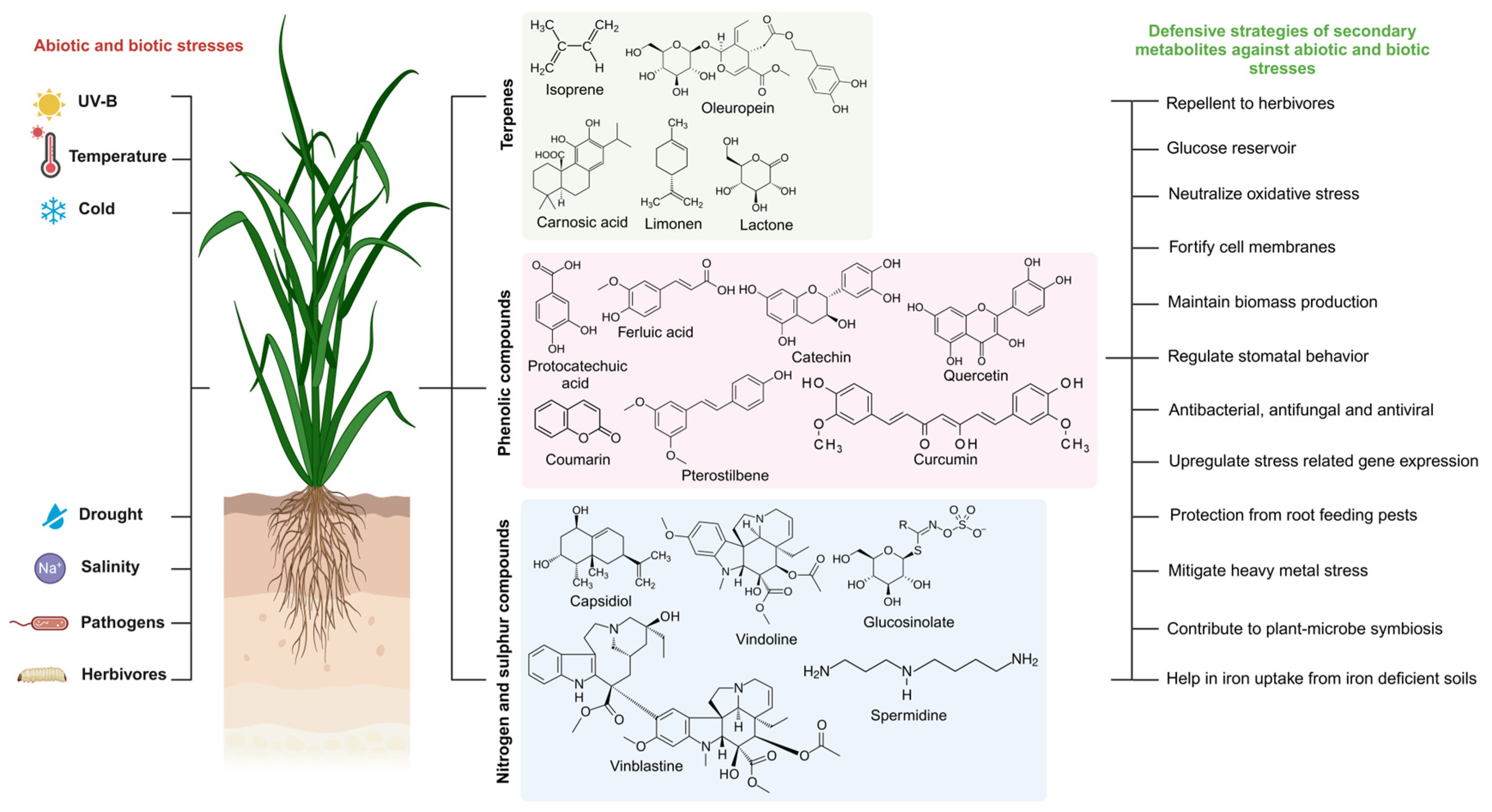
:1. Introduction
2. Diversity of Secondary Metabolites and Their Biosynthesis in Plants
3. The Roles of SMs in Plant Stress Responses
3.1. Terpenes
3.2. Phenolics
3.3. Flavonoids
3.4. Tannins
3.5. Lignans and Lignin
3.6. Stilbenes
3.7. Curcuminoids
3.8. Chitinases
3.9. Nitrogen- and Sulfur-Containing Secondary Metabolites
3.9.1. Nitrogen-Containing Alkaloids
3.9.2. Sulfur-Containing Glucosinolates (GSLs)
3.9.3. Sulfur-Containing Phytoalexins
4. Roles of Secondary Metabolites in Plant–Microbiome Interactions
4.1. Mechanisms of Interaction
4.2. Bi-Directional Influence of Secondary Metabolites and Microbial Activity
4.3. Role of Secondary Metabolites in Sustainable Agriculture
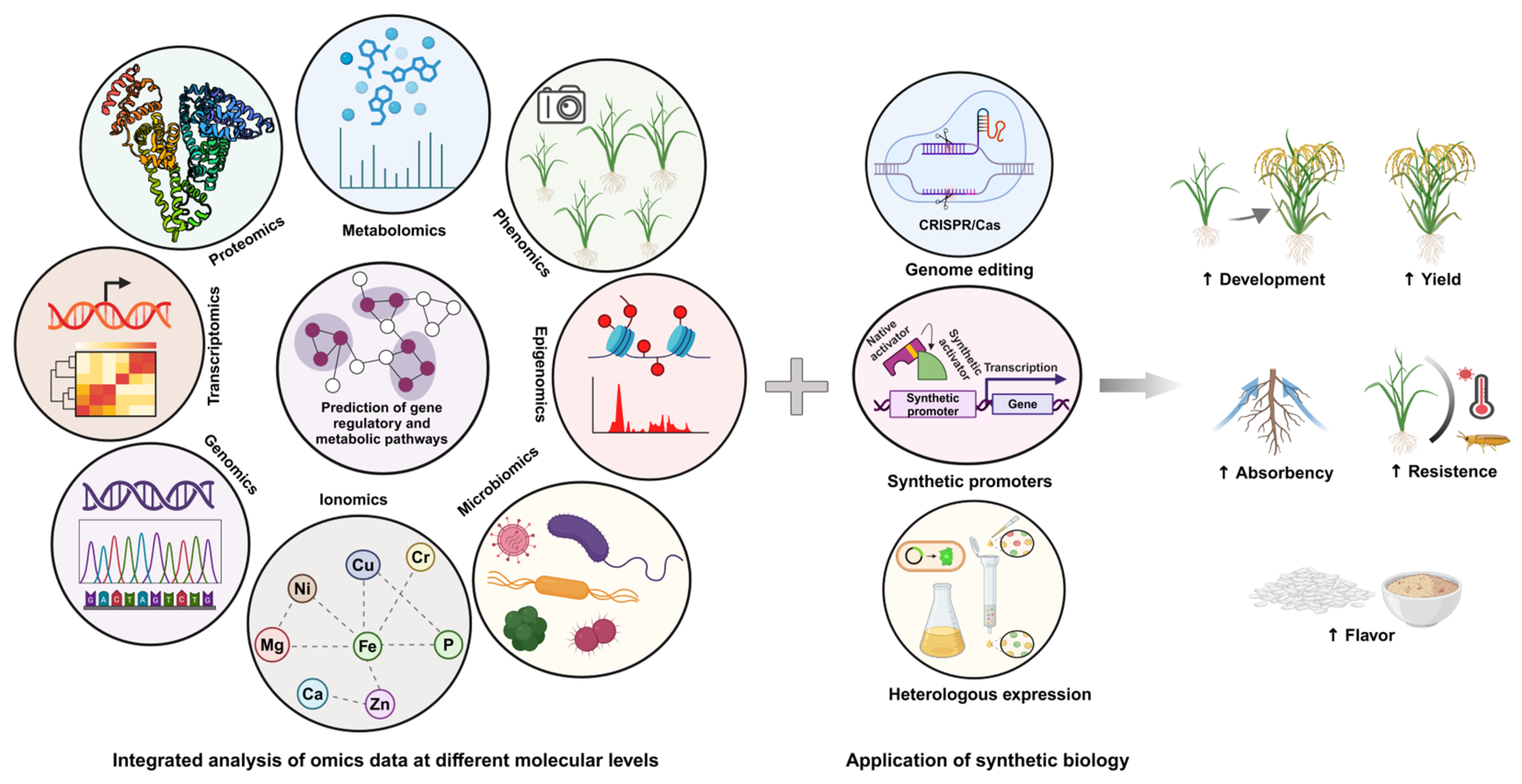
5. Expression and Manipulation of Gene Clusters for Secondary Metabolites Biosynthesis
6. Transcriptional Regulation of Secondary Metabolite Biosynthesis Under Biotic and Abiotic Stress Conditions
6.1. Hormonal and Signal Transduction Pathways
6.2. Epigenetic Regulation and Chromatin Remodeling
7. Biotechnological Advances in Engineering Secondary Metabolite Pathways
8. Conclusions and Prospect
| Name | Related Functions | Plant Specie | References |
|---|---|---|---|
| Terpenes | |||
| Monoterpenes | Chemical products secreted by plants are important against insect toxicity | Chrysanthemum, cumin, pepper, mint, eucalyptus | [150] |
| Diterpenes | Act as epithelium irritants and toxins to insects and mammals | Codiaeum, Hura Phyllanthus | [151] |
| Triterpenes | Triterpenes have some self-protective effects against insects by altering their development | Higher plants Ferns and marine organisms | [152] |
| Polyterpenes | Offer defense as a process for infection repair and as resistance to pests | Bruce banner | [153] |
| Phenolics | |||
| Phenolics flavonoids Coumarin Bioflavonoids Others | Flavanol content is significantly lower under the lower temperature treatment in pygmy smartweed | Polygonum minus Huds. | [154] |
| - | HT has little effect on seed phenolics, but reduces anthocyanins in the skin of grapes | Vitis vinifera L. | [155] |
| - | Monoterpenes and sesquiterpenes increase in thyme in response to DS | Artemisia annua L. | [156] |
| - | Monosubstituted flavanols increase under UVB Flavanols are unaffected; supplemental UVB also increases tannins in some species | Tomato | [54] |
| Nitrogen-containing SMs | |||
| Alkaloids Cyanogenic glycosides Non-Protein Amino Acid | Cause signaling molecule to trigger flavonoid biosynthesis under lower temperatures | Apple (Malus sp.) | [54] |
| - | Temperature causes an upregulation of key enzymes in isoprene production | Carrots (Daucus carota L.) | [157] |
| - | Increased light may have negative consequences on SM production in sensitive plants. Longer photoperiod | Ocimum basilicum L. | [158] |
| - | Plants have more cyanogenic glycosides; variability also observed in alkaloids, which increases in the shade in evergreen tropical trees | Tabernaemontana pachysiphon Stapf | [54] |
| - | Arabidopsis mutants lacking flavonoids; production mechanisms are hypersensitive to UVB radiation; flavonoid production is tolerant to typically lethal UVB levels | Arabidopsis thaliana | [159] |
| Sulfur-containing SMs | |||
| Glutathione | GSH acts as a growth regulator and during stress it acts as an antioxidant, strengthening the defense system of the plants | Spinach Avocados Okara | [160] |
| Glucosinolate GLS | Plays a role in defense by poisoning herbivore insects during damage and as a feeding repellent | Mustar Allium allylcysd plant | [161] |
| Phytoalexins | This is a common defense mechanism against insect pests in numerous plants | Grapevine Vitis vinifera | [162] |
| Defensins, thionins, and lectins | Defensins, thionine, and lectins are stimulated by numerous stresses and show resistance against them | Circulatory white blood cells and tissue cells, wheat, corn, and tomato | [163] |
| Stilbenes | |||
| Resveratrol and pterostilbene) | Increased stilbene accumulation, greater with UV-C compared to fungal inoculum, and shows resistance | Vitis vinifera cvs. Alphonse Lavallée, Dan Ben-Hanna | [164] |
| anthocyanins; flavonoids; hydroxycinnamic acids Napoleon | Increased stilbene accumulation, greater with UV-C compared to UV-B (3- and 2-fold, respectively), and shows resistance | V. vinifera cv. Sangiovese | [164] |
| Stilbenes | Downregulation of STS expression under both low and high temperatures, upregulation of STS expression in response to CuSO4, and shows resistance | V. vinifera cv. Cabernet Sauvignon | [164] |
| Mono-glucosylated derivative resveratrol (trans- and cis-piceid and trans- and cis-resveratroloside) | Increase in trans-resveratrol endogenous accumulation and decreased release into the culture medium Glucosides show response to stress | V. vinifera cv. Barbera | [164] |
| Curcuminoids | |||
| Curcumin | Physical and chemical defense against pathogens as well as other stresses | Curcuma longa. L. | [165] |
| Curcumin/bisdemethoxycurcumin | Volatile compound shows antibacterial mechanism against a wide distribution of Gram-positive bacteria, | Curcuma longa. L. | [166] |
| Demethoxycurcumin | which have antipathogenic action against fungi, bacteria, and other pathogen agents | Turmeric | [167] |
| Chitinases | |||
| Maize chitinase 2 gene | Secondary metabolites considered as molecular targets of selection in plant–pathogen interactions. | Transgenic maize plant | [168] |
| Chitinase I gene | Inhibits phytopathogenic fungi A. solani, R. solani, F. spp., and V. dahliae | Hordeum vulgare cultivar, Haider-93 | [96] |
| Rice class I chitinase gene (Rchit) | Resistance against late leaf spot, rust disease, and A. flavus infection | Oryza sativa (Rice) | [169] |
| Tobacco osmotin (ap24) and rice chitinase (chi 11) gene | Reduce sheath blight disease caused by R. solani | Nicotiana sp. (Tobacco) and Oryza sativa (Rice) | [170] |
| Rice chitinase-3 gene | Resistance against leaf spot in peanut by Cercospora arachidicola | Oryza sativa (Rice) | [171] |
| Peroxidase | |||
| Glutathione peroxidase | Causes a reduction in the substrate to convert H2O2 hydroperoxides into water or oxygen, and shows resistance | Nicotiana sp. (Tobacco) | [96] |
| Horseradish peroxidase | Plants have adopted peroxidase systems to show resistance against numerous stresses | Armoracia rusticana | [172] |
| Cytochrome c peroxidase | These enzymes use peroxides as an electron acceptor for a reduction in oxidative damage due to stress in plants | Yeast | [173] |
| Myeloperoxidase | Includes plant immune responses to biotic stresses | Spinach | [174] |
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fahad, M.; Tariq, L.; Altaf, M.T.; Shahnawaz, M.; Aslam, M.; Liaqat, W.; Ullah, I.; Ullah, I.; Mohamed, H.I.; Basit, A. In Silico Identification and Characterization of Rare Cold Inducible 2 (RCI2) Gene Family in Cotton. Biochem. Genet. 2024, 62, 4567–4590. [Google Scholar] [CrossRef]
- Al-Khayri, J.M.; Rashmi, R.; Surya Ulhas, R.; Sudheer, W.N.; Banadka, A.; Nagella, P.; Aldaej, M.I.; Rezk, A.A.-S.; Shehata, W.F.; Almaghasla, M.I. The role of nanoparticles in response of plants to abiotic stress at physiological, biochemical, and molecular levels. Plants 2023, 12, 292. [Google Scholar] [CrossRef]
- Hou, J.; Pugazhendhi, A.; Phuong, T.N.; Thanh, N.C.; Brindhadevi, K.; Velu, G.; Chi, N.T.L.; Yuan, D. Plant resistance to disease: Using biochar to inhibit harmful microbes and absorb nutrients. Environ. Res. 2022, 214, 113883. [Google Scholar] [CrossRef]
- Khan, A.; Farhan, A.; Maqbool, F.; Maqsood, N.; Qayyum, W.; Haider, A.; Khan, M.Y.; Maleki-baladi, R.; Rahdar, A.; Díez-Pascual, A.M. Exploring the transporters and mechanisms of arsenic detoxification in plants and potential role of nanoparticles in alleviating arsenic stress. Plant Growth Regul. 2024, 104, 95–119. [Google Scholar] [CrossRef]
- War, A.R.; Paulraj, M.G.; Ahmad, T.; Buhroo, A.A.; Hussain, B.; Ignacimuthu, S.; Sharma, H.C. Mechanisms of plant defense against insect herbivores. Plant Signal. Behav. 2012, 7, 1306–1320. [Google Scholar] [CrossRef]
- Dar, S.A.; Hasan, W.; Devi, Y.K.; Tlak Gajger, I.; John, J. Enzyme-mediated adaptation of herbivorous insects to host phytochemicals. Phytochem. Rev. 2024, 23, 1–24. [Google Scholar] [CrossRef]
- Ali, M.Y.; Naseem, T.; Holopainen, J.K.; Liu, T.; Zhang, J.; Zhang, F. Tritrophic interactions among arthropod natural enemies, herbivores and plants considering volatile blends at different scale levels. Cells 2023, 12, 251. [Google Scholar] [CrossRef] [PubMed]
- Akbar, R.; Sun, J.; Bo, Y.; Khattak, W.A.; Khan, A.A.; Jin, C.; Zeb, U.; Ullah, N.; Abbas, A.; Liu, W. Understanding the Influence of Secondary Metabolites in Plant Invasion Strategies: A Comprehensive Review. Plants 2024, 13, 3162. [Google Scholar] [CrossRef] [PubMed]
- Ranner, J.L.; Schalk, S.; Martyniak, C.; Parniske, M.; Gutjahr, C.; Stark, T.D.; Dawid, C. Primary and secondary metabolites in Lotus japonicus. J. Agric. Food Chem. 2023, 71, 11277–11303. [Google Scholar] [CrossRef]
- Khan, A.; Kanwal, F.; Shazad, M.; Naz, S.; Jalil, S.; Zhang, G. Interactions of arsenic and phosphorus in their uptake and transportation in plants: Advances and prospective research on the mechanisms and approaches for alleviating arsenic stress. J. Integr. Agric. 2024. [Google Scholar] [CrossRef]
- Elshafie, H.S.; Camele, I.; Mohamed, A.A. A comprehensive review on the biological, agricultural and pharmaceutical properties of secondary metabolites based-plant origin. Int. J. Mol. Sci. 2023, 24, 3266. [Google Scholar] [CrossRef] [PubMed]
- Al-Khayri, J.M.; Rashmi, R.; Toppo, V.; Chole, P.B.; Banadka, A.; Sudheer, W.N.; Nagella, P.; Shehata, W.F.; Al-Mssallem, M.Q.; Alessa, F.M. Plant secondary metabolites: The weapons for biotic stress management. Metabolites 2023, 13, 716. [Google Scholar] [CrossRef]
- Kajla, M.; Roy, A.; Singh, I.K.; Singh, A. Regulation of the regulators: Transcription factors controlling biosynthesis of plant secondary metabolites during biotic stresses and their regulation by miRNAs. Front. Plant Sci. 2023, 14, 1126567. [Google Scholar] [CrossRef]
- Haghpanah, M.; Hashemipetroudi, S.; Arzani, A.; Araniti, F. Drought Tolerance in Plants: Physiological and Molecular Responses. Plants 2024, 13, 2962. [Google Scholar] [CrossRef] [PubMed]
- Tu, M.; Du, C.; Yu, B.; Wang, G.; Deng, Y.; Wang, Y.; Chen, M.; Chang, J.; Yang, G.; He, G. Current advances in the molecular regulation of abiotic stress tolerance in sorghum via transcriptomic, proteomic, and metabolomic approaches. Front. Plant Sci. 2023, 14, 1147328. [Google Scholar] [CrossRef]
- Zheng, S.; Zeng, T.; Li, C.; Chen, B.; Coley, C.W.; Yang, Y.; Wu, R. Deep learning driven biosynthetic pathways navigation for natural products with BioNavi-NP. Nat. Commun. 2022, 13, 3342. [Google Scholar] [CrossRef] [PubMed]
- Marchiosi, R.; dos Santos, W.D.; Constantin, R.P.; de Lima, R.B.; Soares, A.R.; Finger-Teixeira, A.; Mota, T.R.; de Oliveira, D.M.; Foletto-Felipe, M.d.P.; Abrahão, J. Biosynthesis and metabolic actions of simple phenolic acids in plants. Phytochem. Rev. 2020, 19, 865–906. [Google Scholar] [CrossRef]
- Kaur, S.; Samota, M.K.; Choudhary, M.; Choudhary, M.; Pandey, A.K.; Sharma, A.; Thakur, J. How do plants defend themselves against pathogens-Biochemical mechanisms and genetic interventions. Physiol. Mol. Biol. Plants 2022, 28, 485–504. [Google Scholar] [CrossRef]
- Khan, S.; Sehar, Z.; Albaqami, M.; Khan, N.A. Ethylene crosstalk with isoprenoid-derived signaling molecules in the context of salinity tolerance. Environ. Exp. Bot. 2023, 212, 105379. [Google Scholar] [CrossRef]
- Perez-Gil, J.; Behrendorff, J.; Douw, A.; Vickers, C.E. The methylerythritol phosphate pathway as an oxidative stress sense and response system. Nat. Commun. 2024, 15, 5303. [Google Scholar] [CrossRef]
- Kumar, S.; Korra, T.; Thakur, R.; Arutselvan, R.; Kashyap, A.S.; Nehela, Y.; Chaplygin, V.; Minkina, T.; Keswani, C. Role of plant secondary metabolites in defence and transcriptional regulation in response to biotic stress. Plant Stress 2023, 8, 100154. [Google Scholar]
- Khan, M.; Ali, S.; Al Azzawi, T.N.I.; Saqib, S.; Ullah, F.; Ayaz, A.; Zaman, W. The key roles of ROS and RNS as a signaling molecule in plant–microbe interactions. Antioxidants 2023, 12, 268. [Google Scholar] [CrossRef] [PubMed]
- Divekar, P.A.; Narayana, S.; Divekar, B.A.; Kumar, R.; Gadratagi, B.G.; Ray, A.; Singh, A.K.; Rani, V.; Singh, V.; Singh, A.K. Plant secondary metabolites as defense tools against herbivores for sustainable crop protection. Int. J. Mol. Sci. 2022, 23, 2690. [Google Scholar] [CrossRef]
- Liu, Y.; Singh, S.K.; Pattanaik, S.; Wang, H.; Yuan, L. Light regulation of the biosynthesis of phenolics, terpenoids, and alkaloids in plants. Commun. Biol. 2023, 6, 1055. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Méndez, N.; Miguel-Rojas, C.; Jimenez-Berni, J.A.; Gomez-Candon, D.; Pérez-de-Luque, A.; Fereres, E.; Catala-Forner, M.; Villegas, D.; Sillero, J.C. Plant breeding and management strategies to minimize the impact of water scarcity and biotic stress in cereal crops under Mediterranean conditions. Agronomy 2021, 12, 75. [Google Scholar] [CrossRef]
- Juroszek, P.; von Tiedemann, A. Linking plant disease models to climate change scenarios to project future risks of crop diseases: A review. J. Plant Dis. Prot. 2015, 122, 3–15. [Google Scholar] [CrossRef]
- Wilson, R.T. Coping with catastrophe: Crop diversity and crop production in Tigray National Regional State in Northern Ethiopia. Afr. J. Agric. Res. 2023, 19, 321–336. [Google Scholar]
- Ma, M.; Li, M.; Wu, Z.; Liang, X.; Zheng, Q.; Li, D.; Wang, G.; An, T. The microbial biosynthesis of noncanonical terpenoids. Appl. Microbiol. Biotechnol. 2024, 108, 226. [Google Scholar] [CrossRef]
- Maurya, A.; Mohan, S.; Verma, S.C. Antidiabetic potential of naturally occurring sesquiterpenes: A review. Curr. Top. Med. Chem. 2021, 21, 851–862. [Google Scholar] [CrossRef]
- Rivas-Ubach, A.; Peñuelas, J.; Hódar, J.A.; Oravec, M.; Paša-Tolić, L.; Urban, O.; Sardans, J. We are what we eat: A stoichiometric and ecometabolomic study of caterpillars feeding on two pine subspecies of Pinus sylvestris. Int. J. Mol. Sci. 2018, 20, 59. [Google Scholar] [CrossRef]
- Qian, J.; Zhu, C.; Jian, G.; Zeng, L.; Yang, Y. Release patterns and potential utility of herbivore-induced plant volatiles in crops: A review. Environ. Exp. Bot. 2024, 219, 105659. [Google Scholar] [CrossRef]
- Bont, Z.; Züst, T.; Arce, C.C.; Huber, M.; Erb, M. Heritable variation in root secondary metabolites is associated with recent climate. J. Ecol. 2020, 108, 2611–2624. [Google Scholar] [CrossRef]
- Merchán-Gaitán, J.B.; Mendes, J.H.; Nunes, L.E.; Buss, D.S.; Rodrigues, S.P.; Fernandes, P.M. The Role of Plant Latex in Virus Biology. Viruses 2023, 16, 47. [Google Scholar] [CrossRef]
- Chiquito-Contreras, C.J.; Meza-Menchaca, T.; Guzmán-López, O.; Vásquez, E.C.; Ricaño-Rodríguez, J. Molecular insights into plant–microbe interactions: A comprehensive review of key mechanisms. Front. Biosci. Elite 2024, 16, 9. [Google Scholar] [CrossRef] [PubMed]
- Palm, E.R.; Salzano, A.M.; Vergine, M.; Negro, C.; Nissim, W.G.; Sabbatini, L.; Balestrini, R.; de Pinto, M.C.; Fortunato, S.; Gohari, G. Response to salinity stress in four Olea europaea L. genotypes: A multidisciplinary approach. Environ. Exp. Bot. 2024, 218, 105586. [Google Scholar] [CrossRef]
- Ahmad, A.; Blasco, B.; Martos, V. Combating salinity through natural plant extracts based biostimulants: A review. Front. Plant Sci. 2022, 13, 862034. [Google Scholar] [CrossRef]
- El Yamani, M.; Cordovilla, M.d.P. Tolerance Mechanisms of Olive Tree (Olea europaea) under Saline Conditions. Plants 2024, 13, 2094. [Google Scholar] [CrossRef]
- Shahrajabian, M.H.; Kuang, Y.; Cui, H.; Fu, L.; Sun, W. Metabolic changes of active components of important medicinal plants on the basis of traditional Chinese medicine under different environmental stresses. Curr. Org. Chem. 2023, 27, 782–806. [Google Scholar] [CrossRef]
- Bertamini, M.; Faralli, M.; Varotto, C.; Grando, M.S.; Cappellin, L. Leaf monoterpene emission limits photosynthetic downregulation under heat stress in field-grown grapevine. Plants 2021, 10, 181. [Google Scholar] [CrossRef]
- Tang, H.; Wang, Q.; Xie, H.; Li, W. The function of secondary metabolites in resisting stresses in horticultural plants. Fruit Res. 2024, 4, e021. [Google Scholar] [CrossRef]
- Rezaei-Chiyaneh, E.; Mahdavikia, H.; Alipour, H.; Dolatabadian, A.; Battaglia, M.L.; Maitra, S.; Harrison, M.T. Biostimulants alleviate water deficit stress and enhance essential oil productivity: A case study with savory. Sci. Rep. 2023, 13, 720. [Google Scholar] [CrossRef]
- Mohammadi, V.; Zare Mehrjerdi, M.; Rastogi, A.; Gruda, N.S.; Aliniaeifard, S. Effects of Seed Priming with Gamma Radiation on Growth, Photosynthetic Functionality, and Essential Oil and Phytochemical Contents of Savory Plants. Horticulturae 2024, 10, 677. [Google Scholar] [CrossRef]
- Kumar, S.; Abedin, M.M.; Singh, A.K.; Das, S. Role of phenolic compounds in plant-defensive mechanisms. In Plant Phenolics in Sustainable Agriculture; Springer: Singapore, 2020; Volume 1, pp. 517–532. [Google Scholar]
- Alshibl, H.M.; Al-Abdullah, E.S.; Haiba, M.E.; Alkahtani, H.M.; Awad, G.E.; Mahmoud, A.H.; Ibrahim, B.M.; Bari, A.; Villinger, A. Synthesis and evaluation of new coumarin derivatives as antioxidant, antimicrobial, and anti-inflammatory agents. Molecules 2020, 25, 3251. [Google Scholar] [CrossRef] [PubMed]
- Tsivileva, O.M.; Koftin, O.V.; Evseeva, N.V. Coumarins as fungal metabolites with potential medicinal properties. Antibiotics 2022, 11, 1156. [Google Scholar] [CrossRef] [PubMed]
- Giorni, P.; Rastelli, S.; Fregonara, S.; Bertuzzi, T. Monitoring phenolic compounds in rice during the growing season in relation to fungal and mycotoxin contamination. Toxins 2020, 12, 341. [Google Scholar] [CrossRef]
- Kumar, M.; Tak, Y.; Potkule, J.; Choyal, P.; Tomar, M.; Meena, N.L.; Kaur, C. Phenolics as plant protective companion against abiotic stress. In Plant Phenolics in Sustainable Agriculture; Springer: Singapore, 2020; Volume 1, pp. 277–308. [Google Scholar]
- Madany, M.M.; Saleh, A.M.; Habeeb, T.H.; Hozzein, W.N.; AbdElgawad, H. Silicon dioxide nanoparticles alleviate the threats of broomrape infection in tomato by inducing cell wall fortification and modulating ROS homeostasis. Environ. Sci. Nano 2020, 7, 1415–1430. [Google Scholar] [CrossRef]
- Ali, S.; Khan, N.; Tang, Y. Epigenetic marks for mitigating abiotic stresses in plants. J. Plant Physiol. 2022, 275, 153740. [Google Scholar] [CrossRef]
- Zhang, H.; Song, Y.; Fan, Z.; Ruan, J.; Hu, J.; Zhang, Q. Aluminum Supplementation Mediates the Changes in Tea Plant Growth and Metabolism in Response to Calcium Stress. Int. J. Mol. Sci. 2023, 25, 530. [Google Scholar] [CrossRef]
- Saqib, M.; Shahzad, U.; Zulfiqar, F.; Tiwari, R.K.; Lal, M.K.; Naz, S.; Jahan, M.S.; Awan, Z.A.; El-Sheikh, M.A.; Altaf, M.A. Exogenous melatonin alleviates cadmium-induced inhibition of growth and photosynthesis through upregulating antioxidant defense system in strawberry. S. Afr. J. Bot. 2023, 157, 10–18. [Google Scholar] [CrossRef]
- Rizaludin, M.S.; Stopnisek, N.; Raaijmakers, J.M.; Garbeva, P. The chemistry of stress: Understanding the ‘cry for help’of plant roots. Metabolites 2021, 11, 357. [Google Scholar] [CrossRef]
- Gashu, K.; Verma, P.K.; Acuña, T.; Agam, N.; Bustan, A.; Fait, A. Temperature differences between sites lead to altered phenylpropanoid metabolism in a varietal dependent manner. Front. Plant Sci. 2023, 14, 1239852. [Google Scholar] [CrossRef] [PubMed]
- Qaderi, M.M.; Martel, A.B.; Strugnell, C.A. Environmental factors regulate plant secondary metabolites. Plants 2023, 12, 447. [Google Scholar] [CrossRef] [PubMed]
- Butnariu, M. Plant genome engineering for improved flavonoids production. In Plants as Bioreactors for Industrial Molecules; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2023; pp. 215–240. [Google Scholar]
- Qiu, M.; Jiang, J.; Jiang, W.; Zhang, W.; Jiang, Y.; Xin, F.; Jiang, M. The biosynthesis of L-phenylalanine-derived compounds by engineered microbes. Biotechnol. Adv. 2024, 77, 108448. [Google Scholar] [CrossRef] [PubMed]
- Wei, X.; Xie, B.; Wan, C.; Song, R.; Zhong, W.; Xin, S.; Song, K. Enhancing soil health and plant growth through microbial fertilizers: Mechanisms, benefits, and sustainable agricultural practices. Agronomy 2024, 14, 609. [Google Scholar] [CrossRef]
- Mansoor, S.; Ali, A.; Kour, N.; Bornhorst, J.; AlHarbi, K.; Rinklebe, J.; Abd El Moneim, D.; Ahmad, P.; Chung, Y.S. Heavy metal induced oxidative stress mitigation and ROS scavenging in plants. Plants 2023, 12, 3003. [Google Scholar] [CrossRef]
- Kuljarusnont, S.; Iwakami, S.; Iwashina, T.; Tungmunnithum, D. Flavonoids and Other Phenolic Compounds for Physiological Roles, Plant Species Delimitation, and Medical Benefits: A Promising View. Molecules 2024, 29, 5351. [Google Scholar] [CrossRef]
- Kumar, G.A.; Kumar, S.; Bhardwaj, R.; Swapnil, P.; Meena, M.; Seth, C.S.; Yadav, A. Recent advancements in multifaceted roles of flavonoids in plant–rhizomicrobiome interactions. Front. Plant Sci. 2024, 14, 1297706. [Google Scholar] [CrossRef]
- Chen, M.; Li, Y.; Liu, H.; Zhang, D.; Shi, Q.-S.; Zhong, X.-Q.; Guo, Y.; Xie, X.-B. High value valorization of lignin as environmental benign antimicrobial. Mater. Today Bio 2023, 18, 100520. [Google Scholar] [CrossRef]
- Singh, H.; Singh, R.; Singh, A.; Singh, H.; Singh, G.; Kaur, S.; Singh, B. Role of oxidative stress in diabetes-induced complications and their management with antioxidants. Arch. Physiol. Biochem. 2024, 130, 616–641. [Google Scholar] [CrossRef]
- Shoaib, N.; Pan, K.; Mughal, N.; Raza, A.; Liu, L.; Zhang, J.; Wu, X.; Sun, X.; Zhang, L.; Pan, Z. Potential of UV-B radiation in drought stress resilience: A multidimensional approach to plant adaptation and future implications. Plant Cell Environ. 2024, 47, 387–407. [Google Scholar] [CrossRef]
- Zhou, C.; Mughal, N.; Zhang, X.; Chen, J.; Shoaib, N.; Wang, X.; Yong, T.; Yang, F.; Liu, W.; Wu, X. Soybean plants enhance growth through metabolic regulation under heterogeneous drought stress. Agric. Water Manag. 2024, 303, 109029. [Google Scholar] [CrossRef]
- Singh, P.; Singh, A.; Choudhary, K.K. Revisiting the role of phenylpropanoids in plant defense against UV-B stress. Plant Stress 2023, 7, 100143. [Google Scholar] [CrossRef]
- Mahdavian, K. Effects of Ultraviolet Radiation on Plants and Their Protective Mechanisms. Russ. J. Plant Physiol. 2024, 71, 184. [Google Scholar] [CrossRef]
- Singh, S.; Kaur, I.; Kariyat, R. The multifunctional roles of polyphenols in plant-herbivore interactions. Int. J. Mol. Sci. 2021, 22, 1442. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Guan, Q.; Zhang, H.; Tang, L. Effect of metal ions on the interaction of condensed tannins with protein. Foods 2023, 12, 829. [Google Scholar] [CrossRef]
- Naz, M.; Zhang, D.; Liao, K.; Chen, X.; Ahmed, N.; Wang, D.; Zhou, J.; Chen, Z. The past, present, and future of plant activators targeting the salicylic acid signaling pathway. Genes 2024, 15, 1237. [Google Scholar] [CrossRef]
- Iqbal, N.; Poór, P. Plant Protection by Tannins Depends on Defence-Related Phytohormones. J. Plant Growth Regul. 2024, 44, 22–39. [Google Scholar] [CrossRef]
- Zhang, L.; Guan, Q.; Jiang, J.; Khan, M.S. Tannin complexation with metal ions and its implication on human health, environment and industry: An overview. Int. J. Biol. Macromol. 2023, 253, 127485. [Google Scholar] [CrossRef]
- Plamada, D.; Vodnar, D.C. Polyphenols—Gut microbiota interrelationship: A transition to a new generation of prebiotics. Nutrients 2021, 14, 137. [Google Scholar] [CrossRef]
- Ruan, T.; Li, P.; Wang, H.; Li, T.; Jiang, G. Identification and prioritization of environmental organic pollutants: From an analytical and toxicological perspective. Chem. Rev. 2023, 123, 10584–10640. [Google Scholar] [CrossRef]
- Gill, S.S.; Tuteja, N. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol. Biochem. 2010, 48, 909–930. [Google Scholar] [CrossRef]
- Jha, Y.; Mohamed, H.I. Plant secondary metabolites as a tool to investigate biotic stress tolerance in plants: A review. Gesunde Pflanz. 2022, 74, 771–790. [Google Scholar] [CrossRef]
- Sharma, A.; Shahzad, B.; Rehman, A.; Bhardwaj, R.; Landi, M.; Zheng, B. Response of phenylpropanoid pathway and the role of polyphenols in plants under abiotic stress. Molecules 2019, 24, 2452. [Google Scholar] [CrossRef]
- Riaz, M.W.; Yousaf, M.I.; Hussain, Q.; Yasir, M.; Sajjad, M.; Shah, L. Role of lignin in wheat plant for the enhancement of resistance against lodging and biotic and abiotic stresses. Stresses 2023, 3, 434–453. [Google Scholar] [CrossRef]
- Feduraev, P.; Skrypnik, L.; Riabova, A.; Pungin, A.; Tokupova, E.; Maslennikov, P.; Chupakhina, G. Phenylalanine and tyrosine as exogenous precursors of wheat (Triticum aestivum L.) secondary metabolism through PAL-associated pathways. Plants 2020, 9, 476. [Google Scholar] [CrossRef] [PubMed]
- Barceló, A.R.; Gómez Ros, L.; Gabaldón, C.; López-Serrano, M.; Pomar, F.; Carrión, J.; Pedreño, M. Basic peroxidases: The gateway for lignin evolution? Phytochem. Rev. 2004, 3, 61–78. [Google Scholar] [CrossRef]
- Riseh, R.S.; Fathi, F.; Lagzian, A.; Vatankhah, M.; Kennedy, J.F. Modifying lignin: A promising strategy for plant disease control. Int. J. Biol. Macromol. 2024, 271, 132696. [Google Scholar]
- Wang, L.; Ning, C.; Pan, T.; Cai, K. Role of silica nanoparticles in abiotic and biotic stress tolerance in plants: A review. Int. J. Mol. Sci. 2022, 23, 1947. [Google Scholar] [CrossRef]
- Kumar, P.; Singh, B.; Rajak, S.; Pandey, S.; Pati, P. Dynamics of reactive oxygen species and lignin biosynthesis during leaf spot disease of Withania somnifera (L.) Dunal. Plant Biol. 2023, 25, 757–770. [Google Scholar] [CrossRef]
- Ghosh, S.K.; Ishangulyyeva, G.; Erbilgin, N.; Bonello, P. Terpenoids are involved in the expression of systemic-induced resistance in Austrian pine. Plant Cell Environ. 2024, 47, 2206–2227. [Google Scholar] [CrossRef]
- Jędrzejczak, P.; Collins, M.N.; Jesionowski, T.; Klapiszewski, Ł. The role of lignin and lignin-based materials in sustainable construction—A comprehensive review. Int. J. Biol. Macromol. 2021, 187, 624–650. [Google Scholar] [CrossRef] [PubMed]
- Demis, E. Mechanism of Plant Resistance to Insects, Weeds and Pathogens. Middle East Res. J. Agric. Food Sci. 2024, 4, 76–85. [Google Scholar] [CrossRef]
- Jan, R.; Asaf, S.; Numan, M.; Lubna; Kim, K.-M. Plant secondary metabolite biosynthesis and transcriptional regulation in response to biotic and abiotic stress conditions. Agronomy 2021, 11, 968. [Google Scholar] [CrossRef]
- Kunova, A.; Pinna, C.; Ghosh, S.; Dozio, D.; Pizzatti, C.; Princiotto, S.; Cortesi, P.; Dallavalle, S.; Pinto, A. Stilbenoids as Antifungals to Counteract Rice Blast Pathogen Pyricularia oryzae. ACS Agric. Sci. Technol. 2023, 4, 43–50. [Google Scholar] [CrossRef]
- Lin, F.; Chen, J.; Wang, X.; Ma, H.; Liang, S.; Hu, H.; Fan, H.; Wu, Z.; Chai, T.; Wang, H. Combined analysis of Polygonum cuspidatum transcriptome and metabolome revealed that PcMYB62, a transcription factor, responds to methyl jasmonate and inhibits resveratrol biosynthesis. Int. J. Biol. Macromol. 2024, 270, 132450. [Google Scholar] [CrossRef] [PubMed]
- Gao, Q.; Zheng, R.; Lu, J.; Li, X.; Wang, D.; Cai, X.; Ren, X.; Kong, Q. Trends in the Potential of Stilbenes to Improve Plant Stress Tolerance: Insights of Plant Defense Mechanisms in Response to Biotic and Abiotic Stressors. J. Agric. Food Chem. 2024, 72, 7655–7671. [Google Scholar] [CrossRef] [PubMed]
- Uka, V.; Cary, J.W.; Lebar, M.D.; Puel, O.; De Saeger, S.; Diana Di Mavungu, J. Chemical repertoire and biosynthetic machinery of the Aspergillus flavus secondary metabolome: A review. Compr. Rev. Food Sci. Food Saf. 2020, 19, 2797–2842. [Google Scholar] [CrossRef]
- Vicidomini, C.; Palumbo, R.; Moccia, M.; Roviello, G.N. Oxidative Processes and Xenobiotic Metabolism in Plants: Mechanisms of Defense and Potential Therapeutic Implications. J. Xenobiot. 2024, 14, 1541–1569. [Google Scholar] [CrossRef]
- Qin, T.; Chen, X.; Meng, J.; Guo, Q.; Xu, S.; Hou, S.; Yuan, Z.; Zhang, W. The role of curcumin in the liver-gut system diseases: From mechanisms to clinical therapeutic perspective. Crit. Rev. Food Sci. Nutr. 2024, 64, 8822–8851. [Google Scholar] [CrossRef]
- Nicoliche, T.; Bartolomeo, C.S.; Lemes, R.M.R.; Pereira, G.C.; Nunes, T.A.; Oliveira, R.B.; Nicastro, A.L.M.; Soares, É.N.; da Cunha Lima, B.F.; Rodrigues, B.M. Antiviral, anti-inflammatory and antioxidant effects of curcumin and curcuminoids in SH-SY5Y cells infected by SARS-CoV-2. Sci. Rep. 2024, 14, 10696. [Google Scholar] [CrossRef]
- Plaza, V.; Silva-Moreno, E.; Castillo, L. Breakpoint: Cell wall and glycoproteins and their crucial role in the phytopathogenic fungi infection. Curr. Protein Pept. Sci. 2020, 21, 227–244. [Google Scholar] [CrossRef] [PubMed]
- Chouhan, R.; Ahmed, S.; Gandhi, S.G. Over-expression of PR proteins with chitinase activity in transgenic plants for alleviation of fungal pathogenesis. J. Plant Pathol. 2023, 105, 69–81. [Google Scholar] [CrossRef]
- Vaghela, B.; Vashi, R.; Rajput, K.; Joshi, R. Plant chitinases and their role in plant defense: A comprehensive review. Enzym. Microb. Technol. 2022, 159, 110055. [Google Scholar] [CrossRef]
- Singh, H.R.; Deka, M.; Das, S. Enhanced resistance to blister blight in transgenic tea (Camellia sinensis [L.] O. Kuntze) by overexpression of class I chitinase gene from potato (Solanum tuberosum). Funct. Integr. Genom. 2015, 15, 461–480. [Google Scholar] [CrossRef] [PubMed]
- Wu, G. Amino acids: Metabolism, functions, and nutrition. Amino Acids 2009, 37, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Basagni, F.; Marotta, G.; Rosini, M.; Minarini, A. Polyamine–Drug Conjugates: Do They Boost Drug Activity? Molecules 2023, 28, 4518. [Google Scholar] [CrossRef]
- Künstler, A.; Gullner, G.; Ádám, A.L.; Kolozsváriné Nagy, J.; Király, L. The versatile roles of sulfur-containing biomolecules in plant defense—A road to disease resistance. Plants 2020, 9, 1705. [Google Scholar] [CrossRef]
- Gogoi, K.; Gogoi, H.; Borgohain, M.; Saikia, R.; Chikkaputtaiah, C.; Hiremath, S.; Basu, U. The molecular dynamics between reactive oxygen species (ROS), reactive nitrogen species (RNS) and phytohormones in plant’s response to biotic stress. Plant Cell Rep. 2024, 43, 263. [Google Scholar] [CrossRef]
- Lv, Q.; Li, X.; Fan, B.; Zhu, C.; Chen, Z. The cellular and subcellular organization of the glucosinolate–myrosinase system against herbivores and pathogens. Int. J. Mol. Sci. 2022, 23, 1577. [Google Scholar] [CrossRef]
- Nabaei, M.; Amooaghaie, R.; Ghorbanpour, M.; Ahadi, A. Crosstalk between melatonin and nitric oxide restrains Cadmium-induced oxidative stress and enhances vinblastine biosynthesis in Catharanthus roseus (L) G Don. Plant Cell Rep. 2024, 43, 139. [Google Scholar] [CrossRef]
- Chhajed, S.; Mostafa, I.; He, Y.; Abou-Hashem, M.; El-Domiaty, M.; Chen, S. Glucosinolate biosynthesis and the glucosinolate–myrosinase system in plant defense. Agronomy 2020, 10, 1786. [Google Scholar] [CrossRef]
- Chripkova, M.; Zigo, F.; Mojzis, J. Antiproliferative effect of indole phytoalexins. Molecules 2016, 21, 1626. [Google Scholar] [CrossRef] [PubMed]
- Mathur, V.; Ulanova, D. Microbial metabolites beneficial to plant hosts across ecosystems. Microb. Ecol. 2023, 86, 25–48. [Google Scholar] [CrossRef]
- Saini, N.; Anmol, A.; Kumar, S.; Bakshi, M.; Dhiman, Z. Exploring phenolic compounds as natural stress alleviators in plants—A comprehensive review. Physiol. Mol. Plant Pathol. 2024, 133, 102383. [Google Scholar] [CrossRef]
- Shah, A.; Smith, D.L. Flavonoids in agriculture: Chemistry and roles in, biotic and abiotic stress responses, and microbial associations. Agronomy 2020, 10, 1209. [Google Scholar] [CrossRef]
- Pandey, P.; Tripathi, A.; Dwivedi, S.; Lal, K.; Jhang, T. Deciphering the mechanisms, hormonal signaling, and potential applications of endophytic microbes to mediate stress tolerance in medicinal plants. Front. Plant Sci. 2023, 14, 1250020. [Google Scholar] [CrossRef] [PubMed]
- Plaszkó, T.; Szűcs, Z.; Vasas, G.; Gonda, S. Interactions of fungi with non-isothiocyanate products of the plant glucosinolate pathway: A review on product formation, antifungal activity, mode of action and biotransformation. Phytochemistry 2022, 200, 113245. [Google Scholar] [CrossRef]
- Amani Machiani, M.; Javanmard, A.; Habibi Machiani, R.; Sadeghpour, A. Arbuscular mycorrhizal fungi and changes in primary and secondary metabolites. Plants 2022, 11, 2183. [Google Scholar] [CrossRef]
- Rani, A.; Guleria, M.; Sharma, Y.; Sharma, S.; Chaudhary, A.; Sharma, R.; Kumar, P. Insights into elicitor’s role in augmenting secondary metabolites production and climate resilience in genus Ocimum—A globally important medicinal and aromatic crop. Ind. Crops Prod. 2023, 202, 117078. [Google Scholar] [CrossRef]
- Shumilina, J.; Soboleva, A.; Abakumov, E.; Shtark, O.Y.; Zhukov, V.A.; Frolov, A. Signaling in Legume–Rhizobia Symbiosis. Int. J. Mol. Sci. 2023, 24, 17397. [Google Scholar] [CrossRef]
- Raza, A.; Hassan, A.; Akram, W.; Anjum, T.; Ali, B. Seed coating with the synthetic consortium of beneficial Bacillus microbes improves seedling growth and manages Fusarium wilt disease. Sci. Hortic. 2024, 325, 112645. [Google Scholar] [CrossRef]
- Maserumule, M.; Rauwane, M.; Madala, N.E.; Ncube, E.; Figlan, S. Defence-related metabolic changes in wheat (Triticum aestivum L.) seedlings in response to infection by Puccinia graminis f. sp. tritici. Front. Plant Sci. 2023, 14, 1166813. [Google Scholar] [CrossRef]
- Kiani, R.; Arzani, A.; Mirmohammady Maibody, S. Polyphenols, flavonoids, and antioxidant activity involved in salt tolerance in wheat, Aegilops cylindrica and their amphidiploids. Front. Plant Sci. 2021, 12, 646221. [Google Scholar] [CrossRef] [PubMed]
- Ji, W.; Osbourn, A.; Liu, Z. Understanding metabolic diversification in plants: Branchpoints in the evolution of specialized metabolism. Philos. Trans. B 2024, 379, 20230359. [Google Scholar] [CrossRef]
- Li, S.; Khoso, M.A.; Xu, H.; Zhang, C.; Liu, Z.; Wagan, S.; Dinislam, K.; Liu, L. WRKY Transcription Factors (TFs) as Key Regulators of Plant Resilience to Environmental Stresses: Current Perspective. Agronomy 2024, 14, 2421. [Google Scholar] [CrossRef]
- Abdulraheem, M.I.; Xiong, Y.; Moshood, A.Y.; Cadenas-Pliego, G.; Zhang, H.; Hu, J. Mechanisms of plant epigenetic regulation in response to plant stress: Recent discoveries and implications. Plants 2024, 13, 163. [Google Scholar] [CrossRef] [PubMed]
- Cravens, A.; Payne, J.; Smolke, C.D. Synthetic biology strategies for microbial biosynthesis of plant natural products. Nat. Commun. 2019, 10, 2142. [Google Scholar] [CrossRef]
- Scherlach, K.; Hertweck, C. Mining and unearthing hidden biosynthetic potential. Nat. Commun. 2021, 12, 3864. [Google Scholar] [CrossRef]
- Li, W.; Zou, G.; Bao, D.; Wu, Y. Current Advances in the Functional Genes of Edible and Medicinal Fungi: Research Techniques, Functional Analysis, and Prospects. J. Fungi 2024, 10, 311. [Google Scholar] [CrossRef]
- Wang, M.; Chen, L.; Zhang, Z.; Wang, Q. Recent advances in genome mining and synthetic biology for discovery and biosynthesis of natural products. Crit. Rev. Biotechnol. 2025, 45, 236–256. [Google Scholar] [CrossRef]
- Bhuyan, S.J.; Kumar, M.; Ramrao Devde, P.; Rai, A.C.; Mishra, A.K.; Singh, P.K.; Siddique, K.H. Progress in gene editing tools, implications and success in plants: A review. Front. Genome Ed. 2023, 5, 1272678. [Google Scholar] [CrossRef]
- Rabeh, K.; Hnini, M.; Oubohssaine, M. A comprehensive review of transcription factor-mediated regulation of secondary metabolites in plants under environmental stress. Stress Biol. 2025, 5, 15. [Google Scholar] [CrossRef]
- Liu, C.; Wen, L.; Cui, Y.; Ahammed, G.J.; Cheng, Y. Metal transport proteins and transcription factor networks in plant responses to cadmium stress. Plant Cell Rep. 2024, 43, 218. [Google Scholar] [CrossRef] [PubMed]
- Alfieri, M.; Vaccaro, M.C.; Cappetta, E.; Ambrosone, A.; De Tommasi, N.; Leone, A. Coactivation of MEP-biosynthetic genes and accumulation of abietane diterpenes in Salvia sclarea by heterologous expression of WRKY and MYC2 transcription factors. Sci. Rep. 2018, 8, 11009. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Singh, S.K.; Patra, B.; Liu, Y.; Pattanaik, S.; Yuan, L. Mitogen-activated protein kinase-mediated regulation of plant specialized metabolism. J. Exp. Bot. 2025, 76, 262–276. [Google Scholar] [CrossRef]
- Chen, C.; Liu, F.; Zhang, K.; Niu, X.; Zhao, H.; Liu, Q.; Georgiev, M.I.; Xu, X.; Zhang, X.; Zhou, M. MeJA-responsive bHLH transcription factor LjbHLH7 regulates cyanogenic glucoside biosynthesis in Lotus japonicus. J. Exp. Bot. 2022, 73, 2650–2665. [Google Scholar] [CrossRef]
- Cavalieri, V. The expanding constellation of histone post-translational modifications in the epigenetic landscape. Genes 2021, 12, 1596. [Google Scholar] [CrossRef]
- Yadav, V.; Wang, Z.; Wei, C.; Amo, A.; Ahmed, B.; Yang, X.; Zhang, X. Phenylpropanoid pathway engineering: An emerging approach towards plant defense. Pathogens 2020, 9, 312. [Google Scholar] [CrossRef]
- Pérez-Llorca, M.; Pollmann, S.; Müller, M. Ethylene and jasmonates signaling network mediating secondary metabolites under abiotic stress. Int. J. Mol. Sci. 2023, 24, 5990. [Google Scholar] [CrossRef]
- Javed, T.; Shabbir, R.; Ali, A.; Afzal, I.; Zaheer, U.; Gao, S.-J. Transcription factors in plant stress responses: Challenges and potential for sugarcane improvement. Plants 2020, 9, 491. [Google Scholar] [CrossRef]
- Patra, P.; Disha, B.; Kundu, P.; Das, M.; Ghosh, A. Recent advances in machine learning applications in metabolic engineering. Biotechnol. Adv. 2023, 62, 108069. [Google Scholar] [CrossRef]
- Liu, H.; Li, J.; Singh, B.K. Harnessing co-evolutionary interactions between plants and Streptomyces to combat drought stress. Nat. Plants 2024, 10, 1159–1171. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Xu, J.; Li, R.; Ge, Y.; Li, Y.; Li, R. Plants’ response to abiotic stress: Mechanisms and strategies. Int. J. Mol. Sci. 2023, 24, 10915. [Google Scholar] [CrossRef] [PubMed]
- Anas, M.; Khalid, A.; Saleem, M.H.; Ali Khan, K.; Ahmed Khattak, W.; Fahad, S. Symbiotic Synergy: Unveiling Plant-Microbe Interactions in Stress Adaptation. J. Crop Health 2025, 77, 18. [Google Scholar] [CrossRef]
- Yeshi, K.; Crayn, D.; Ritmejerytė, E.; Wangchuk, P. Plant secondary metabolites produced in response to abiotic stresses has potential application in pharmaceutical product development. Molecules 2022, 27, 313. [Google Scholar] [CrossRef]
- Li, T.; Zhang, S.; Li, Y.; Zhang, L.; Song, W.; Chen, C. Overexpression of AtMYB2 Promotes Tolerance to Salt Stress and Accumulations of Tanshinones and Phenolic Acid in Salvia miltiorrhiza. Int. J. Mol. Sci. 2024, 25, 4111. [Google Scholar] [CrossRef]
- Singh, S.; Ramakrishna, W. Application of CRISPR–Cas9 in plant–plant growth-promoting rhizobacteria interactions for next Green Revolution. 3 Biotech 2021, 11, 492. [Google Scholar] [CrossRef]
- Mashabela, M.D.; Masamba, P.; Kappo, A.P. Applications of metabolomics for the elucidation of abiotic stress tolerance in plants: A special focus on osmotic stress and heavy metal toxicity. Plants 2023, 12, 269. [Google Scholar] [CrossRef]
- Ali, S.; Akhtar, M.S.; Siraj, M.; Zaman, W. Molecular Communication of Microbial Plant Biostimulants in the Rhizosphere Under Abiotic Stress Conditions. Int. J. Mol. Sci. 2024, 25, 12424. [Google Scholar] [CrossRef]
- Lu, J.; Chen, H.; Yang, Z.; Sun, S.; Luo, Q.; Xie, J.; Tan, J. Physiological and molecular mechanisms of the response of roots of Pinus massoniana Lamb. to low-temperature stress. Front. Plant Sci. 2022, 13, 954324. [Google Scholar] [CrossRef]
- Roychowdhury, R.; Das, S.P.; Gupta, A.; Parihar, P.; Chandrasekhar, K.; Sarker, U.; Kumar, A.; Ramrao, D.P.; Sudhakar, C. Multi-omics pipeline and omics-integration approach to decipher plant’s abiotic stress tolerance responses. Genes 2023, 14, 1281. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Bai, X.; Joong Oh, E. Strategic approaches for designing yeast strains as protein secretion and display platforms. Crit. Rev. Biotechnol. 2024, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Bhojiya, A.A.; Joshi, H. Crispr Gene Editing for Secondary Metabolite Production: A Review. In Gene Editing in Plants: CRISPR-Cas and Its Applications; Springer: Singapore, 2024; pp. 437–475. [Google Scholar]
- Liu, Y.; Xue, B.; Liu, H.; Wang, S.; Su, H. Rational construction of synthetic consortia: Key considerations and model-based methods for guiding the development of a novel biosynthesis platform. Biotechnol. Adv. 2024, 72, 108348. [Google Scholar] [CrossRef]
- Fan, B.-L.; Chen, L.-H.; Chen, L.-L.; Guo, H. Integrative Multi-Omics Approaches for Identifying and Characterizing Biological Elements in Crop Traits: Current Progress and Future Prospects. Int. J. Mol. Sci. 2025, 26, 1466. [Google Scholar] [CrossRef] [PubMed]
- Reisz, J.A.; Dzieciatkowska, M.; Stephenson, D.; Gamboni, F.; Morton, D.H.; D’Alessandro, A. Red Blood Cells from Individuals with Lesch–Nyhan Syndrome: Multi-Omics Insights into a Novel S162N Mutation Causing Hypoxanthine-Guanine Phosphoribosyltransferase Deficiency. Antioxidants 2023, 12, 1699. [Google Scholar] [CrossRef]
- Nikolaou, P.; Marciniak, P.; Adamski, Z.; Ntalli, N. Controlling stored products’ pests with plant secondary metabolites: A review. Agriculture 2021, 11, 879. [Google Scholar] [CrossRef]
- Sokan-Adeaga, A.A.; Sokan-Adeaga, M.A.; Sokan-Adeaga, E.D.; Oparaji, A.N.; Edris, H.; Tella, E.O.; Balogun, F.A.; Aledeh, M.; Amubieya, O.E. Environmental toxicants and health adversities: A review on interventions of phytochemicals. J. Public Health Res. 2023, 12, 22799036231181226. [Google Scholar] [CrossRef]
- Chen, X.; Dai, X.; Liu, Y.; Yang, Y.; Yuan, L.; He, X.; Gong, G. Solanum nigrum Linn.: An insight into current research on traditional uses, phytochemistry, and pharmacology. Front. Pharmacol. 2022, 13, 918071. [Google Scholar] [CrossRef]
- Qasim, M.; Islam, W.; Rizwan, M.; Hussain, D.; Noman, A.; Khan, K.A.; Ghramh, H.A.; Han, X. Impact of plant monoterpenes on insect pest management and insect-associated microbes. Heliyon 2024, 10, e39120. [Google Scholar] [CrossRef]
- Jurčević Šangut, I.; Pavličević, L.; Šamec, D. Influence of Air Drying, Freeze Drying and Oven Drying on the Biflavone Content in Yellow Ginkgo (Ginkgo biloba L.) Leaves. Appl. Sci. 2024, 14, 2330. [Google Scholar] [CrossRef]
- Ryu, S.; Han, J.H.; Cho, J.G.; Jeong, J.H.; Lee, S.K.; Lee, H.J. High temperature at veraison inhibits anthocyanin biosynthesis in berry skins during ripening in ‘Kyoho’grapevines. Plant Physiol. Biochem. 2020, 157, 219–228. [Google Scholar] [CrossRef]
- Khalid, K.A.; El-Gohary, A.E.; Ahmed, A.M. Raising the efficiency of lemon trees to produce essential oil by exogenous cysteine under various soil structures. J. Essent. Oil Bear. Plants 2020, 23, 194–203. [Google Scholar] [CrossRef]
- Mukherjee, S.; Kutty, N.N.; Bera, P.; Mitra, A. Impact of light and sucrose supplementation on cellular differentiation, metabolic shift and modulation of gene expression in hairy roots of Daucus carota. Plant Cell Tissue Organ Cult. (PCTOC) 2019, 136, 383–397. [Google Scholar] [CrossRef]
- Fayezizadeh, M.R.; Ansari, N.A.; Sourestani, M.M.; Hasanuzzaman, M. Variations in photoperiods and their impact on yield, photosynthesis and secondary metabolite production in basil microgreens. BMC Plant Biol. 2024, 24, 712. [Google Scholar] [CrossRef]
- Peng, M.; Shahzad, R.; Gul, A.; Subthain, H.; Shen, S.; Lei, L.; Zheng, Z.; Zhou, J.; Lu, D.; Wang, S. Differentially evolved glucosyltransferases determine natural variation of rice flavone accumulation and UV-tolerance. Nat. Commun. 2017, 8, 1975. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Waheed, A.; Aili, A.; Xu, H.; Kuerban, A.; Muhammad, M.; Ali, S. Copper sulfate-induced stress in Spinach: Metabolic pathway disruption and plant response. Sci. Hortic. 2024, 337, 113575. [Google Scholar] [CrossRef]
- Sun, R.; Gols, R.; Harvey, J.A.; Reichelt, M.; Gershenzon, J.; Pandit, S.S.; Vassão, D.G. Detoxification of plant defensive glucosinolates by an herbivorous caterpillar is beneficial to its endoparasitic wasp. Mol. Ecol. 2020, 29, 4014–4031. [Google Scholar] [CrossRef] [PubMed]
- Jeandet, P.; Trotel-Aziz, P.; Jacquard, C.; Clément, C.; Mohan, C.; Morkunas, I.; Khan, H.; Aziz, A. Use of Elicitors and Beneficial Bacteria to Induce and Prime the Stilbene Phytoalexin Response: Applications to Grapevine Disease Resistance. Agronomy 2023, 13, 2225. [Google Scholar] [CrossRef]
- Roy-Barman, S.; Raut, R.A.; Sarkar, A.; Sabnam, N.; Chakraborty, S.; Saha, P. Recent advances in the development of transgenic crop plants, biosafety aspects, and future perspectives. In Plant Biotechnology; Apple Academic Press: Palm Bay, FL, USA, 2017; Volume 2, pp. 271–411. [Google Scholar]
- Valletta, A.; Iozia, L.; Leonelli, F. Impact of environmental factors on stilbene biosynthesis. Plants 2021, 10, 90. [Google Scholar] [CrossRef]
- Fuloria, S.; Mehta, J.; Chandel, A.; Sekar, M.; Rani, N.N.I.M.; Begum, M.Y.; Subramaniyan, V.; Chidambaram, K.; Thangavelu, L.; Nordin, R. A comprehensive review on the therapeutic potential of Curcuma longa Linn. in relation to its major active constituent curcumin. Front. Pharmacol. 2022, 13, 820806. [Google Scholar] [CrossRef]
- Jyotirmayee, B.; Mahalik, G. A review on selected pharmacological activities of Curcuma longa L. Int. J. Food Prop. 2022, 25, 1377–1398. [Google Scholar] [CrossRef]
- Kępińska-Pacelik, J.; Biel, W. Turmeric and Curcumin—Health-Promoting Properties in Humans versus Dogs. Int. J. Mol. Sci. 2023, 24, 14561. [Google Scholar] [CrossRef]
- El-Sayed, G.M.; Emam, M.T.; Hammad, M.A.; Mahmoud, S.H. Gene Cloning, Heterologous Expression, and In Silico Analysis of Chitinase B from Serratia marcescens for Biocontrol of Spodoptera frugiperda Larvae Infesting Maize Crops. Molecules 2024, 29, 1466. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.; Brar, A.; Yadav, M.; Chawade, A.; Vivekanand, V.; Pareek, N. Chitinases—Potential candidates for enhanced plant resistance towards fungal pathogens. Agriculture 2018, 8, 88. [Google Scholar] [CrossRef]
- Manghwar, H.; Hussain, A. Mechanism of tobacco osmotin gene in plant responses to biotic and abiotic stress tolerance: A brief history. Biocell 2022, 46, 623. [Google Scholar] [CrossRef]
- Gullner, G.; Komives, T.; Király, L.; Schröder, P. Glutathione S-transferase enzymes in plant-pathogen interactions. Front. Plant Sci. 2018, 9, 1836. [Google Scholar] [CrossRef]
- Gleń-Karolczyk, K.; Boligłowa, E.; Filipiak-Florkiewicz, A.; Florkiewicz, A.; Luty, L. The Effect of Biopreparations and Biostimulants on the Chemical Composition and Microorganisms Associated with Verticillium Wilt of Horseradish Roots (Armoracia rusticana Gaertn.). Appl. Sci. 2021, 11, 680. [Google Scholar] [CrossRef]
- Kaya, A.; Mariotti, M.; Gladyshev, V.N. Cytochrome c peroxidase facilitates the beneficial use of H2O2 in prokaryotes. Proc. Natl. Acad. Sci. USA 2017, 114, 8678–8680. [Google Scholar] [CrossRef]
- Szechyńska-Hebda, M.; Ghalami, R.Z.; Kamran, M.; Van Breusegem, F.; Karpiński, S. To be or not to be? Are reactive oxygen species, antioxidants, and stress signalling universal determinants of life or death? Cells 2022, 11, 4105. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khan, A.; Kanwal, F.; Ullah, S.; Fahad, M.; Tariq, L.; Altaf, M.T.; Riaz, A.; Zhang, G. Plant Secondary Metabolites—Central Regulators Against Abiotic and Biotic Stresses. Metabolites 2025, 15, 276. https://doi.org/10.3390/metabo15040276
Khan A, Kanwal F, Ullah S, Fahad M, Tariq L, Altaf MT, Riaz A, Zhang G. Plant Secondary Metabolites—Central Regulators Against Abiotic and Biotic Stresses. Metabolites. 2025; 15(4):276. https://doi.org/10.3390/metabo15040276
Chicago/Turabian StyleKhan, Ameer, Farah Kanwal, Sana Ullah, Muhammad Fahad, Leeza Tariq, Muhammad Tanveer Altaf, Asad Riaz, and Guoping Zhang. 2025. "Plant Secondary Metabolites—Central Regulators Against Abiotic and Biotic Stresses" Metabolites 15, no. 4: 276. https://doi.org/10.3390/metabo15040276
APA StyleKhan, A., Kanwal, F., Ullah, S., Fahad, M., Tariq, L., Altaf, M. T., Riaz, A., & Zhang, G. (2025). Plant Secondary Metabolites—Central Regulators Against Abiotic and Biotic Stresses. Metabolites, 15(4), 276. https://doi.org/10.3390/metabo15040276








