Defining the Baseline and Oxidant Perturbed Lipidomic Profiles of Daphnia magna
Abstract
:1. Introduction
2. Results
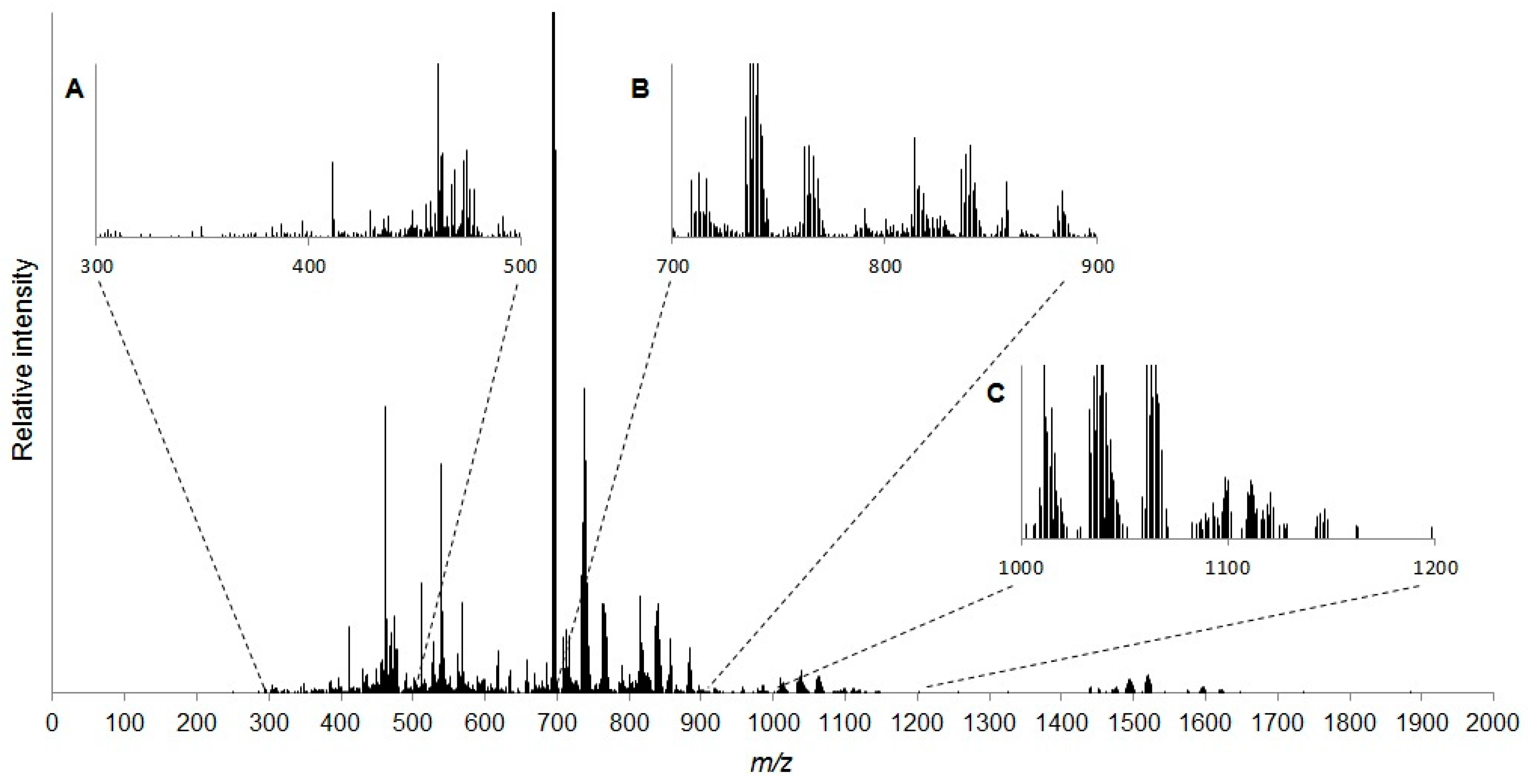
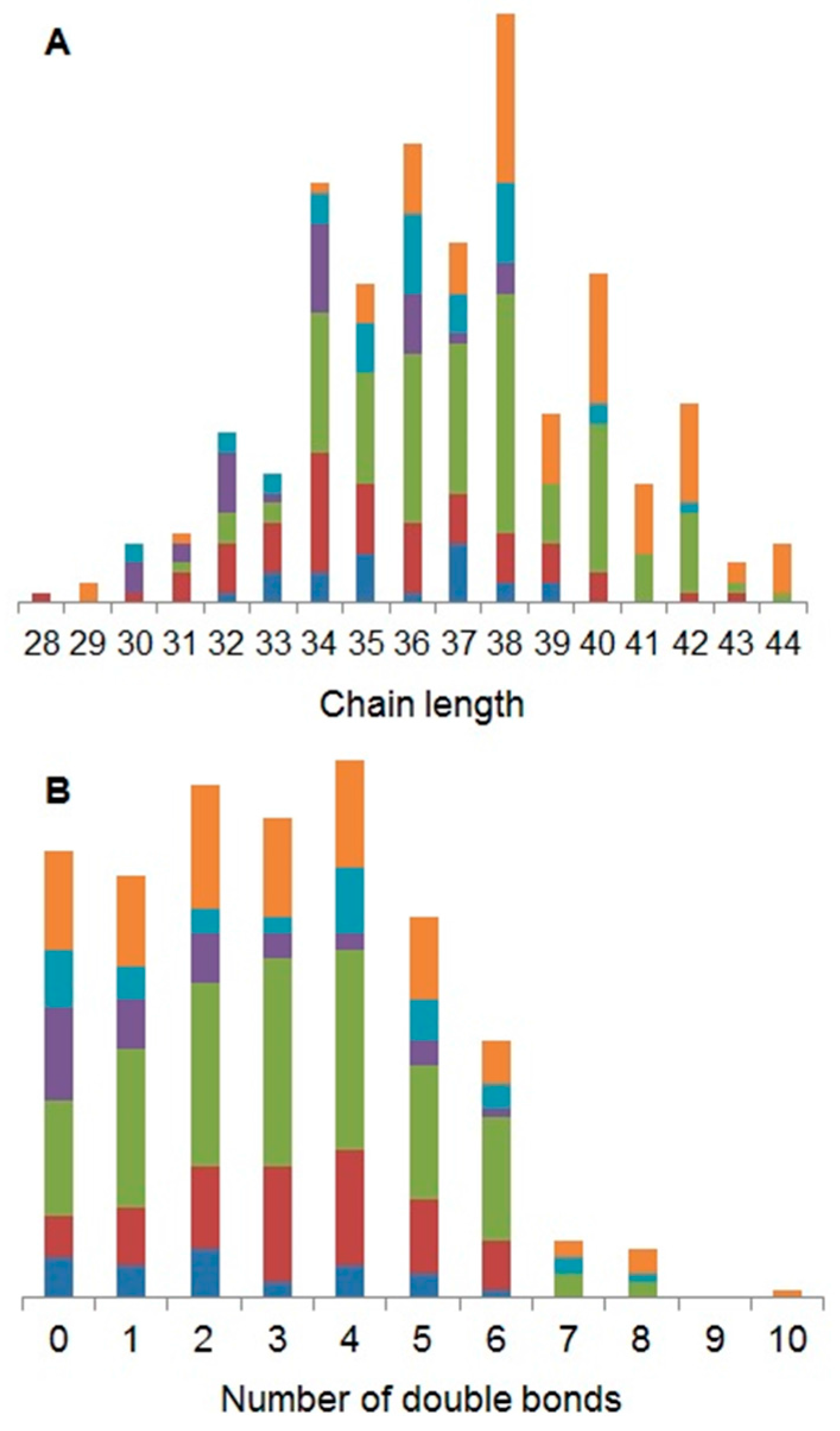
2.1. Characterisation of the Daphnia magna Baseline Lipidome
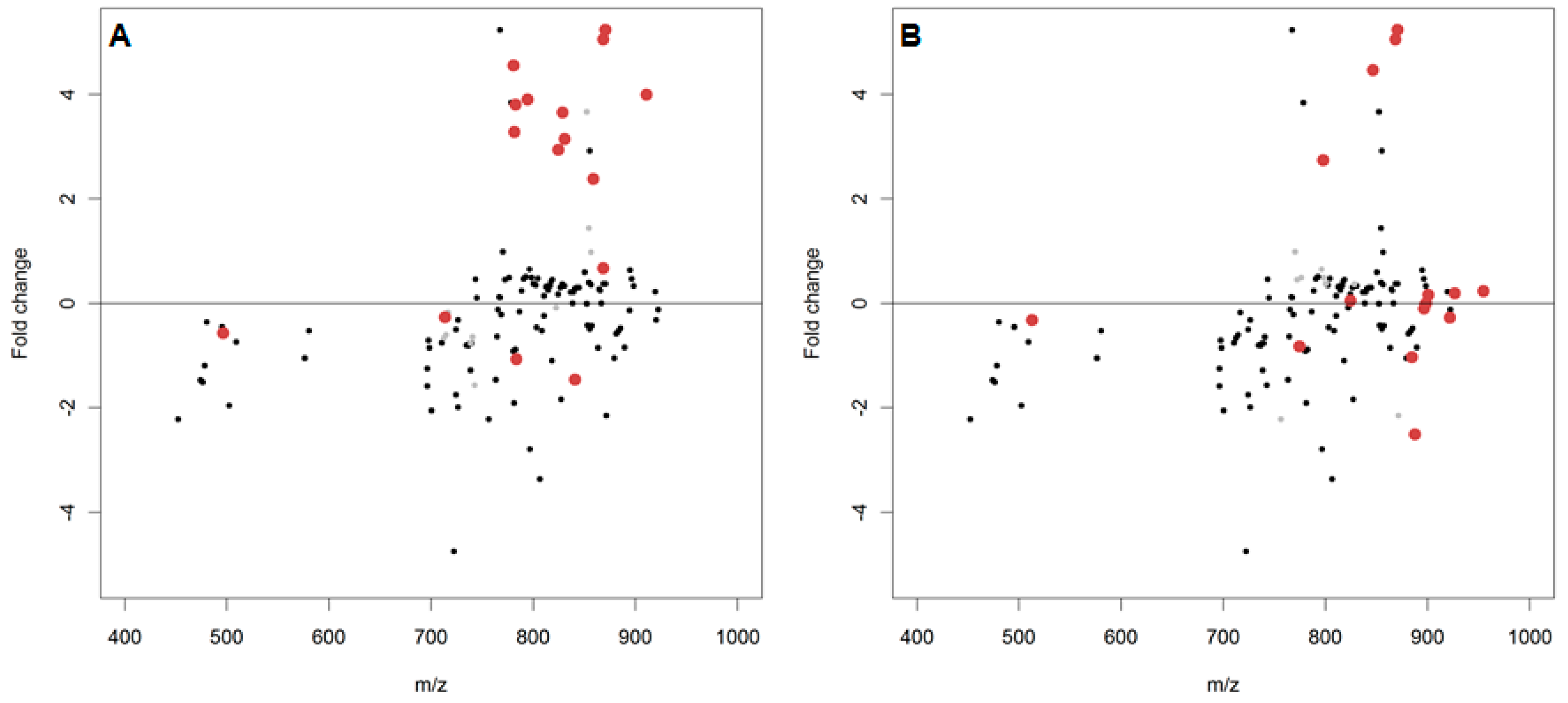
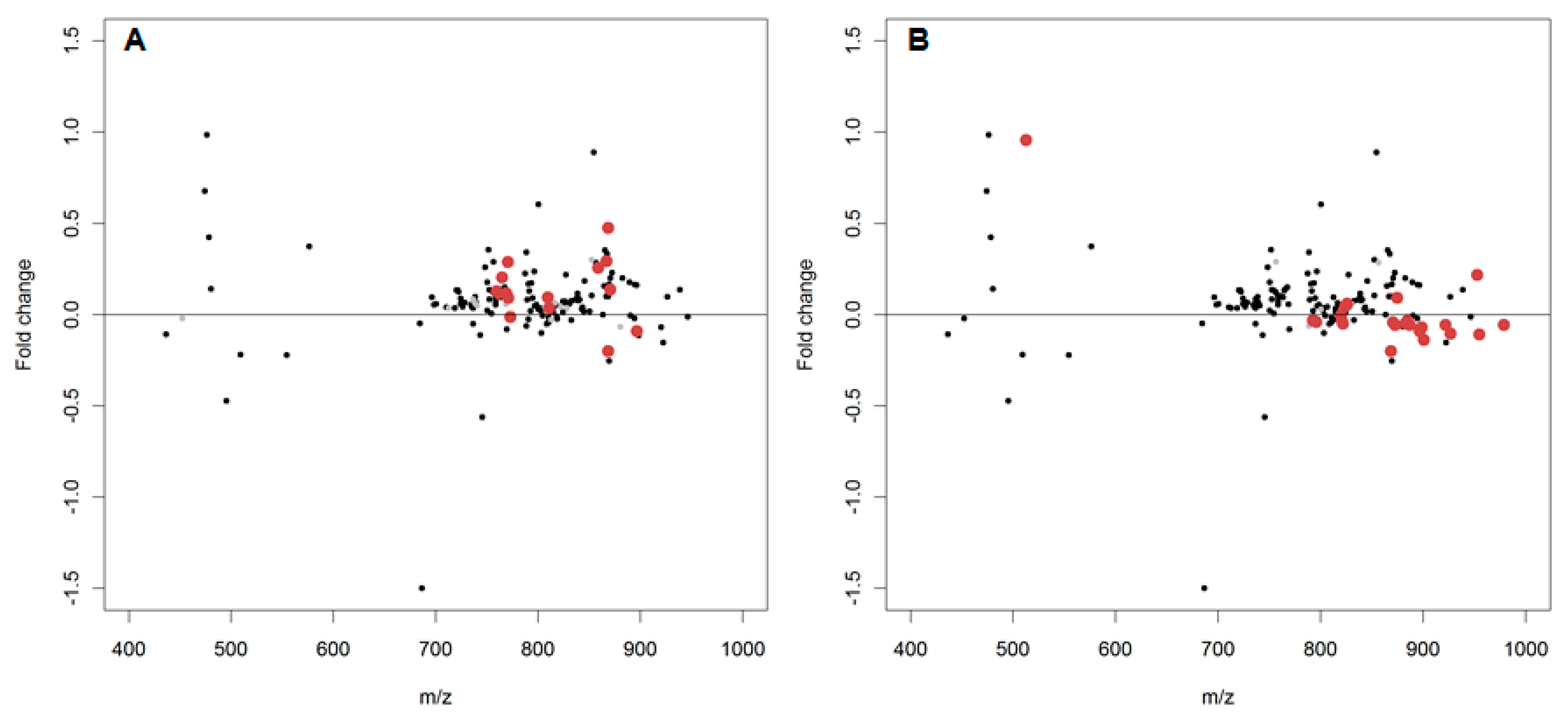
2.2. In Vitro Oxidation of the Daphnia magna Lipidome
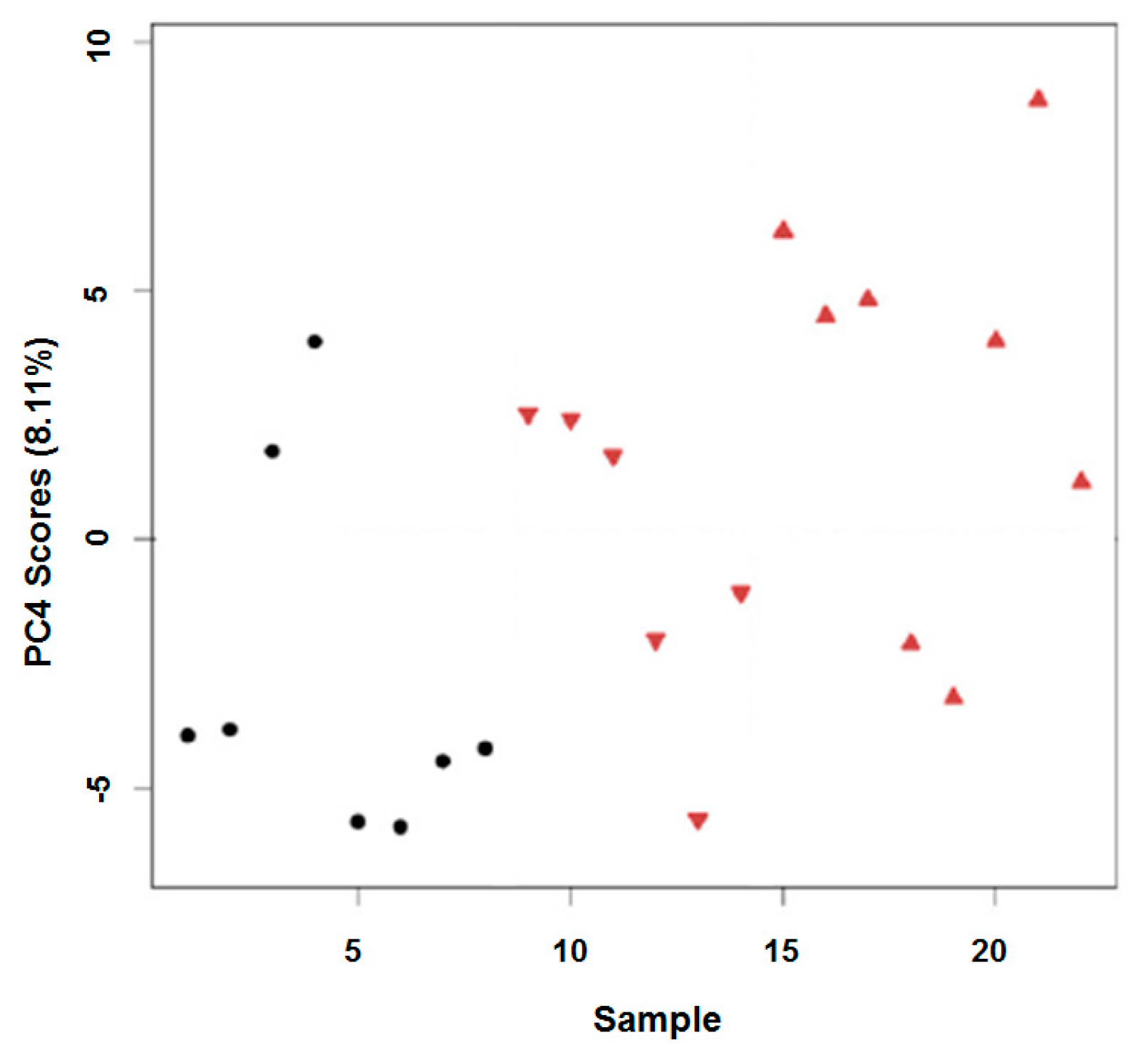
2.3. In Vivo Oxidation of the Daphnia magna Lipidome by Copper
3. Discussion
3.1. Characterisation of the Daphnia magna Baseline Lipidome
3.2. In Vitro Oxidation of the Daphnia magna Lipidome
3.3. In Vivo Oxidation of the Daphnia magna Lipidome by Copper
4. Materials and Methods
4.1. Daphnia Culturing and Lipid Extraction
4.2. In Vitro Oxidation of Daphnia Lipid Extracts
4.3. In Vivo Oxidation of the Daphnia lipidome
4.4. Direct Infusion FT-ICR Mass Spectrometry
4.5. Data Processing and Statistical Analysis
4.6. Determining the Baseline Lipidome of D. magna
4.7. Putative Annotation of Potential Oxidation Products
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Ankley, G.T.; Bennet, R.S.; Erickson, R.J.; Hoff, D.J.; Hornung, M.W.; Johnson, R.D.; Mount, D.R.; Nichols, J.W.; Russom, C.L.; Schmieder, P.K.; et al. Adverse outcome pathways: A conceptual framework to support ecotoxicology research and risk assessment. Environ. Toxicol. Chem. 2010, 29, 730–741. [Google Scholar] [CrossRef] [PubMed]
- Burden, N.; Mahoney, C.; Müller, B.P.; Terry, C.; Westmoreland, C.; Kimber, I. Aligning the 3Rs with new paradigms in the safety assessment of chemicals. Toxicology 2015, 330, 62–66. [Google Scholar] [CrossRef] [PubMed]
- Tollefsen, K.E.; Scholz, S.; Cronin, M.T.; Edwards, S.W.; de Knecht, J.; Crofton, K.; Garcia-Reyero, N.; Hartung, T.; Worth, A.; Patlewicz, G. Applying Adverse Outcome Pathways (AOPs) to support Integrated Approaches to Testing and Assessment (IATA). Regul. Toxicol. Pharm. 2014, 70, 629–640. [Google Scholar] [CrossRef] [PubMed]
- Water Framework Directive (WFD). Directive 2000/60/EC of the European Parliament and of the Council of 23 October 2000 Establishing a Framework for Community Action in the Field of Water Policy (Water Framework Directive); Official Journal of the European Communities: Luxembourg, 2000. [Google Scholar]
- Ejsing, C.S.; Sampaio, J.L.; Surendranath, V.; Duchoslav, E.; Ekroos, K.; Klemm, R.W.; Simons, K.; Shevchenko, A. Global analysis of the yeast lipidome by quantitative shotgun mass spectrometry. Proc. Natl. Acad. Sci. USA 2009, 106, 2136–2141. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Gross, R.W. Shotgun lipidomics: Electrospray ionization mass spectrometric analysis and quantitation of cellular lipidomes directly from crude biological samples. Mass Spectrom. Rev. 2005, 24, 367–412. [Google Scholar] [CrossRef] [PubMed]
- Viant, M.R.; Rosenblum, E.; Tjeerdema, R.S. NMR-Based Metabolomics: A Powerful Approach for Characterizing the Effects of Environmental Stressors on Organism Health. Environ. Sci. Technol. 2003, 37, 4982–4989. [Google Scholar] [CrossRef] [PubMed]
- Williams, T.D.; Gensberg, K.; Minchin, S.D.; Chipman, J.K. A DNA expression array to detect toxic stress response in European flounder (Platichthys flesus). Aquat. Toxicol. 2003, 65, 141–157. [Google Scholar] [CrossRef]
- Wenk, M.R. Lipidomics: New Tools and Applications. Cell 2010, 143, 888–895. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dennis, E.A. Lipidomics joins the omics evolution. Proc. Natl. Acad. Sci. USA 2009, 106, 2089–2090. [Google Scholar] [CrossRef] [PubMed]
- Navas-Iglesias, N.; Carrasco-Pancorbo, A.; Cuadros-Rodrı´guez, L. From lipids analysis towards lipidomics, a new challenge for the analytical chemistry of the 21st century. Part II: Analytical lipidomics. Trends Anal. Chem. 2009, 28, 393–403. [Google Scholar] [CrossRef]
- Lísa, M.; Holčapek, M. High-throughput and comprehensive lipidomics analysis using ultrahigh-performance supercritical fluid chromatography-mass spectrometry. Anal. Chem. 2015, 87, 7187–7195. [Google Scholar] [CrossRef] [PubMed]
- Cajika, T.; Fiehn, O. Comprehensive analysis of lipids in biological systems by liquid chromatography-mass spectrometry. Trends Anal. Chem. 2014, 61, 192–206. [Google Scholar] [CrossRef] [PubMed]
- Jung, H.R.; Sylvänne, T.; Koistinen, K.M.; Tarasov, K.; Kauhanen, D.; Ekroos, K. High throughput quantitative molecular lipidomics. Biochim. Biophys. Acta 2011, 1811, 925–934. [Google Scholar] [CrossRef] [PubMed]
- Schevchenko, A.; Simons, K. Lipidomics: Coming to grips with lipid diversity. Nat. Rev. Mol. Cell Biol. 2010, 11, 593–598. [Google Scholar] [CrossRef] [PubMed]
- Degenkolbe, T.; Giavalisco, P.; Zuther, E.; Seiwert, B.; Hincha, D.K.; Willmitzer, L. Differential remodeling of the lipidome during cold acclimation in natural accessions of Arabidopsis thaliana. Plant J. 2012, 72, 972–982. [Google Scholar] [CrossRef] [PubMed]
- Shen, Q.; Wang, Y.; Gong, L.; Guo, R.; Dong, W.; Cheung, H.Y. Shotgun lipidomics strategy for fast analysis of phospholipids in fisheries waste and its potential in species differentiation. J. Agric. Food Chem. 2012, 60, 9384–9393. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.; Xu, J.; Chen, J.; Chen, D.; Xu, S.; Luo, Q.; Wang, Y. Lipidomics focusing on serum polar lipids reveals species dependent stress resistance of fish under tropical storm. Metabolomics 2012, 8, 299–309. [Google Scholar] [CrossRef]
- Sengupta, N.; Gerard, P.D.; Baldwin, W.S. Perturbations in polar lipids, starvation survival and reproduction following exposure to unsaturated fatty acids or environmental toxicants in Daphnia magna. Chemosphere 2016, 144, 2302–2311. [Google Scholar] [CrossRef] [PubMed]
- Scanlan, L.D.; Loguinov, A.V.; Teng, Q.; Antczak, P.; Dailey, K.P.; Nowinski, D.T.; Kornbluh, J.; Lin, X.X.; Lachenauer, E.; Arai, A.; et al. Gene Transcription, Metabolite and Lipid Profiling in Eco-Indicator Daphnia magna Indicate Diverse Mechanisms of Toxicity by Legacy and Emerging Flame-Retardants. Environ. Sci. Technol. 2015, 49, 7400–7410. [Google Scholar] [CrossRef] [PubMed]
- Stanley, J.K.; Perkins, E.J.; Habib, T.; Sims, J.G.; Chappell, P.; Escalon, B.L.; Wilbanks, M.; Garcia-Reyero, N. The Good, the Bad, and the Toxic: Approaching Hormesis in Daphnia magna Exposed to an Energetic Compound. Environ. Sci. Technol. 2013, 47, 9424–9433. [Google Scholar] [CrossRef] [PubMed]
- Bychek, E.A.; Dobson, G.A.; Harwood, J.L.; Guschina, I.A. Daphnia magna can tolerate short-term starvation without major changes in lipid metabolism. Lipids 2005, 40, 599–608. [Google Scholar] [CrossRef] [PubMed]
- Lampert, W. Daphnia: Development of a Model Organism in Ecology and Evolution. Excellence in Ecology; International Ecology Institute: Luhe, Germany, 2011; Volume 21. [Google Scholar]
- Colbourne, J.K.; Pfrender, M.E.; Gilbert, D.; Thomas, W.K.; Tucker, A.; Oakley, T.H.; Tokishita, S.; Aerts, A.; Arnold, G.J.; Basu, M.K.; et al. The ecoresponsive genome of Daphnia pulex. Science 2011, 331, 555–561. [Google Scholar] [CrossRef] [PubMed]
- Niki, E. Lipid peroxidation products as oxidative stress biomarkers. Biofactors 2008, 34, 171–180. [Google Scholar] [CrossRef] [PubMed]
- Horton, A.A.; Fairhurst, S. Lipid-peroxidation and mechanisms of toxicity. CRC Crit. Rev. Toxicol. 1987, 18, 27–79. [Google Scholar] [CrossRef] [PubMed]
- Reis, A.; Spickett, C.M. Chemistry of phospholipid oxidation. BBA-Biomembranes 2012, 1818, 2374–2387. [Google Scholar] [CrossRef] [PubMed]
- Lunec, J. Oxygen Radicals: Their Measurement In Vivo. Anal. Proc. 1989, 26, 130–131. [Google Scholar]
- Korizis, K.N.; Exarchou, A.; Michalopoulos, E.; Georgakopoulos, C.D.; Kolonitsiou, F.; Mantagos, S.; Gartaganis, S.P.; Karamanos, N.K. Determination of malondialdehyde by capillary electrophoresis, application to human plasma and relation of its levels with prematurity. Biomed. Chromatogr. 2001, 15, 287–291. [Google Scholar] [CrossRef] [PubMed]
- Spickett, C.M.; Wiswedel, I.; Siems, W.; Zarkovic, K.; Zarkovic, N. Advances in methods for the determination of biologically relevant lipid peroxidation products. Free Radic. Res. 2010, 44, 1172–1202. [Google Scholar] [CrossRef] [PubMed]
- Schuhmann, K.; Almeida, R.; Baumert, M.; Herzog, R.; Bornstein, S.R.; Shevchenko, A. Shotgun lipidomics on a LTQ Orbitrap mass spectrometer by successive switching between acquisition polarity modes. J. Mass Spectrom. 2012, 47, 96–104. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ji, J.; Kline, A.E.; Amoscato, A.; Samhan-Arias, A.K.; Sparvero, L.J.; Tyurin, V.A.; Tyurina, Y.Y.; Fink, B.; Manole, M.D.; Puccio, A.M.; et al. Lipidomics identifies cardiolipin oxidation as a mitochondrial target for redox therapy of brain injury. Nat. Neurosci. 2012, 15, 1407–1415. [Google Scholar] [CrossRef] [PubMed]
- Sparvero, L.J.; Amoscato, A.A.; Kochanek, P.M.; Pitt, B.R.; Kagan, V.E.; Bayir, H. Mass-spectrometry based oxidative lipidomics and lipid imaging: Applications in traumatic brain injury. J. Neurochem. 2010, 115, 1322–1336. [Google Scholar] [CrossRef] [PubMed]
- Kagan, V.E.; Borisenko, G.G.; Tyurina, Y.Y.; Tyurin, V.A.; Jiang, J.; Potapovich, A.I.; Kini, V.; Amoscato, A.; Fujii, Y. Oxidative lipidomics of apoptosis: Redox catalytic interactions of cytochrome C with Cardiolipin and phosphatidylserine. Free Radic. Biol. Med. 2004, 37, 1963–1985. [Google Scholar] [CrossRef] [PubMed]
- Jordão, R.; Casas, J.; Fabrias, G.; Campos, B.; Piña, B.; Lemos, M.F.L.; Soares, A.M.V.M.; Tauler, R.; Barata, C. Obesogens beyond Vertebrates: Lipid Perturbation by Tributyltin in the Crustacean Daphnia magna. Environ. Health Perspect. 2015, 123, 813–819. [Google Scholar] [CrossRef] [PubMed]
- Southam, A.D.; Weber, R.J.M.; Engel, J.; Jones, M.R.; Viant, M.R. A complete workflow for high-resolution spectral-stitching nanoelectrospray direct-infusion mass-spectrometry-based metabolomics and lipidomics. Nat. Protoc. 2016, 12, 255–273. [Google Scholar] [CrossRef] [PubMed]
- Weber, R.J.M.; Southam, A.D.; Sommer, U.; Viant, M.R. Characterization of Isotopic Abundance Measurements in High Resolution FT-ICR and Orbitrap Mass Spectra for Improved Confidence of Metabolite Identification. Anal. Chem. 2011, 83, 3737–3743. [Google Scholar] [CrossRef] [PubMed]
- Taylor, N.S.; Weber, R.J.M.; Southam, A.D.; Payne, T.G.; Hrydziuszko, O.; Arvanitis, T.N.; Viant, M.R. A new approach to toxicity testing in Daphnia magna: Application of high throughput FT-ICR mass spectrometry metabolomics. Metabolomics 2009, 5, 44–58. [Google Scholar] [CrossRef]
- Taylor, N.S.; Weber, R.J.M.; White, T.A.; Viant, M.R. Discriminating between different acute chemical toxicities via changes in the Daphnid metabolome. Toxicol. Sci. 2010, 118, 307–317. [Google Scholar] [CrossRef] [PubMed]
- Poynton, H.C.; Taylor, N.S.; Hicks, J.; Colson, K.; Chan, S.; Scanlan, L.; Loguinov, A.V.; Vulpe, C.; Viant, M.R. Metabolomics of microliter hemolymph samples enables an improved understanding of the combined metabolic and transcriptional responses of Daphnia magna to cadmium. Environ. Sci. Technol. 2011, 45, 3710–3717. [Google Scholar] [CrossRef] [PubMed]
- Gaetke, L.M.; Chow, C.K. Copper toxicity, oxidative stress, and antioxidant nutrients. Toxicology 2003, 189, 147–163. [Google Scholar] [CrossRef]
- Persson, J.; Vrede, T. Polyunsaturated fatty acids in zooplankton: Variation due to taxonomy and trophic position. Freshw. Biol. 2006, 51, 887–900. [Google Scholar] [CrossRef]
- Masclaux, H.; Bec, A.; Kainz, M.J.; Perriere, F.; Desvilettes, C.; Bourdier, G. Accumulation of polyunsaturated fatty acids by cladocerans: Effects of taxonomy, temperature and food. Freshw. Biol. 2012, 57, 696–703. [Google Scholar] [CrossRef]
- Tyurin, V.A.; Tyurina, Y.Y.; Feng, W.; Mnuskin, A.; Jiang, J.; Tang, M.; Zhang, X.; Zhao, Q.; Kochanek, P.M.; Clark, R.S.; et al. Mass spectrometric characterization of phospholipids and their primary peroxidation products in rat cortical neurons during staurosporine-induced apoptosis. J. Neurochem. 2008, 107, 1614–1633. [Google Scholar] [CrossRef] [PubMed]
- Vance, D.E.; Vance, J.E. Biochemistry of Lipids, Lipoproteins and Membranes, 5th ed.; Elsevie: Amsterdam, The Netherlands, 2008. [Google Scholar]
- Brandão, F.; Cappello, T.; Raimundo, J.; Santos, M.A.; Maisano, M.; Mauceri, A.; Pacheco, M.; Pereira, P. Unravelling the mechanisms of mercury hepatotoxicity in wild fish (Liza aurata) through a triad approach: Biaccumulations, metabolomic profiles and oxidative stress. Metallomics 2015, 7, 1352–1363. [Google Scholar] [PubMed]
- Cappello, T.; Brandao, F.; Guilherme, S.; Santos, M.A.; Maisano, M.; Mauceri, A.; Canário, J.; Pacheco, M.; Pereira, P. Insights into the mechanisms underlying mercury-induced oxidative stress in gills of wild fish (Liza aurata) combining 1H metabolomics and conventional biochemical assays. Sci. Total Environ. 2016, 548–549, 13–24. [Google Scholar] [PubMed]
- USEPA. ECOTOX Database. Available online: http://cfpub.epa.gov/ecotox/ (accessed on 13 January 2017).
- Watanabe, H.; Takahashi, E.; Nakamura, Y.; Oda, S.; Tatarazako, N.; Iguchi, T. Development of a Daphnia magna DNA microarray for evaluating the toxicity of environmental chemicals. Environ. Toxicol. Chem. 2007, 26, 669–676. [Google Scholar] [CrossRef] [PubMed]
- Organization for Economic Cooperation and Development (OECD). OECD Guidelines for Testing of Chemicals, No. 202, Daphnia sp. Acute Immobilisation Test; Organisation for Economic Cooperation and Development: Paris, France, 2004; p. 12. [Google Scholar]
- Schwarz, J.A.; Mitchelmore, C.L.; Jones, R.; O’Dea, A.; Seymour, S. Exposure to copper induces oxidative and stress responses and DNA damage in the coral Montastraea franksi. Comp. Biochem. Phys. C 2013, 157, 272–279. [Google Scholar] [CrossRef] [PubMed]
- Rhee, J.-S.; Yu, I.T.; Kim, B.M.; Jeong, C.B.; Lee, K.W.; Kim, M.J.; Lee, S.J.; Park, G.S.; Lee, J.S. Copper induces apoptotic cell death through reactive oxygen species-triggered oxidative stress in the intertidal copepod Tigriopus japonicus. Aquat. Toxicol. 2013, 132, 182–189. [Google Scholar] [CrossRef] [PubMed]
- Bligh, E.G.; Dyer, W.J. A rapid method of total lipid extraction and purification. Can. J. Biochem. Phys. 1959, 37, 911–917. [Google Scholar] [CrossRef] [PubMed]
- Yamada, N.; Murakami, N.; Morimoto, T.; Sakakibara, J. Auto-growth inhibitory substance from the fresh-water cyanobacterium Phormidium-tenue. Chem. Pharm. Bull. 1993, 41, 1863–1865. [Google Scholar] [CrossRef] [PubMed]
- Southam, A.D.; Payne, T.G.; Cooper, H.J.; Arvanitis, T.N.; Viant, M.R. Dynamic range and mass accuracy of wide-scan direct infusion nanoelectrospray fourier transform ion cyclotron resonance mass spectrometry-based metabolomics increased by the spectral stitching method. Anal. Chem. 2007, 79, 4595–4602. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Payne, T.G.; Southam, A.D.; Arvanitis, T.N.; Viant, M.R. A signal filtering method for improved quantification and noise discrimination in fourier transform ion cyclotron resonance mass spectrometry-based metabolomics data. J. Mass Spectrom. 2009, 20, 1087–1095. [Google Scholar] [CrossRef] [PubMed]
- Comisarow, M.B.; Melka, J.D. Error estimates for finite zero-filling in fourier transform spectrometry. Anal. Chem. 1979, 51, 2198–2203. [Google Scholar] [CrossRef]
- Dieterle, F.; Ross, A.; Schlotterbeck, G.; Senn, H. Probabilistic quotient normalization as robust method to account for dilution of complex biological mixtures. Application in H-1 NMR metabonomics. Anal. Chem. 2006, 78, 4281–4290. [Google Scholar] [CrossRef] [PubMed]
- Purohit, P.V.; Rocke, D.M.; Viant, M.R.; Woodruff, D.L. Discrimination models using variance-stabilizing transformation of metabolomic NMR data. Omics 2004, 8, 118–130. [Google Scholar] [CrossRef] [PubMed]
- Benjami, Y.; Hochberg, Y. Controlling the false discovery rate—A practical and powerful approach to multiple testing. J. R. Stat. Soc. B 1995, 57, 289–300. [Google Scholar]
- Sumner, L.W.; Amberg, A.; Barrett, D.; Beale, M.H.; Beger, R.; Daykin, C.A.; Fan, T.W.-M.; Fiehn, O.; Goodacre, R.; Griffin, J.L.; et al. Proposed minimum reporting standards for chemical analysis. Metabolomics 2007, 3, 211–221. [Google Scholar] [CrossRef] [PubMed]
- Weber, R.J.M.; Viant, M.R. MI-Pack: Increased confidence of metabolite identification in mass spectra by integrating accurate masses and metabolic pathways. Chemometr. Intell. Lab. 2010, 104, 75–82. [Google Scholar] [CrossRef]
- Fahy, E.; Subramaniam, S.; Murphy, R.C.; Nishijima, M.; Raetz, C.R.; Shimizu, T.; Spener, F.; van Meer, G.; Wakelam, M.J.; Dennis, E.A. Update of the LIPID MAPS comprehensive classification system for lipids. J. Lipid Res. 2009, 50, S9–S14. [Google Scholar] [CrossRef] [PubMed]
- Kerwin, J.L.; Wiens, A.M.; Ericsson, L.H. Identification of fatty acids by electrospray mass spectrometry and tandem mass spectrometry. J. Mass Spectrom. 1996, 31, 184–192. [Google Scholar] [CrossRef]

| Lipid Class | PA | PC | PE | PG | PI | PS | MGDG | Total |
|---|---|---|---|---|---|---|---|---|
| Number of putatively annotated lipid species | 30 | 87 | 107 | 32 | 35 | 80 | 3 | 374 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Taylor, N.S.; White, T.A.; Viant, M.R. Defining the Baseline and Oxidant Perturbed Lipidomic Profiles of Daphnia magna. Metabolites 2017, 7, 11. https://doi.org/10.3390/metabo7010011
Taylor NS, White TA, Viant MR. Defining the Baseline and Oxidant Perturbed Lipidomic Profiles of Daphnia magna. Metabolites. 2017; 7(1):11. https://doi.org/10.3390/metabo7010011
Chicago/Turabian StyleTaylor, Nadine S., Thomas A. White, and Mark R. Viant. 2017. "Defining the Baseline and Oxidant Perturbed Lipidomic Profiles of Daphnia magna" Metabolites 7, no. 1: 11. https://doi.org/10.3390/metabo7010011
APA StyleTaylor, N. S., White, T. A., & Viant, M. R. (2017). Defining the Baseline and Oxidant Perturbed Lipidomic Profiles of Daphnia magna. Metabolites, 7(1), 11. https://doi.org/10.3390/metabo7010011






