Effects of a 6-Week Concurrent Training Program Combining Resistance and Various Modalities of Aerobic Exercise in Obese Women with Prehypertension: A Randomized Controlled Trial
Abstract
:1. Introduction
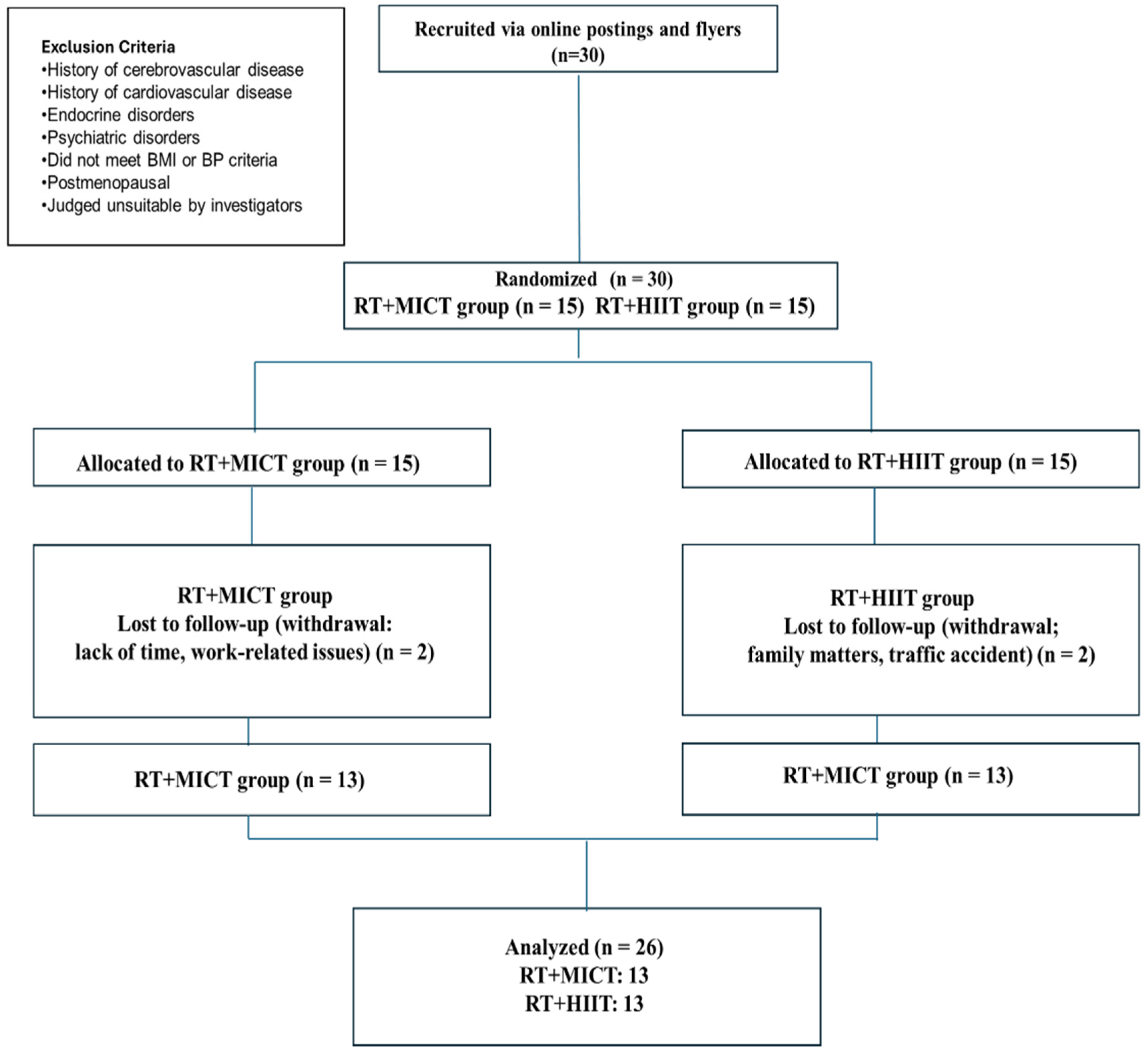
2. Materials and Methods
2.1. Participants
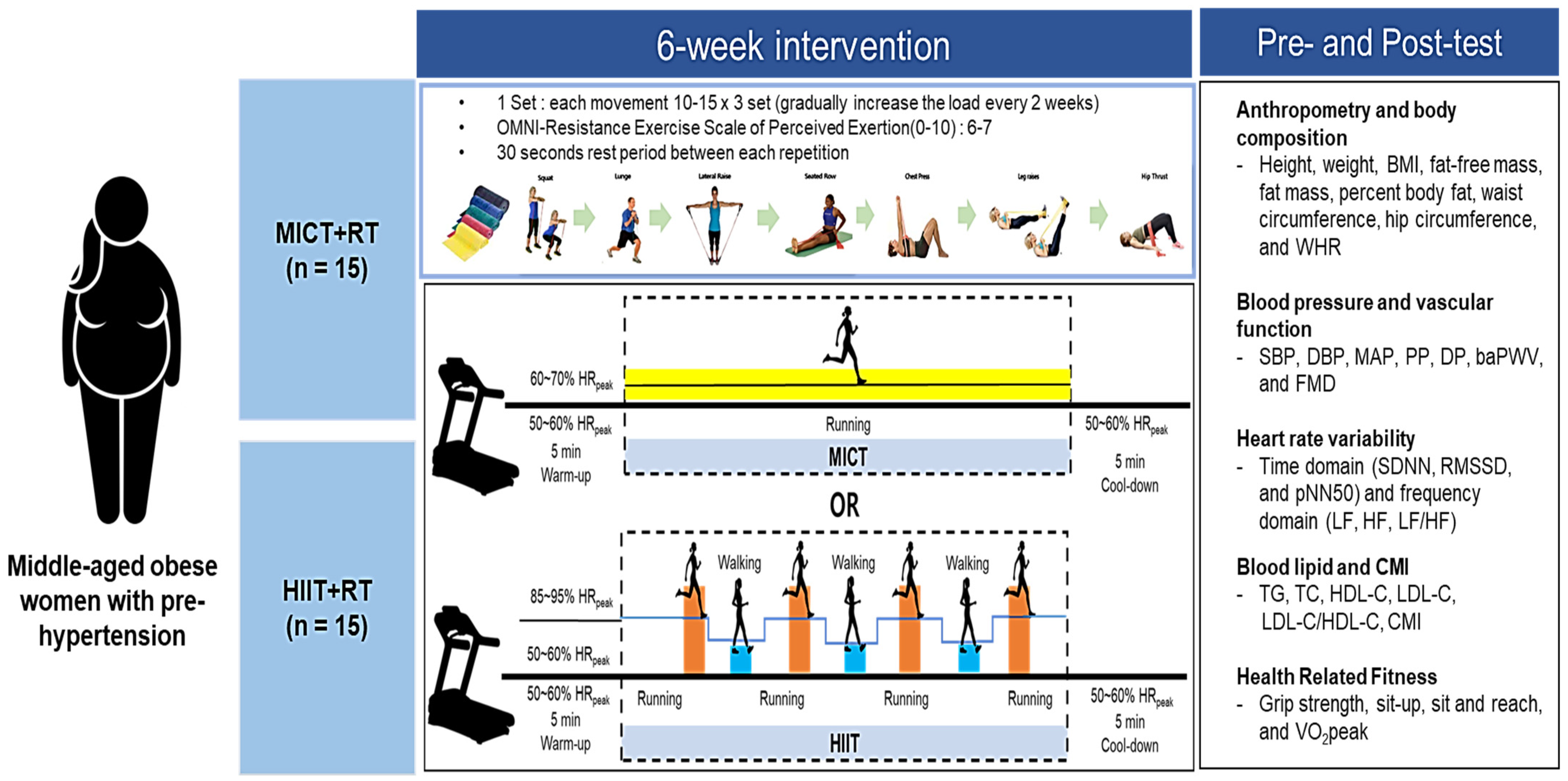
2.2. Study Design
2.3. Measurement
2.4. Statistical Analysis
3. Results
3.1. Body Composition
3.2. Blood Pressure and Vascular Function
3.3. Autonomic Nervous System Function
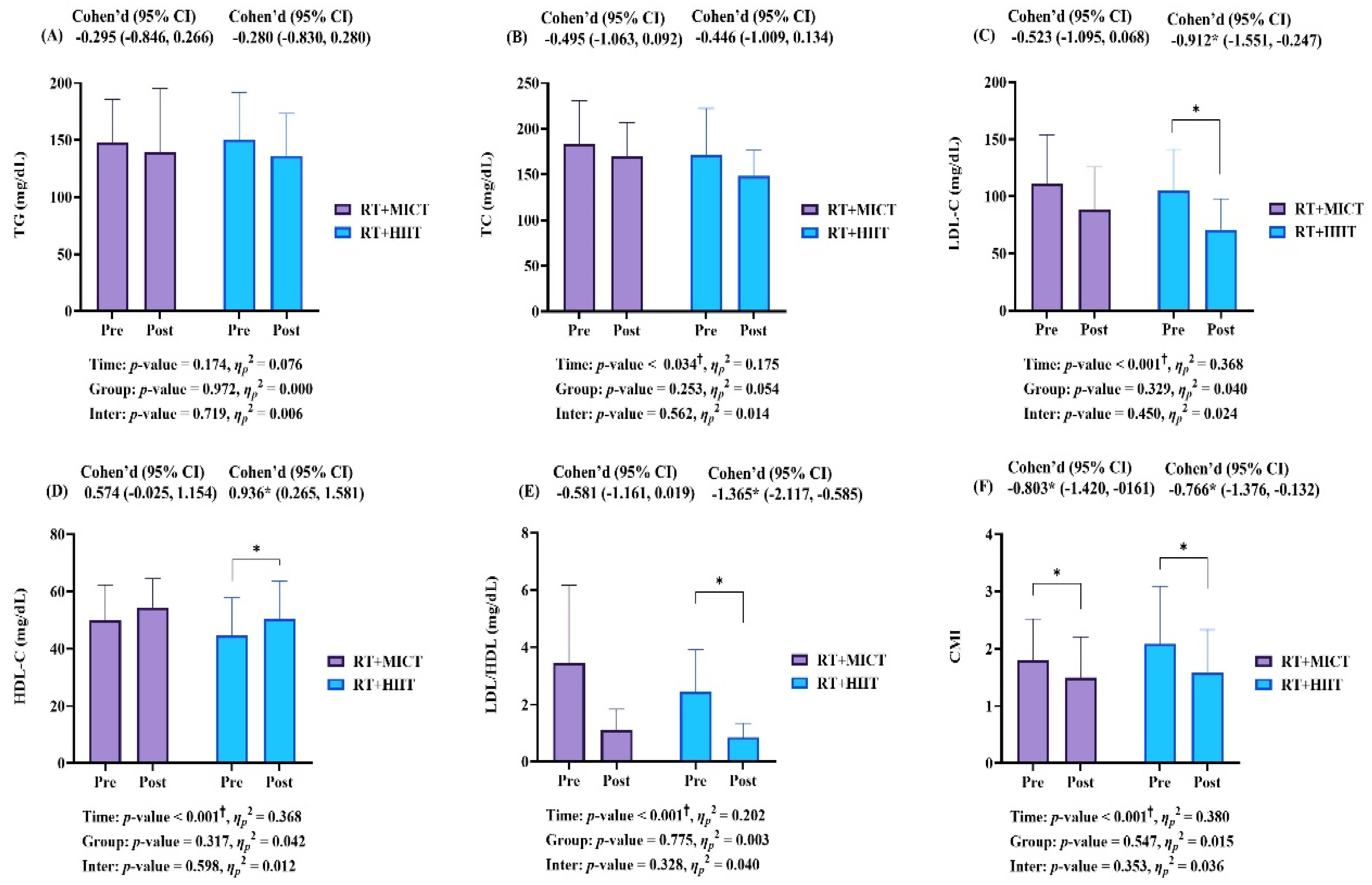
3.4. Blood Lipid Level and Cardiometabolic Index
3.5. Health-Related Fitness
4. Discussion
5. Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kim, B.Y.; Kang, S.M.; Kang, J.H.; Kang, S.Y.; Kim, K.K.; Kim, K.B.; Kim, B.; Kim, S.J.; Kim, Y.H.; Kim, J.H.; et al. 2020 Korean society for the study of obesity guidelines for the management of obesity in Korea. J. Obes. Metab. Syndr. 2021, 30, 81–92. [Google Scholar] [CrossRef] [PubMed]
- Jeong, S.M.; Jung, J.H.; Yang, Y.S.; Kim, W.; Cho, I.Y.; Lee, Y.B.; Park, K.Y.; Nam, G.E.; Han, K.; on Behalf of the Taskforce Team of the Obesity Fact Sheet of the Korean Society for the Study of Obesity. 2023 Obesity Fact Sheet: Prevalence of Obesity and Abdominal Obesity in Adults, Adolescents, and Children in Korea from 2012 to 2021. J. Obes. Metab. Syndr. 2024, 33, 27–35. [Google Scholar] [CrossRef] [PubMed]
- Nunes, P.R.; Barcelos, L.C.; Oliveira, A.A.; Furlanetto Júnior, R.; Martins, F.M.; Orsatti, C.L.; Resende, E.A.; Orsatti, F.L. Effect of resistance training on muscular strength and indicators of abdominal adiposity, metabolic risk, and inflammation in postmenopausal women: Controlled and randomized clinical trial of efficacy of training volume. Age 2016, 38, 40. [Google Scholar] [CrossRef] [PubMed]
- Wright, S.M.; Aronne, L.J. Causes of obesity. Abdom. Imaging 2012, 37, 730–732. [Google Scholar] [CrossRef]
- Apovian, C.M. Obesity: Definition, comorbidities, causes, and burden. Am. J. Manag. Care 2016, 22, s176–s185. [Google Scholar]
- Leggio, M.; Lombardi, M.; Caldarone, E.; Severi, P.; D’Emidio, S.; Armeni, M.; Bravi, V.; Bendini, M.G.; Mazza, A. The relationship between obesity and hypertension: An updated comprehensive overview on vicious twins. Hypertens. Res. 2017, 40, 947–963. [Google Scholar] [CrossRef]
- Korea Disease Control and Prevention Agency. 2022 National Health Statistics: Results of the 9th Korea National Health and Nutrition Examination Survey, 1st year; 2023.
- Lamirault, G.; Artifoni, M.; Daniel, M.; Barber-Chamoux, N.; Nantes University Hospital Working Group on Hypertension. Resistant Hypertension: Novel Insights. Curr. Hypertens. Rev. 2020, 16, 61–72. [Google Scholar] [CrossRef]
- McCarthy, D.; Berg, A. Weight loss strategies and the risk of skeletal muscle mass loss. Nutrients 2021, 13, 2473. [Google Scholar] [CrossRef]
- Passos, G.S.; Poyares, D.; Santana, M.G.; Teixeira, A.A.D.S.; Lira, F.S.; Youngstedt, S.D.; Santos, R.V.T.D.; Tufik, S.; De Mello, M.T. Exercise improves immune function, antidepressive response, and sleep quality in patients with chronic primary insomnia. BioMed Res. Int. 2014, 2014, 498961. [Google Scholar] [CrossRef]
- Petridou, A.; Siopi, A.; Mougios, V. Exercise in the management of obesity. Metab. Clin. Exp. 2019, 92, 163–169. [Google Scholar] [CrossRef]
- Schjerve, I.E.; Tyldum, G.A.; Tjønna, A.E.; Stølen, T.; Loennechen, J.P.; Hansen, H.E.M.; Haram, P.M.; Heinrich, G.; Bye, A.; Najjar, S.M.; et al. Both aerobic endurance and strength training programmes improve cardiovascular health in obese adults. Clin. Sci. 2008, 115, 283–293. [Google Scholar] [CrossRef] [PubMed]
- Xi, H.; He, Y.; Niu, Y.; Sui, X.; Zhang, J.; Zhu, R.; Xu, H.; Zhang, S.; Li, Y.; Yuan, Y.; et al. Effect of combined aerobic and resistance exercise on blood pressure in postmenopausal women: A systematic review and meta-analysis of randomized controlled trials. Exp. Gerontol. 2021, 155, 111560. [Google Scholar] [CrossRef] [PubMed]
- Achten, J.; Jeukendrup, A.E. Optimizing fat oxidation through exercise and diet. Nutrition 2004, 20, 716–727. [Google Scholar] [CrossRef] [PubMed]
- Mann, S.; Beedie, C.; Jimenez, A. Differential effects of aerobic exercise, resistance training and combined exercise modalities on cholesterol and the lipid profile: Review, synthesis and recommendations. Sports Med. 2014, 44, 211–221. [Google Scholar] [CrossRef]
- Yavari, A.; Najafipoor, F.; Aliasgarzadeh, A.; Niafar, M.; Mobasseri, M. Effect of aerobic exercise, resistance training or combined training on glycaemic control and cardio-vascular risk factors in patients with type 2 diabetes. Biol. Sport 2012, 29, 135–143. [Google Scholar] [CrossRef]
- Mendonça, F.R.; Ferreira de Faria, W.; Marcio da Silva, J.; Massuto, R.B.; Castilho dos Santos, G.; Correa, R.C.; Ferreira dos Santos, C.; Sasaki, J.E.; Neto, A.S. Effects of aerobic exercise combined with resistance training on health-related physical fitness in adolescents: A randomized controlled trial. J. Exerc. Sci. Fit. 2022, 20, 182–189. [Google Scholar] [CrossRef]
- Pashaei, Z.; Malandish, A.; Alipour, S.; Jafari, A.; Laher, I.; Hackney, A.C.; Suzuki, K.; Granacher, U.; Saeidi, A.; Zouhal, H. Effects of HIIT training and HIIT combined with circuit resistance training on measures of physical fitness, miRNA expression, and metabolic risk factors in overweight/obese middle-aged women. BMC Sports Sci. Med. Rehabil. 2024, 16, 123. [Google Scholar] [CrossRef]
- Dupuit, M.; Rance, M.; Morel, C.; Bouillon, P.; Pereira, B.; Bonnet, A.; Maillard, F.; Duclos, M.; Boisseau, N. Moderate-Intensity Continuous Training or High-Intensity Interval Training with or without Resistance Training for Altering Body Composition in Postmenopausal Women. Med. Sci. Sports Exerc. 2020, 52, 736–745. [Google Scholar] [CrossRef]
- He, J.; Wang, Y.; Zhang, J.; Gao, C.; Ma, Y.; Dong, C.; Chen, L. Effects of Combined Training with Different Intensities on Cardiac Structure and Function in Patients with Type 2 Diabetes: A Randomised Controlled Trial. 2023. Available online: https://assets-eu.researchsquare.com/files/rs-3629093/v1/fa7084c0-7232-40c1-9d7b-78d5c02f64ba.pdf?c=1700827651 (accessed on 15 April 2025).
- Mir, E.; Shamseddini, A.; Rahimi, N.; Bazgir, B. Impacts of a 12-week aerobic, resistance, and combined exercise training on serum FAM19A5, glucose homeostasis, and novel cardiovascular risk factors among adults with obesity. Int. J. Diabetes Dev. Ctries. 2024, 45, 175–186. [Google Scholar] [CrossRef]
- Collins, K.A.; Huffman, K.M.; Wolever, R.Q.; Smith, P.J.; Siegler, I.C.; Ross, L.M.; Hauser, E.R.; Jiang, R.; Jakicic, J.M.; Costa, P.T.; et al. Determinants of Dropout from and Variation in Adherence to an Exercise Intervention: The STRRIDE Randomized Trials. Transl. J. Am. Coll. Sports Med. 2022, 7, e000190. [Google Scholar] [CrossRef]
- Sadja, J.; Tomfohr, L.; Jiménez, J.A.; Edwards, K.M.; Rock, C.L.; Calfas, K.; Mills, P.J. Higher physical fatigue predicts adherence to a 12-week exercise intervention in women with elevated blood pressure. Health Psychol. 2012, 31, 156–163. [Google Scholar] [CrossRef] [PubMed]
- Costa, E.C.; Hay, J.L.; Kehler, D.S.; Boreskie, K.F.; Arora, R.C.; Umpierre, D.; Szwajcer, A.; Duhamel, T.A. Effects of High-Intensity Interval Training Versus Moderate-Intensity Continuous Training On Blood Pressure in Adults with Pre- to Established Hypertension: A Systematic Review and Meta-Analysis of Randomized Trials. Sports Med. 2018, 48, 2127–2142. [Google Scholar] [CrossRef] [PubMed]
- Alghanim, S.; Alablani, M.F.; Alqutami, A.; Alotaibi, R.T.; Jung, H.C.; Stoner, L.; Alansare, A.B. Effects of Exercise Interventions on Estimated Pulse Wave Velocity and Mean Arterial Pressure in Overweight Adults: The Role of Modality. Rev. Cardiovasc. Med. 2024, 25, 139. [Google Scholar] [CrossRef]
- Sun, F.; Williams, C.A.; Sun, Q.; Hu, F.; Zhang, T. Effect of eight-week high-intensity interval training versus moderate-intensity continuous training programme on body composition, cardiometabolic risk factors in sedentary adolescents. Front. Physiol. 2024, 15, 1450341. [Google Scholar] [CrossRef]
- Wewege, M.; van den Berg, R.; Ward, R.E.; Keech, A. The effects of high-intensity interval training vs. moderate-intensity continuous training on body composition in overweight and obese adults: A systematic review and meta-analysis. Obes. Rev. 2017, 18, 635–646. [Google Scholar] [CrossRef]
- Sanca-Valeriano, S.; Espinola-Sánchez, M.; Caballero-Alvarado, J.; Canelo-Aybar, C. Effect of high-intensity interval training compared to moderate-intensity continuous training on body composition and insulin sensitivity in overweight and obese adults: A systematic review and meta-analysis. Heliyon 2023, 9, e20402. [Google Scholar] [CrossRef]
- Li, L.; Liu, X.; Shen, F.; Xu, N.; Li, Y.; Xu, K.; Li, J.; Liu, Y. Effects of high-intensity interval training versus moderate-intensity continuous training on blood pressure in patients with hypertension: A meta-analysis. Medicine 2022, 101, e32246. [Google Scholar] [CrossRef]
- Sabouri, M.; Amirshaghaghi, F.; Hesari, M.M. High-intensity interval training improves the vascular endothelial function comparing moderate-intensity interval training in overweight or obese adults: A meta-analysis. Clin. Nutr. ESPEN 2023, 53, 100–106. [Google Scholar] [CrossRef]
- Costache, A.D.; Maștaleru, A.; Leon, M.M.; Roca, M.; Gavril, R.S.; Cosău, D.E.; Rotundu, A.; Amagdalinei, A.I.; Mitu, O.; Costache, E., II; et al. High-Intensity Interval Training vs. Medium-Intensity Continuous Training in Cardiac Rehabilitation Programs: A Narrative Review. Medicina 2024, 60, 1875. [Google Scholar] [CrossRef]
- Shishira, K.B.; Vaishali, K.; Kadavigere, R.; Sukumar, S.; Shivashankara, K.N.; Pullinger, S.A.; Bommasamudram, T. Effects of high-intensity interval training versus moderate-intensity continuous training on vascular function among individuals with overweight and obesity-a systematic review. Int. J. Obes. 2024, 48, 1517–1533. [Google Scholar] [CrossRef]
- Taylor, J.L.; Keating, S.E.; Holland, D.J.; Green, D.J.; Coombes, J.S.; Bailey, T.G. Comparison of high intensity interval training with standard cardiac rehabilitation on vascular function. Scand. J. Med. Sci. Sports 2022, 32, 512–520. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, M.W.; Johns, J.A.; Robinson, S.A.; Bungay, A.; Mekary, S.; Kimmerly, D.S. Impact of High-Intensity Interval Training, Moderate-Intensity Continuous Training, and Resistance Training on Endothelial Function in Older Adults. Med. Sci. Sports Exerc. 2020, 52, 1057–1067. [Google Scholar] [CrossRef] [PubMed]
- Kolmos, M.; Krawcyk, R.S.; Kruuse, C. Effect of high-intensity training on endothelial function in patients with cardiovascular and cerebrovascular disease: A systematic review. SAGE Open Med. 2016, 4, 2050312116682253. [Google Scholar] [CrossRef] [PubMed]
- Du, Z.; Qian, J.; Yang, J.; Xue, C.; Xu, Z. Comparing the Impact of High-Intensity Interval Training and Moderate-Intensity Continuous Training on Metabolic Syndrome Risk Factors: A Meta-Analytic Review. In Proceedings of the 2024 International Conference on Biomedicine and Intelligent Technology, Houston, TX, USA, 10–13 November 2024; pp. 225–229. [Google Scholar]
- Ashor, A.W.; Lara, J.; Siervo, M.; Celis-Morales, C.; Mathers, J.C. Effects of exercise modalities on arterial stiffness and wave reflection: A systematic review and meta-analysis of randomized controlled trials. PLoS ONE 2014, 9, e110034. [Google Scholar] [CrossRef]
- Gupta, A.K.; Ravussin, E.; Johannsen, D.L.; Stull, A.J.; Cefalu, W.T.; Johnson, W.D. Endothelial Dysfunction: An Early Cardiovascular Risk Marker in Asymptomatic Obese Individuals with Prediabetes. Br. J. Med. Med. Res. 2012, 2, 413–423. [Google Scholar] [CrossRef]
- Park, H.Y.; Jung, W.S.; Kim, S.W.; Lim, K. Effects of Interval Training Under Hypoxia on the Autonomic Nervous System and Arterial and Hemorheological Function in Healthy Women. Int. J. Women’s Health 2022, 14, 79–90. [Google Scholar] [CrossRef]
- Jung, W.S.; Kim, S.W.; Park, H.Y. Interval Hypoxic Training Enhances Athletic Performance and Does Not Adversely Affect Immune Function in Middle- and Long-Distance Runners. Int. J. Environ. Res. Public Health 2020, 17, 1934. [Google Scholar] [CrossRef]
- Park, H.Y.; Jung, W.S.; Kim, J.; Lim, K. Twelve weeks of exercise modality in hypoxia enhances health-related function in obese older Korean men: A randomized controlled trial. Geriatr. Gerontol. Int. 2019, 19, 311–316. [Google Scholar] [CrossRef]
- Philbois, S.V.; Ribeiro, V.B.; Tank, J.; Dos Reis, R.M.; Gerlach, D.A.; Souza, H.C.D. Cardiovascular autonomic modulation differences between moderate-intensity continuous and high-intensity interval aerobic training in women with PCOS: A randomized trial. Front. Endocrinol. 2022, 13, 1024844. [Google Scholar] [CrossRef]
- Su, Z.Y.; Yu, W.L.; Yan, Z.W.; Ding, D.D.; Fang, C.C.; Luo, Q.L.; Liu, X.; Cao, L.Z. Comparison of high-intensity interval training and moderate-intensity continuous training on cardiopulmonary function, cardiac autonomic function and vascular function in adolescent boys with obesity: A randomized controlled trial. Eur. J. Sport. Sci. 2024, 24, 1871–1882. [Google Scholar] [CrossRef]
- Alansare, A.; Alford, K.; Lee, S.; Church, T.; Jung, H.C. The Effects of High-Intensity Interval Training vs. Moderate-Intensity Continuous Training on Heart Rate Variability in Physically Inactive Adults. Int. J. Environ. Res. Public Health 2018, 15, 1508. [Google Scholar] [CrossRef] [PubMed]
- Masroor, S.; Bhati, P.; Verma, S.; Khan, M.; Hussain, M.E. Heart Rate Variability following Combined Aerobic and Resistance Training in Sedentary Hypertensive Women: A Randomised Control Trial. Indian Heart J. 2018, 70 (Suppl. S3), S28–S35. [Google Scholar] [CrossRef] [PubMed]
- Zaki, S.; Alam, M.F.; Sharma, S.; El-Ashker, S.; Ahsan, M.; Nuhmani, S. Impact of Concurrent Exercise Training on Cardiac Autonomic Modulation, Metabolic Profile, Body Composition, Cardiorespiratory Fitness, and Quality of Life in Type 2 Diabetes with Cardiac Autonomic Neuropathy: A Randomized Controlled Trial. J. Clin. Med. 2024, 13, 3910. [Google Scholar] [CrossRef]
- Da Silva, M.A.R.; Baptista, L.C.; Neves, R.S.; De França, E.; Loureiro, H.; Lira, F.S.; Caperuto, E.C.; Veríssimo, M.T.; Martins, R.A. The Effects of Concurrent Training Combining Both Resistance Exercise and High-Intensity Interval Training or Moderate-Intensity Continuous Training on Metabolic Syndrome. Front. Physiol. 2020, 11, 572. [Google Scholar] [CrossRef]
- Mc, C.C.; Mamikunian, G.; Thorp, D.B. The Effects of HIIT vs. MICT and Sedentary Controls on Blood Lipid Concentrations in Nondiabetic Overweight and Obese Young Adults: A Meta-analysis. Int. J. Exerc. Sci. 2023, 16, 791–813. [Google Scholar] [CrossRef]
- Coswig, V.S.; Barbalho, M.; Raiol, R.; Del Vecchio, F.B.; Ramirez-Campillo, R.; Gentil, P. Effects of high vs moderate-intensity intermittent training on functionality, resting heart rate and blood pressure of elderly women. J. Transl. Med. 2020, 18, 88. [Google Scholar] [CrossRef]
- Guo, Z.; Li, M.; Cai, J.; Gong, W.; Liu, Y.; Liu, Z. Effect of High-Intensity Interval Training vs. Moderate-Intensity Continuous Training on Fat Loss and Cardiorespiratory Fitness in the Young and Middle-Aged a Systematic Review and Meta-Analysis. Int. J. Environ. Res. Public Health 2023, 20, 4741. [Google Scholar] [CrossRef]
- Yakut, H.; Dursun, H.; Felekoğlu, E.; Başkurt, A.A.; Alpaydın, A.; Özalevli, S. Effect of home-based high-intensity interval training versus moderate-intensity continuous training in patients with myocardial infarction: A randomized controlled trial. Ir. J. Med. Sci. 2022, 191, 2539–2548. [Google Scholar] [CrossRef]
- Squeo, M.R.; Di Giacinto, B.; Perrone, M.A.; Santini, M.; Sette, M.L.; Fabrizi, E.; Vaquer, A.; Parisi, A.; Spataro, A.; Biffi, A. Efficacy and Safety of a Combined Aerobic, Strength and Flexibility Exercise Training Program in Patients with Implantable Cardiac Devices. J. Cardiovasc. Dev. Dis. 2022, 9, 182. [Google Scholar] [CrossRef]
- Villareal, D.T.; Aguirre, L.; Gurney, A.B.; Waters, D.L.; Sinacore, D.R.; Colombo, E.; Armamento-Villareal, R.; Qualls, C. Aerobic or Resistance Exercise, or Both, in Dieting Obese Older Adults. N. Engl. J. Med. 2017, 376, 1943–1955. [Google Scholar] [CrossRef]
- Morales-Palomo, F.; Moreno-Cabañas, A.; Alvarez-Jimenez, L.; Mora-Gonzalez, D.; Ortega, J.F.; Mora-Rodriguez, R. Efficacy of morning versus afternoon aerobic exercise training on reducing metabolic syndrome components: A randomized controlled trial. J. Physiol. 2024, 602, 6463–6477. [Google Scholar] [CrossRef] [PubMed]
| Parameters | RT + MICT (n = 13) | RT + HIIT (n = 13) | p-Value |
|---|---|---|---|
| Age (year) | 47.08 ± 6.36 | 47.00 ± 7.58 | 0.978 |
| Height (cm) | 159.60 ± 3.01 | 160.75 ± 3.81 | 0.421 |
| Weight (kg) | 70.66 ± 8.77 | 70.54 ± 9.76 | 0.973 |
| BMI (kg/m2) | 27.68 ± 3.86 | 27.34 ± 3.30 | 0.808 |
| Lean body mass (kg) | 43.72 ± 2.85 | 43.42 ± 4.71 | 0.842 |
| Fat mass (kg) | 26.82 ± 7.49 | 27.25 ± 5.52 | 0.869 |
| Percent body fat (%) | 37.42 ± 5.09 | 38.30 ± 4.10 | 0.633 |
| Body fat mass (kg) | 127.00 ± 6.12 | 128.15 ± 7.79 | 0.678 |
| Percent body fat (%) | 81.62 ± 6.78 | 80.15 ± 5.93 | 0.564 |
| Parameters | Groups | Test | Cohen’s d (95% CI) | p (η2) | ||
|---|---|---|---|---|---|---|
| Pre | Post | |||||
| Body weight (kg) | RT + MICT | 70.66 ± 8.77 | 69.80 ± 9.00 | −0.336 (−0.889, 0.230) | T G T × G | 0.770 (0.124) 0.944 (0.000) 0.820 (0.002) |
| RT + HIIT | 70.54 ± 9.76 | 69.43 ± 8.48 | −0.434 (−0.996. 0.143) | |||
| BMI (kg/m2) | RT + MICT | 27.68 ± 3.86 | 27.23 ± 3.12 | −0.339 (−0.892, 0.227) | T G T × G | 0.770 (0.124) 0.831 (0.002) 0.790 (0.003) |
| RT + HIIT | 27.34 ± 3.30 | 27.00 ± 3.45 | −0.435 (−0.998, 0.143) | |||
| Lean body mass (kg) | RT + MICT | 43.72 ± 2.85 | 43.80 ± 2.61 | 0.035 (−0.509, 0.578) | T G T × G | 0.620 (0.010) 0.889 (0.001) 0.772 (0.004) |
| RT + HIIT | 43.42 ± 4.71 | 43.71 ± 4.27 | 0.197 (−0.356, 0.742) | |||
| Fat mass (kg) | RT + MICT | 26.82 ± 7.49 | 26.14 ± 6.51 | −0.254 (−0.802, 0.304) | T G T × G | 0.175 (0.075) 0.868 (0.001) 0.988 (0.000) |
| RT + HIIT | 27.25 ± 5.52 | 26.55 ± 6.44 | −0.299 (−0.850, 0.263) | |||
| Percent body fat (%) | RT + MICT | 37.42 ± 5.09 | 37.18 ± 4.78 | −0.127 (−1.751, −0.371) | T G T × G | 0.218 (0.063) 0.757 (0.004) 0.491 (0.020) |
| RT + HIIT | 38.30 ± 4.10 | 37.45 ± 5.51 | −0.346 (−0.900, 0.221) | |||
| WC (cm) | RT + MICT | 89.82 ± 6.68 | 87.95 ± 6.32 | −1.075 * (−1.751, −0.371) | T G T × G | <0.001 † (0.604) 0.544 (0.016) 0.787 (0.003) |
| RT + HIIT | 88.02 ± 8.92 | 85.98 ± 9.05 | −1.313 * (−2.051, −0.547) | |||
| HC (cm) | RT + MICT | 104.35 ± 6.61 | 101.65 ± 6.90 | −1.579 * (−2.392, −0.737) | T G T × G | <0.001 † (0.753) 0.889 (0.001) 0.754 (0.004) |
| RT + HIIT | 103.88 ± 6.80 | 101.38 ± 6.93 | −1.272 * (−2.716, −0.909) | |||
| WHR | RT + MICT | 0.86 ± 0.05 | 0.87 ± 0.05 | 0.256 (−0.302, 0.804) | T G T × G | 0.030 † (0.394) 0.889 (0.001) 0.555 (0.015) |
| RT + HIIT | 0.85 ± 0.06 | 0.85 ± 0.07 | 0.061 (−0.484, 0.604) | |||
| Parameters | Groups | Test | Cohen’s d (95% CI) | p (η2) | ||
|---|---|---|---|---|---|---|
| Pre | Post | |||||
| HR (bpm) | RT + MICT | 72.20 ± 8.99 | 66.75 ± 11.59 | −0.680 * (−1.275, −0.062) | T G T × G | <0.001 † (0.482) 0.727 (0.005) 0.514 (0.018) |
| RT + HIIT | 71.91 ± 7.10 | 64.68 ± 8.47 | −1.334 * (−2.078, −0.563) | |||
| SBP (mmHg) | RT + MICT | 127.00 ± 6.12 | 123.77 ± 6.94 | −1.106 * (−1.791, −0.395) | T G T × G | <0.001 † (0.656) 0.968 (0.000) 0.069 (0.132) |
| RT + HIIT | 128.15 ± 7.79 | 122.38 ± 8.45 | −1.514 * (−2.308, −0.024) | |||
| DBP (mmHg) | RT + MICT | 81.62 ± 6.78 | 77.85 ± 5.46 | −1.153 * (−1.848, 0.429) | T G T × G | <0.001 † (0.383) 0.416 (0.028) 0.799 (0.003) |
| RT + HIIT | 80.15 ± 5.93 | 75.85 ± 5.58 | −0.634 * (−1.221, −0.024) | |||
| MAP (mmHg) | RT + MICT | 96.77 ± 6.13 | 93.08 ± 5.24 | −1.406 * (−2.169, −0.614) | T G T × G | <0.001 † (0.543) 0.559 (0.014) 0.588 (0.012) |
| RT + HIIT | 96.00 ± 5.15 | 91.46 ± 5.39 | −0.928 * (−1.570, −0.259) | |||
| PP (mmHg) | RT + MICT | 45.38 ± 4.81 | 45.92 ± 6.33 | 0.143 (−0406, 0.687) | T G T × G | 0.636 (0.009) 0.522 (0.017) 0.348 (0.037) |
| RT + HIIT | 48.15 ± 8.75 | 46.54 ± 8.31 | −0.225 (−0.771, 0.330) | |||
| DP (mmHg ·bpm) | RT + MICT | 8932.23 ± 1169.78 | 5425.85 ± 941.12 | −3.339 * (−4.756, −1.904) | T G T × G | <0.001 † (0.928) 0.551 (0.015) 0.824 (0.002) |
| RT + HIIT | 8783.85 ± 891.30 | 5186.69 ± 827.34 | −3.550 * (−5.044, −2.038) | |||
| baPWV (cm/s) | RT + MICT | 1316.92 ± 128.52 | 1254.69 ± 163.17 | −0.921 * (−1.562, −1.562) | T G T × G | <0.001 † (0.576) 0.952 (0.000) 0.371 (0.034) |
| RT + HIIT | 1325.08 ± 170.14 | 1239.15 ± 166.56 | −1.326 * (−2.067, −0.557) | |||
| FMD (%) | RT + MICT | 5.14 ± 0.98 | 5.73 ± 0.69 | 1.741 * (0.850, 2.604) | T G T × G | <0.001 † (0.807) 0.473 (0.022) 0.189 (0.071) |
| RT + HIIT | 5.30 ± 1.08 | 6.08 ± 0.82 | 2.181 * (1.150, 3.187) | |||
| Parameters | Groups | Test | Cohen’s d (95% CI) | p (η2) | ||
|---|---|---|---|---|---|---|
| Pre | Post | |||||
| SDNN (ms) | RT + MICT | 24.32 ± 11.04 | 30.91 ± 16.23 | 0.433 (−0.145, 0.995) | T G T × G | 0.023 † (0.196) 0.986 (0.000) 0.846 (0.002) |
| RT + HIIT | 24.75 ± 7.89 | 30.35 ± 10.10 | 0.565 (−0.032, 1.143) | |||
| RMSSD (ms) | RT + MICT | 26.67 ± 20.51 | 31.18 ± 14.90 | 0.225 (−0.331, 0.771) | T G T × G | 0.222 (0.061) 0.950 (0.000) 0.819 (0.002) |
| RT + HIIT | 27.70 ± 9.52 | 30.80 ± 13.26 | 0.356 (−0.213, 0.910) | |||
| pNN50 (%) | RT + MICT | 8.83 ± 13.56 | 10.66 ± 16.90 | 0.188 (−0.365, 0.733) | T G T × G | 0.155 (0.082) 0.741 (0.005) 0.632 (0.010) |
| RT + HIIT | 9.61 ± 7.50 | 13.24 ± 14.94 | 0.393 (−0.180, 0.951) | |||
| LF (ms2) | RT + MICT | 394.77 ± 280.91 | 253.34 ± 105.96 | −0.612 * (−1.197, −0.007) | T G T × G | 0.020 † (0.207) 0.986 (0.000) 0.954 (0.000) |
| RT + HIIT | 397.03 ± 301.33 | 248.81 ± 138.52 | −0.426 (−0.987, 0.151) | |||
| HF (ms2) | RT + MICT | 124.70 ± 49.96 | 284.27 ± 153.35 | 0.943 * (0.270, 1.589) | T G T × G | <0.001 † (0.593) 0.120 (0.098) 0.427 (0.027) |
| RT + HIIT | 161.81 ± 75.50 | 371.97 ± 180.98 | 1.409 * (0.617, 2.174) | |||
| LF/HF (%) | RT + MICT | 3.45 ± 2.72 | 1.12 ± 0.73 | −0.856 * (−1,484, −0.203) | T G T × G | <0.001 † (0.447) 0.168 (0.078) 0.421 (0.027) |
| RT + HIIT | 2.45 ± 1.46 | 0.85 ± 0.49 | −0.933 * (−1.577, −0.263) | |||
| Parameters | Groups | Test | Cohen’s d (95% CI) | p (η2) | ||
|---|---|---|---|---|---|---|
| Pre | Post | |||||
| Grip strength (kg) | RT + MICT | 24.62 ± 4.31 | 24.95 ± 4.10 | 0.230 (−0.326, 0.776) | T G T × G | 0.150 (0.084) 0.644 (0.009) 0.579 (0.013) |
| RT + HIIT | 25.26 ± 4.89 | 26.00 ± 5.48 | 0.342 (−0.225, 0.896) | |||
| Sit-up (n) | RT + MICT | 11.31 ± 6.87 | 13.23 ± 7.84 | 0.627 (0.019, 1.214) | T G T × G | 0.006 † (0.275) 0.635 (0.010) 0.901 (0.001) |
| RT + HIIT | 12.77 ± 7.37 | 14.54 ± 7.88 | 0.559 (−0.038, 1.136) | |||
| Sit and reach (cm) | RT + MICT | 10.24 ± 6.33 | 12.83 ± 6.95 | 1.640 * (0.780, 2.472) | T G T × G | <0.001 † (0.555) 0.785 (0.003) 0.291 (0.046) |
| RT + HIIT | 10.02 ± 5.17 | 11.75 ± 5.79 | 0.732 * (0.104, 1.336) | |||
| VO2peak (mL/kg/min) | RT + MICT | 31.84 ± 3.39 | 39.73 ± 5.84 | 1.100 * (0.390, 1.783) | T G T × G | 0.001 † (0.679) 0.574 (0.013) 0.546 (0.015) |
| RT + HIIT | 31.78 ± 3.28 | 41.16 ± 4.40 | 1.882 * (0.947, 2.789) | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, J.; Lee, E.; Choi, J.-H.; Sun, Y.; Woo, S.; Cho, S.; Hwang, D.; Kim, S.-W.; Kim, J.; Lim, K.; et al. Effects of a 6-Week Concurrent Training Program Combining Resistance and Various Modalities of Aerobic Exercise in Obese Women with Prehypertension: A Randomized Controlled Trial. Metabolites 2025, 15, 278. https://doi.org/10.3390/metabo15040278
Yu J, Lee E, Choi J-H, Sun Y, Woo S, Cho S, Hwang D, Kim S-W, Kim J, Lim K, et al. Effects of a 6-Week Concurrent Training Program Combining Resistance and Various Modalities of Aerobic Exercise in Obese Women with Prehypertension: A Randomized Controlled Trial. Metabolites. 2025; 15(4):278. https://doi.org/10.3390/metabo15040278
Chicago/Turabian StyleYu, Jinhyuk, Eunjoo Lee, Jae-Ho Choi, Yerin Sun, Seungyeon Woo, Sohyang Cho, Deunsol Hwang, Sung-Woo Kim, Jisu Kim, Kiwon Lim, and et al. 2025. "Effects of a 6-Week Concurrent Training Program Combining Resistance and Various Modalities of Aerobic Exercise in Obese Women with Prehypertension: A Randomized Controlled Trial" Metabolites 15, no. 4: 278. https://doi.org/10.3390/metabo15040278
APA StyleYu, J., Lee, E., Choi, J.-H., Sun, Y., Woo, S., Cho, S., Hwang, D., Kim, S.-W., Kim, J., Lim, K., & Park, H.-Y. (2025). Effects of a 6-Week Concurrent Training Program Combining Resistance and Various Modalities of Aerobic Exercise in Obese Women with Prehypertension: A Randomized Controlled Trial. Metabolites, 15(4), 278. https://doi.org/10.3390/metabo15040278







