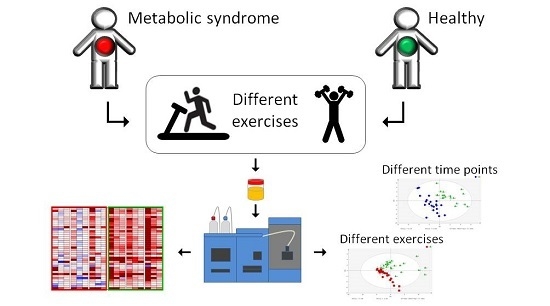
Effects of Different Exercise Modes on the Urinary Metabolic Fingerprint of Men with and without Metabolic Syndrome
Abstract
:1. Introduction
2. Results
2.1. Univariate Analysis
2.2. Multivariate Analysis
3. Discussion
4. Materials and Methods
4.1. Participants
4.2. Preliminary Testing
4.3. Experimental Protocol
4.4. Sample Collection and Preparation
4.5. UPLS-MS/MS Analysis
4.6. Data Handling and Statistical Analysis
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| AMP | adenosine monophosphate |
| ANOVA | analysis of variance |
| ATP | adenosine triphosphate |
| BCAAs | branched-chain amino acids |
| BMI | body mass index |
| CME | continuous moderate-intensity exercise |
| CrAT | carnitine O-acetyltransferase |
| HDL | high-density lipoprotein |
| HIIE | high-intensity interval exercise |
| HILIC | hydrophilic interaction |
| HOMA-IR | homeostasis model assessment–insulin resistance |
| HRmax | maximal heart rate |
| LC-MS | liquid chromatography–mass spectrometry |
| LDL | low-density lipoprotein |
| MetS | metaboli syndrome |
| PLS-DA | partial least square discriminant analysis |
| PTFE | polytetrafluoroethylene |
| RE | resistance exercise |
| RM | repetition maximum |
| UDP | uridine diphosphate |
| UPLC-MS/MS | ultraperformance liquid chromatography–tandem mass spectrometry |
| O2max | maximal oxygen uptake |
| VIP | variable importance on projection |
References
- Alberti, K.G.; Eckel, R.H.; Grundy, S.M.; Zimmet, P.Z.; Cleeman, J.I.; Donato, K.A.; Fruchart, J.C.; James, W.P.; Loria, C.M.; Smith, S.C., Jr.; et al. Harmonizing the metabolic syndrome: A joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation 2009, 120, 1640–1645. [Google Scholar] [PubMed]
- Braun, S.; Bitton-Worms, K.; LeRoith, D. The link between the metabolic syndrome and cancer. Int. J. Biol. Sci. 2011, 7, 1003–1015. [Google Scholar] [CrossRef] [PubMed]
- Ford, E.S. Risks for all-cause mortality, cardiovascular disease, and diabetes associated with the metabolic syndrome: A summary of the evidence. Diabetes Care 2005, 28, 1769–1778. [Google Scholar] [CrossRef] [PubMed]
- O’Neill, S.; O’Driscoll, L. Metabolic syndrome: A closer look at the growing epidemic and its associated pathologies. Obes. Rev. 2015, 16, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Eckel, R.H.; Grundy, S.M.; Zimmet, P.Z. The metabolic syndrome. Lancet 2005, 365, 1415–1428. [Google Scholar] [CrossRef]
- Batch, B.C.; Shah, S.H.; Newgard, C.B.; Turer, C.B.; Haynes, C.; Bain, J.R.; Muehlbauer, M.; Patel, M.J.; Stevens, R.D.; Appel, L.J.; et al. Branched chain amino acids are novel biomarkers for discrimination of metabolic wellness. Metabolism 2013, 62, 961–969. [Google Scholar] [CrossRef] [PubMed]
- Newgard, C.B.; An, J.; Bain, J.R.; Muehlbauer, M.J.; Stevens, R.D.; Lien, L.F.; Haqq, A.M.; Shah, S.H.; Arlotto, M.; Slentz, C.A.; et al. A branched-chain amino acid-related metabolic signature that differentiates obese and lean humans and contributes to insulin resistance. Cell Metab. 2009, 9, 311–326. [Google Scholar] [CrossRef] [PubMed]
- Bogl, L.H.; Kaye, S.M.; Ramo, J.T.; Kangas, A.J.; Soininen, P.; Hakkarainen, A.; Lundbom, J.; Lundbom, N.; Ortega-Alonso, A.; Rissanen, A.; et al. Abdominal obesity and circulating metabolites: A twin study approach. Metabolism 2016, 65, 111–121. [Google Scholar] [CrossRef] [PubMed]
- Wurtz, P.; Soininen, P.; Kangas, A.J.; Ronnemaa, T.; Lehtimaki, T.; Kahonen, M.; Viikari, J.S.; Raitakari, O.T.; Ala-Korpela, M. Branched-chain and aromatic amino acids are predictors of insulin resistance in young adults. Diabetes Care 2013, 36, 648–655. [Google Scholar] [CrossRef] [PubMed]
- Cheng, S.; Rhee, E.P.; Larson, M.G.; Lewis, G.D.; McCabe, E.L.; Shen, D.; Palma, M.J.; Roberts, L.D.; Dejam, A.; Souza, A.L.; et al. Metabolite profiling identifies pathways associated with metabolic risk in humans. Circulation 2012, 125, 2222–2231. [Google Scholar] [CrossRef] [PubMed]
- Wang-Sattler, R.; Yu, Z.; Herder, C.; Messias, A.C.; Floegel, A.; He, Y.; Heim, K.; Campillos, M.; Holzapfel, C.; Thorand, B.; et al. Novel biomarkers for pre-diabetes identified by metabolomics. Mol. Syst. Biol. 2012, 8, 615. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.F.; Cheng, M.L.; Fan, C.M.; Hong, C.Y.; Shiao, M.S. Nicotinuric acid: A potential marker of metabolic syndrome through a metabolomics-based approach. Diabetes Care 2013, 36, 1729–1731. [Google Scholar] [CrossRef] [PubMed]
- Gall, W.E.; Beebe, K.; Lawton, K.A.; Adam, K.P.; Mitchell, M.W.; Nakhle, P.J.; Ryals, J.A.; Milburn, M.V.; Nannipieri, M.; Camastra, S.; et al. alpha-hydroxybutyrate is an early biomarker of insulin resistance and glucose intolerance in a nondiabetic population. PLoS ONE 2010, 5, e10883. [Google Scholar] [CrossRef] [PubMed]
- Koves, T.R.; Ussher, J.R.; Noland, R.C.; Slentz, D.; Mosedale, M.; Ilkayeva, O.; Bain, J.; Stevens, R.; Dyck, J.R.; Newgard, C.B.; et al. Mitochondrial overload and incomplete fatty acid oxidation contribute to skeletal muscle insulin resistance. Cell Metab. 2008, 7, 45–56. [Google Scholar] [CrossRef] [PubMed]
- Pietilainen, K.H.; Sysi-Aho, M.; Rissanen, A.; Seppanen-Laakso, T.; Yki-Jarvinen, H.; Kaprio, J.; Oresic, M. Acquired obesity is associated with changes in the serum lipidomic profile independent of genetic effects—A monozygotic twin study. PLoS ONE 2007, 2, e218. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Z.; Klipfell, E.; Bennett, B.J.; Koeth, R.; Levison, B.S.; Dugar, B.; Feldstein, A.E.; Britt, E.B.; Fu, X.; Chung, Y.M.; et al. Gut flora metabolism of phosphatidylcholine promotes cardiovascular disease. Nature 2011, 472, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Lakka, T.A.; Laaksonen, D.E. Physical activity in prevention and treatment of the metabolic syndrome. Appl. Physiol. Nutr. Metab. 2007, 32, 76–88. [Google Scholar] [CrossRef] [PubMed]
- Roberts, C.K.; Hevener, A.L.; Barnard, R.J. Metabolic syndrome and insulin resistance: Underlying causes and modification by exercise training. Compr. Physiol. 2013, 3. [Google Scholar] [CrossRef]
- Whyte, L.J.; Gill, J.M.; Cathcart, A.J. Effect of 2 weeks of sprint interval training on health-related outcomes in sedentary overweight/obese men. Metabolism 2010, 59, 1421–1428. [Google Scholar] [CrossRef] [PubMed]
- Jorge, M.L.; de Oliveira, V.N.; Resende, N.M.; Paraiso, L.F.; Calixto, A.; Diniz, A.L.; Resende, E.S.; Ropelle, E.R.; Carvalheira, J.B.; Espindola, F.S.; et al. The effects of aerobic, resistance, and combined exercise on metabolic control, inflammatory markers, adipocytokines, and muscle insulin signaling in patients with type 2 diabetes mellitus. Metabolism 2011, 60, 1244–1252. [Google Scholar] [CrossRef] [PubMed]
- Pechlivanis, A.; Kostidis, S.; Saraslanidis, P.; Petridou, A.; Tsalis, G.; Mougios, V.; Gika, H.G.; Mikros, E.; Theodoridis, G.A. 1H NMR-based metabonomic investigation of the effect of two different exercise sessions on the metabolic fingerprint of human urine. J. Proteome Res. 2010, 9, 6405–6416. [Google Scholar] [CrossRef] [PubMed]
- Pechlivanis, A.; Kostidis, S.; Saraslanidis, P.; Petridou, A.; Tsalis, G.; Veselkov, K.; Mikros, E.; Mougios, V.; Theodoridis, G.A. 1H NMR study on the short- and long-term impact of two training programs of sprint running on the metabolic fingerprint of human serum. J. Proteome Res. 2013, 12, 470–480. [Google Scholar] [CrossRef] [PubMed]
- Lewis, G.D.; Farrell, L.; Wood, M.J.; Martinovic, M.; Arany, Z.; Rowe, G.C.; Souza, A.; Cheng, S.; McCabe, E.L.; Yang, E.; et al. Metabolic signatures of exercise in human plasma. Sci. Transl. Med. 2010, 2, 33ra7. [Google Scholar] [CrossRef] [PubMed]
- Oberbach, A.; Bluher, M.; Wirth, H.; Till, H.; Kovacs, P.; Kullnick, Y.; Schlichting, N.; Tomm, J.M.; Rolle-Kampczyk, U.; Murugaiyan, J.; et al. Combined proteomic and metabolomic profiling of serum reveals association of the complement system with obesity and identifies novel markers of body fat mass changes. J. Proteome Res. 2011, 10, 4769–4788. [Google Scholar] [CrossRef] [PubMed]
- Kuehnbaum, N.L.; Gillen, J.B.; Gibala, M.J.; Britz-McKibbin, P. Personalized metabolomics for predicting glucose tolerance changes in sedentary women after high-intensity interval training. Sci. Rep. 2014, 4. [Google Scholar] [CrossRef] [PubMed]
- Huffman, K.M.; Slentz, C.A.; Bateman, L.A.; Thompson, D.; Muehlbauer, M.J.; Bain, J.R.; Stevens, R.D.; Wenner, B.R.; Kraus, V.B.; Newgard, C.B.; et al. Exercise-induced changes in metabolic intermediates, hormones, and inflammatory markers associated with improvements in insulin sensitivity. Diabetes Care 2011, 34, 174–176. [Google Scholar] [CrossRef] [PubMed]
- Huffman, K.M.; Koves, T.R.; Hubal, M.J.; Abouassi, H.; Beri, N.; Bateman, L.A.; Stevens, R.D.; Ilkayeva, O.R.; Hoffman, E.P.; Muoio, D.M.; et al. Metabolite signatures of exercise training in human skeletal muscle relate to mitochondrial remodelling and cardiometabolic fitness. Diabetologia 2014, 57, 2282–2295. [Google Scholar] [CrossRef] [PubMed]
- Campbell, C.; Grapov, D.; Fiehn, O.; Chandler, C.J.; Burnett, D.J.; Souza, E.C.; Casazza, G.A.; Gustafson, M.B.; Keim, N.L.; Newman, J.W.; et al. Improved metabolic health alters host metabolism in parallel with changes in systemic xeno-metabolites of gut origin. PLoS ONE 2014, 9, e84260. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Gao, Y. Physiological conditions can be reflected in human urine proteome and metabolome. Expert Rev. Proteomics 2015, 12, 623–636. [Google Scholar] [CrossRef] [PubMed]
- Yokota, T.; Kinugawa, S.; Okita, K.; Hirabayashi, K.; Suga, T.; Hattori, M.; Nakagawa, Y.; Oyama-Manabe, N.; Shirato, H.; Tsutsui, H. Lower aerobic capacity was associated with abnormal intramuscular energetics in patients with metabolic syndrome. Hypertens. Res. 2011, 34, 1029–1034. [Google Scholar] [CrossRef] [PubMed]
- Sheffield-Moore, M.; Dillon, E.L.; Randolph, K.M.; Casperson, S.L.; White, G.R.; Jennings, K.; Rathmacher, J.; Schuette, S.; Janghorbani, M.; Urban, R.J.; et al. Isotopic decay of urinary or plasma 3-methylhistidine as a potential biomarker of pathologic skeletal muscle loss. J. Cachexia Sarcopenia Muscle 2014, 5, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Elliott, P.; Posma, J.M.; Chan, Q.; Garcia-Perez, I.; Wijeyesekera, A.; Bictash, M.; Ebbels, T.M.; Ueshima, H.; Zhao, L.; van Horn, L.; et al. Urinary metabolic signatures of human adiposity. Sci. Transl. Med. 2015, 7. [Google Scholar] [CrossRef] [PubMed]
- Haus, J.M.; Miller, B.F.; Carroll, C.C.; Weinheimer, E.M.; Trappe, T.A. The effect of strenuous aerobic exercise on skeletal muscle myofibrillar proteolysis in humans. Scand. J. Med. Sci. Sports 2007, 17, 260–266. [Google Scholar] [PubMed]
- Trappe, T.; Williams, R.; Carrithers, J.; Raue, U.; Esmarck, B.; Kjaer, M.; Hickner, R. Influence of age and resistance exercise on human skeletal muscle proteolysis: A microdialysis approach. J. Physiol. 2004, 554, 803–813. [Google Scholar] [CrossRef] [PubMed]
- Muoio, D.M.; Noland, R.C.; Kovalik, J.P.; Seiler, S.E.; Davies, M.N.; DeBalsi, K.L.; Ilkayeva, O.R.; Stevens, R.D.; Kheterpal, I.; Zhang, J.; et al. Muscle-specific deletion of carnitine acetyltransferase compromises glucose tolerance and metabolic flexibility. Cell Metab. 2012, 15, 764–777. [Google Scholar] [CrossRef] [PubMed]
- Seiler, S.E.; Martin, O.J.; Noland, R.C.; Slentz, D.H.; DeBalsi, K.L.; Ilkayeva, O.R.; An, J.; Newgard, C.B.; Koves, T.R.; Muoio, D.M. Obesity and lipid stress inhibit carnitine acetyltransferase activity. J. Lipid. Res. 2014, 55, 635–644. [Google Scholar] [CrossRef] [PubMed]
- Thyfault, J.P.; Rector, R.S.; Noland, R.C. Metabolic inflexibility in skeletal muscle: A prelude to the cardiometabolic syndrome? J. Cardiometab. Syndr. 2006, 1, 184–189. [Google Scholar] [CrossRef] [PubMed]
- Prior, S.J.; Ryan, A.S.; Stevenson, T.G.; Goldberg, A.P. Metabolic inflexibility during submaximal aerobic exercise is associated with glucose intolerance in obese older adults. Obesity (Silver Spring) 2014, 22, 451–457. [Google Scholar] [CrossRef] [PubMed]
- Ekman, C.; Elgzyri, T.; Strom, K.; Almgren, P.; Parikh, H.; Dekker Nitert, M.; Ronn, T.; Manderson Koivula, F.; Ling, C.; Tornberg, A.B.; et al. Less pronounced response to exercise in healthy relatives to type 2 diabetic subjects compared with controls. J. Appl. Physiol. 2015, 119, 953–960. [Google Scholar] [CrossRef] [PubMed]
- Baker, J.S.; McCormick, M.C.; Robergs, R.A. Interaction among Skeletal Muscle Metabolic Energy Systems during Intense Exercise. J. Nutr. Metab. 2010, 2010, 905612. [Google Scholar] [CrossRef] [PubMed]
- Peake, J.M.; Tan, S.J.; Markworth, J.F.; Broadbent, J.A.; Skinner, T.L.; Cameron-Smith, D. Metabolic and hormonal responses to isoenergetic high-intensity interval exercise and continuous moderate-intensity exercise. Am. J. Physiol. Endocrinol. Metab. 2014, 307, E539–E552. [Google Scholar] [CrossRef] [PubMed]
- Gerber, T.; Borg, M.L.; Hayes, A.; Stathis, C.G. High-intensity intermittent cycling increases purine loss compared with workload-matched continuous moderate intensity cycling. Eur. J. Appl. Physiol. 2014, 114, 1513–1520. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, T.; Koyama, H.; Kurajoh, M.; Shoji, T.; Tsutsumi, Z.; Moriwaki, Y. Biochemistry of uridine in plasma. Clin. Chim. Acta 2011, 412, 1712–1724. [Google Scholar] [CrossRef] [PubMed]
- Fritzen, A.M.; Lundsgaard, A.M.; Jeppesen, J.; Christiansen, M.L.; Bienso, R.; Dyck, J.R.; Pilegaard, H.; Kiens, B. 5’-AMP activated protein kinase alpha2 controls substrate metabolism during post-exercise recovery via regulation of pyruvate dehydrogenase kinase 4. J. Physiol. 2015, 593, 4765–4780. [Google Scholar] [CrossRef] [PubMed]
- Pechlivanis, A.; Papaioannou, K.G.; Tsalis, G.; Saraslanidis, P.; Mougios, V.; Theodoridis, G.A. Monitoring the Response of the Human Urinary Metabolome to Brief Maximal Exercise by a Combination of RP-UPLC-MS and 1H NMR Spectroscopy. J. Proteome Res. 2015, 14, 4610–4622. [Google Scholar] [CrossRef] [PubMed]
- Daskalaki, E.; Easton, C.; Watson, D.G. The application of metabolomic profiling to the effects of physical activity. Curr. Metab. 2014, 2, 233–263. [Google Scholar] [CrossRef]
- Grundy, S.M.; Cleeman, J.I.; Daniels, S.R.; Donato, K.A.; Eckel, R.H.; Franklin, B.A.; Gordon, D.J.; Krauss, R.M.; Savage, P.J.; Smith, S.C., Jr.; et al. Diagnosis and management of the metabolic syndrome: An American Heart Association/National Heart, Lung, and Blood Institute scientific statement: Executive Summary. Crit. Pathw. Cardiol. 2005, 4, 198–203. [Google Scholar] [CrossRef] [PubMed]
- Huh, J.Y.; Siopi, A.; Mougios, V.; Park, K.H.; Mantzoros, C.S. Irisin in response to exercise in humans with and without metabolic syndrome. J. Clin. Endocrinol. Metab. 2015, 100, E453–E457. [Google Scholar] [CrossRef] [PubMed]
- Dohoney, P.; Chromiak, J.A.; Lemire, D.; Abadie, B.R.; Kovacs, C. Prediction of one repetition maximum (1-RM) strength from a 4–6 RM and a 7–8 RM submaximal strength test in healthy young adult males. J. Exerc. Physiol. Online 2002, 5, 54–59. [Google Scholar]
- Harris, J.A.; Benedict, F.G. A Biometric Study of Human Basal Metabolism. Proc. Natl. Acad. Sci. USA 1918, 4, 370–373. [Google Scholar] [CrossRef] [PubMed]
- Lin, P.H.; Proschan, M.A.; Bray, G.A.; Fernandez, C.P.; Hoben, K.; Most-Windhauser, M.; Karanja, N.; Obarzanek, E.; Group, D.C.R. Estimation of energy requirements in a controlled feeding trial. Am. J. Clin. Nutr. 2003, 77, 639–645. [Google Scholar] [PubMed]
- Virgiliou, C.; Sampsonidis, I.; Gika, H.G.; Raikos, N.; Theodoridis, G.A. Development and validation of a HILIC- MS/MS multi-targeted method for metabolomics applications. Electrophoresis 2015, 36, 2215–2225. [Google Scholar] [CrossRef] [PubMed]
- Levy, J.C.; Matthews, D.R.; Hermans, M.P. Correct homeostasis model assessment (HOMA) evaluation uses the computer program. Diabetes Care 1998, 21, 2191–2192. [Google Scholar] [CrossRef] [PubMed]
- Helal, I.; Fick-Brosnahan, G.M.; Reed-Gitomer, B.; Schrier, R.W. Glomerular hyperfiltration: Definitions, mechanisms and clinical implications. Nat. Rev. Nephrol. 2012, 8, 293–300. [Google Scholar] [CrossRef] [PubMed]




| MetS (n = 9) | Healthy (n = 14) | |
|---|---|---|
| Age (years) | 46 ± 8 | 41 ± 7 |
| Weight (kg) | 100 ± 10 | 91 ± 15 |
| Height (m) | 1.80 ± 0.07 | 1.80 ± 0.07 |
| BMI (kg·m−2) | 31.0 ± 3.7 | 28.1 ± 4.2 |
| Waist circumference (cm) | 110 (103–117) | 99 (94–108) * |
| Body fat (% body weight) | 26.9 ± 5.5 | 22.7 ± 5.0 |
| Trunk fat (% area) | 37.7 ± 4.9 | 33.1 ± 7.3 |
| Visceral fat rating | 21 (15–24) | 15 (12–19) * |
| Serum glucose (mmol·L−1) | 5.9 (5.6–6.6) | 5.2 (5.0–5.4) *** |
| Serum triacylglycerols (mmol·L−1) | 1.9 (1.3–2.9) | 1.1 (0.9–1.5) ** |
| Serum total cholesterol (mmol·L−1) | 6.4 ± 1.3 | 5.2 ± 0.8 ** |
| HDL cholesterol (mmol·L−1) | 1.2 ± 0.3 | 1.4 ± 0.3 |
| LDL cholesterol (mmol·L−1) | 4.2 ± 1.2 | 3.1 ± 0.8 * |
| Systolic pressure (mm·Hg) | 140 ± 15 | 120 ± 9 *** |
| Diastolic pressure (mm·Hg) | 87 (84–98) | 76 (72–80) *** |
| O2max (mL·kg−1·min−1) | 31.1 ± 4.2 | 37.0 ± 4.1 ** |
| Resting heart rate (bpm) | 67 ± 10 | 64 ± 9 |
| HRmax (bpm) | 176 ± 11 | 179 ± 13 |
| HOMA2-IR | 3.06 ± 1.04 | 2.22 ± 0.82 * |
| Group | Exercise Mode | Time | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HIIE, 2 h | HIIE, 4 h | RE, 2 h | 2 h | 4 h | HIIE | RE | ||||||||||
| MetS vs. Healthy | MetS vs. Healthy | MetS vs. Healthy | HIIE vs. CME | CME vs. RE | HIIE vs. RE | CME vs. RE | 0 vs. 2 h | 0 vs. 4 h | 2 vs. 4 h | 2 vs. 24 h | 0 vs. 2 h | 0 vs. 4 h | 2 vs. 4 h | 2 vs. 24 h | 4 vs. 24 h | |
| 3-Methylhistidine | 1.69 ** | 1.03 ** | 1.63 | |||||||||||||
| 4-Hydroxyphenyllactate | −0.49 * | |||||||||||||||
| Acetylcarnitine | 1.07 * | |||||||||||||||
| Alanine | 1.84 ** | −0.58 *** | −0.61 *** | 3.16 *** | −0.65 *** | −0.73 *** | ||||||||||
| Arginine | −0.64 * | −0.35 * | −0.43 *** | 0.32 * | ||||||||||||
| Citrate | 0.26 ** | −0.35 *** | 0.48 *** | |||||||||||||
| Creatine | −0.45 *** | |||||||||||||||
| Creatinine | −0.53 *** | |||||||||||||||
| Cystine | −0.25 * | |||||||||||||||
| Cytosine | −0.29 | |||||||||||||||
| Dimethylamine | 0.51 *** | −0.22 *** | ||||||||||||||
| Glucose | 0.57 *** | −0.40 *** | ||||||||||||||
| Glutamate | −0.30 | |||||||||||||||
| Glutamine | 1.79 ** | 1.43 *** | −0.46 *** | |||||||||||||
| Guanine | 1.31 *** | 1.97 *** | 0.91 * | −0.74 *** | 2.69 *** | −0.77 *** | −0.76 * | |||||||||
| Histamine | −0.26 ** | |||||||||||||||
| Histidine | −0.29 * | |||||||||||||||
| Hypotaurine | 2.14 *** | |||||||||||||||
| Hypoxanthine | −0.39 | −0.77 *** | 10.21 *** | 1.57 | 6.38 *** | 7.01 *** | 1.85 *** | −0.55 *** | −0.89 *** | 16.78 *** | 7.45 *** | −0.94 *** | −0.90 *** | |||
| Inosine | −0.71 *** | 37.43 ** | 4.73 ** | 2.40 *** | 7.28 *** | −0.91 *** | ||||||||||
| Kynurenate | 0.81 | −0.55 *** | −0.51 *** | |||||||||||||
| Lactate | −0.93 ** | 60.84 *** | 21.59 *** | −0.92 ** | −0.96 *** | 58.70 *** | 5.83 | −0.87 *** | −0.98 *** | |||||||
| Lysine | 1.51 ** | 0.69 * | ||||||||||||||
| Methylamine | 0.65 ** | |||||||||||||||
| Monoisoamylamine | 0.68 * | −0.40 * | −0.36 *** | 1.41 *** | −0.50 *** | |||||||||||
| Proline | 0.81 * | |||||||||||||||
| Pyroglutamate | 1.47 *** | −0.50 *** | 1.85 *** | |||||||||||||
| Pyruvate | −0.75 *** | 16.54 *** | 18.25 *** | −0.90 *** | −0.94 *** | |||||||||||
| Riboflavin | −0.81 * | −0.76 | −0.69 * | |||||||||||||
| Serine | 1.13 *** | |||||||||||||||
| Sucrose | −0.72 * | |||||||||||||||
| Thiamine | −0.58 | −0.51 * | 0.42 | |||||||||||||
| Threonine | 1.70 ** | |||||||||||||||
| Thymine | 1.88 ** | |||||||||||||||
| Trimethylamine | −0.44 *** | 0.19 | ||||||||||||||
| Uracil | −0.27 * | |||||||||||||||
| Uridine | 0.53 | −0.41 * | −0.44 * | −0.43 ** | −0.13 | −0.39 * | ||||||||||
| Xanthine | 0.94 *** | −0.55 *** | −0.51 *** | −0.51 *** | ||||||||||||
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Siopi, A.; Deda, O.; Manou, V.; Kellis, S.; Kosmidis, I.; Komninou, D.; Raikos, N.; Christoulas, K.; Theodoridis, G.A.; Mougios, V. Effects of Different Exercise Modes on the Urinary Metabolic Fingerprint of Men with and without Metabolic Syndrome. Metabolites 2017, 7, 5. https://doi.org/10.3390/metabo7010005
Siopi A, Deda O, Manou V, Kellis S, Kosmidis I, Komninou D, Raikos N, Christoulas K, Theodoridis GA, Mougios V. Effects of Different Exercise Modes on the Urinary Metabolic Fingerprint of Men with and without Metabolic Syndrome. Metabolites. 2017; 7(1):5. https://doi.org/10.3390/metabo7010005
Chicago/Turabian StyleSiopi, Aikaterina, Olga Deda, Vasiliki Manou, Spyros Kellis, Ioannis Kosmidis, Despina Komninou, Nikolaos Raikos, Kosmas Christoulas, Georgios A. Theodoridis, and Vassilis Mougios. 2017. "Effects of Different Exercise Modes on the Urinary Metabolic Fingerprint of Men with and without Metabolic Syndrome" Metabolites 7, no. 1: 5. https://doi.org/10.3390/metabo7010005
APA StyleSiopi, A., Deda, O., Manou, V., Kellis, S., Kosmidis, I., Komninou, D., Raikos, N., Christoulas, K., Theodoridis, G. A., & Mougios, V. (2017). Effects of Different Exercise Modes on the Urinary Metabolic Fingerprint of Men with and without Metabolic Syndrome. Metabolites, 7(1), 5. https://doi.org/10.3390/metabo7010005