Metabolic Alterations in Male-Sterile Potato as Compared to Male-Fertile
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Sample Preparation
2.3. Gas Chromatography Coupled with Mass Spectrometry (Gc-Ms)
2.4. Metabolite Identification and Quantification
2.5. Statistical Analysis of Metabolomic Data
2.6. Assessment of the Frequency of Abnormalities in the First and the Second Meiotic Divisions
2.7. Pollen Viability Assay
2.8. Aniline Blue Staining
2.9. Measurement and Morphological Observation of Pollen Grains
3. Results
3.1. Metabolome Profiling of Anthers at the Mature Pollen Stage in Male-Sterile and Male-Fertile Potato Genotypes
3.2. Assessment of the Frequency of Abnormalities in the First and the Second Meiotic Divisions
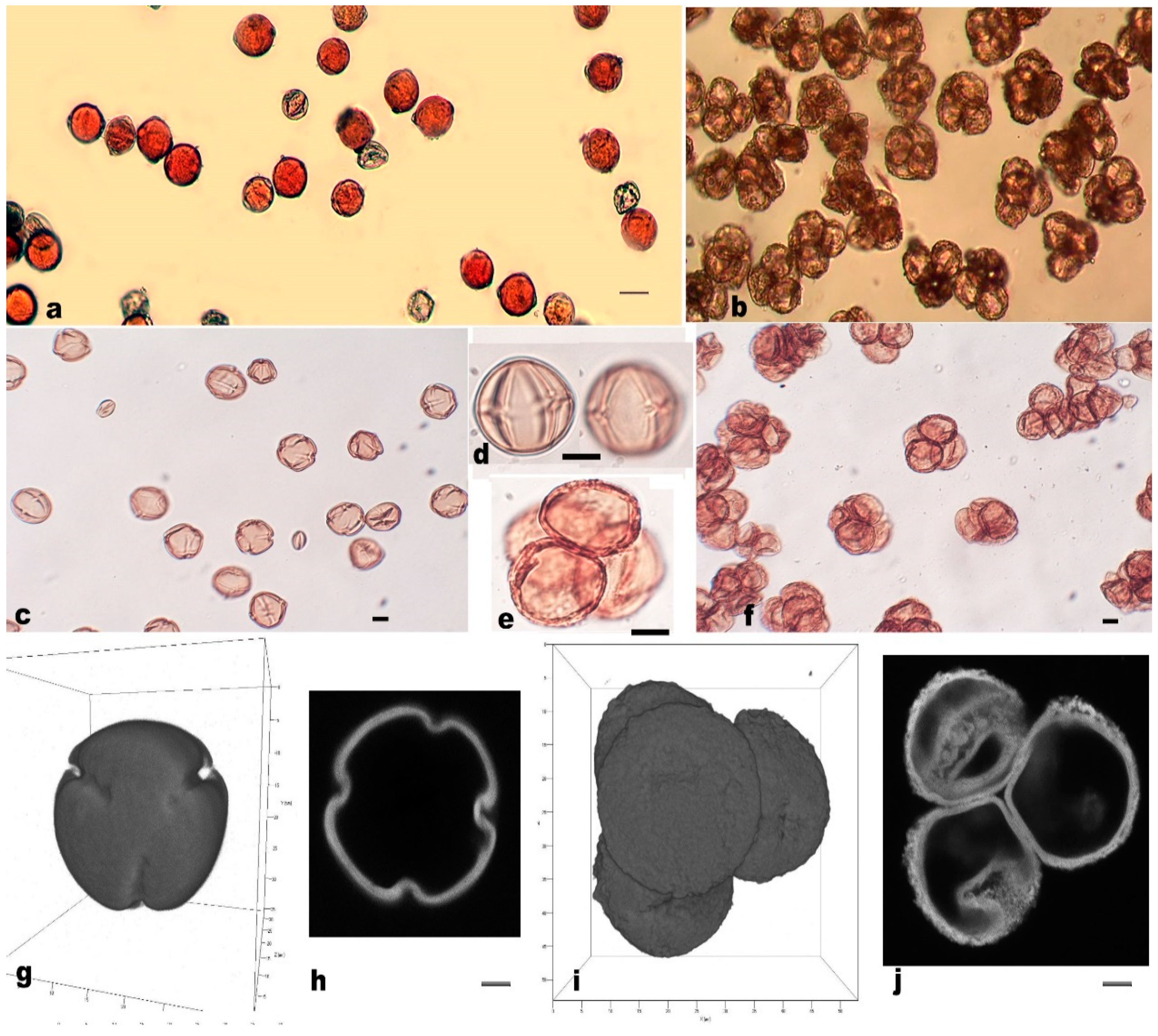
3.3. Measurement and Morphological Observation of Pollen Grains
4. Discussion
5. Conclusion and Out Look
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of interest
References
- Lindhout, P.; Meijer, D.; Schotte, T.; Hutten, R.C.B.; Visser, R.G.F.; Van Eck, H.J. Towards F1 hybrid seed potato breeding. Potato Res. 2011, 54, 301–312. [Google Scholar] [CrossRef]
- Jansky, S.H.; Charkowski, A.O.; Douches, D.S.; Gusmini, G.; Richael, C.; Bethke, P.C.; Spooner, D.M.; Novy, R.G.; DeJong, H.; DeJong, W.S.; et al. Reinventing potato as a diploid inbred line-basedcrop. Crop. Sci. 2016, 56, 1412–1422. [Google Scholar] [CrossRef]
- Lindhout, P.; De Vries, M.; Ter Maat, M.; Ying, S.; Viquez-Zamora, M.; Van Heusden, S. Hybrid potato breeding for improved varieties. In Achieving Sustainable Cultivation of Potatoes; Wang-Pruski, G., Ed.; Breeding, Nutritional and Sensory Quality; Burleigh Dodds Science Publishing: Cambridge, UK, 2017; Volume 1, pp. 99–124. [Google Scholar]
- Hosaka, K.; Sanetomo, R. Development of a rapid identification method for potato cytoplasm and its use for evaluating Japanese collections. Theor. Appl. Genet. 2012, 125, 1237–1251. [Google Scholar] [CrossRef] [PubMed]
- Mihovilovich, E.; Sanetomo, R.; Hosaka, K.; Ordonez, B.; Aponte, M.; Bonierbale, M. Cytoplasmic diversity in potato breeding: Case study from the International Potato Center. Mol. Breed. 2015, 35, 137–146. [Google Scholar] [CrossRef]
- Anisimova, I.N.; Gavrilenko, T.A. Cytoplasmic male sterility and prospects for its utilization in potato breeding, genetic studies and hybrid seed production. Russ. J. Genet. Appl. Res. 2017, 7, 721–735. [Google Scholar] [CrossRef]
- Sanetomo, R.; Gebhardt, C. Cytoplasmic genome types of European potatoes and their effects on complex agronomic traits. BMC Plant Biol. 2015, 15, 162. [Google Scholar] [CrossRef] [PubMed]
- Lössl, A.; Adler, N.; Horn, R.; Frei, U.; Wenzel, G. Chondriome type characterization of potato: Mt α, β, γ, δ, ε, and novel plastid-mitochondrial configurations. Theor. Appl. Genet. 1999, 99, 1–10. [Google Scholar] [CrossRef]
- Song, Y.-S.; Schwarzfischer, A. Development of STS markers for selection of extreme resistance (Rysto) to PVY and maternal pedigree analysis of extremely resistant cultivars. Am. J. Potato Res. 2008, 85, 159–170. [Google Scholar] [CrossRef]
- Abdalla, M.M.F.; Hermsen, J.G.T. Plasmon and male sterility types in Solanum verrucosum and its interspecific hybrid derivate. Euphytica 1972, 21, 209–220. [Google Scholar] [CrossRef]
- Ortiz, R.; Iwanaga, M.; Peloquin, S.J. Male sterility and 2n pollen in 4x progenies derived from 4x × 2x and 4x × 4x crosses in potatoes. Potato Res. 1993, 36, 227–236. [Google Scholar] [CrossRef]
- Zoteyeva, N.M.; Antonova, O.Y.; Klimenko, N.S.; Apalikova, O.V.; Carlson-Nilsson, U.; Karabitsina, Y.I.; Ukhatova, Y.V.; Gavrilenko, T.A. Facilitation of introgressive hybridization of wild polyploid mexican potato species using DNA markers of R genes and of different cytoplasmic types. Agric. Biol. 2017, 52, 964–975. [Google Scholar] [CrossRef]
- Rhee, S.Y.; Osborne, E.; Poindexter, P.D.; Somerville, C.R. Microspore separation in the quartet 3 mutants of Arabidopsis is impaired by a defect in a developmentally regulated polygalacturonase required for pollen mother. Plant Physiol. 2003, 133, 1170–1180. [Google Scholar] [CrossRef] [PubMed]
- Lu, P.; Chai, M.; Yang, J.; Ning, G.; Wang, G.; Ma, H. The Arabidopsis CALLOSE DEFECTIVE MICROSPORE1 gene is required for male fertility through regulating callose metabolism during microsporogenesis. Plant Physiol. 2014, 164, 1893–1904. [Google Scholar] [CrossRef] [PubMed]
- Omidvar, V.; Mohorianu, I.; Dalmay, T.; Zheng, Y.; Fei, Z.; Pucci, A.; Mazzucato, A.; Večeřová, V.; Sedlářova, M.; Fellner, M. Transcriptional regulation of male-sterility in 7B-1 male-sterile tomato mutant. PLoS ONE 2017, 12, e0170715. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Dun, X.; Zhou, Z.; Xia, S.; Yi, B.; Wen, J.; Shen, J.; Ma, C.; Tu, J.; Fu, T. A separation defect of tapetum cells and microspore mother cells results in male sterility in Brassica napus: The role of abscisic acid in early anther development. Plant Mol. Biol 2009, 72, 111–123. [Google Scholar] [CrossRef] [PubMed]
- Engelke, T.; Tatlioglu, T. A PCR-marker for the CMS(1) inducing cytoplasm in chives derived from recombination events affecting the mitochondrial gene atp9. Theor. Appl. Genet. 2002, 104, 698–702. [Google Scholar] [CrossRef] [PubMed]
- Winiarczyk, K.; Jaroszuk-Ściseł, J.; Kupisz, K. Characterization of callase (β-1,3-d-glucanase) activity during microsporogenesis in the sterile anthers of Allium sativum L. and the fertile anthers of A. atropurpureum. Sex. Plant Reprod. 2012, 25, 123–131. [Google Scholar] [CrossRef] [PubMed]
- Jin, W.; Horner, H.T.; Reid, G.; Shoemaker, R.C. Analysis and mapping of gene families encoding β-13-glucanases of soybean. Genetics 1999, 153, 445–452. [Google Scholar] [PubMed]
- Dorion, S.; Lalonde, S.; Saini, H.S. Induction of male sterility in wheat by meiotic-stage water deficit is preceded by a decline in invertase activity and changes in carbohydrate metabolism in anthers. Plant Physiol. 1996, 111, 137–145. [Google Scholar] [CrossRef]
- Goetz, M.; Godt, D.E.; Guivarch, A.; Kahmann, U.; Chriqui, D.; Roitsch, T. Induction of male sterility in plants by metabolic engineering of the carbohydrate supply. Proc. Natl. Acad. Sci. USA 2001, 98, 6522–6527. [Google Scholar] [CrossRef]
- Pressman, E.; Shaked, R.; Shen, S.; Altahan, L.; Firon, N. Variations in carbohydrate content and sucrose-metabolizing enzymes in tomato (Solanum lycopersicum L.) stamen parts during pollen maturation. Am. J. Plant Sci. 2012, 3, 252–260. [Google Scholar] [CrossRef]
- Yang, K.; Zhou, X.; Wang, Y.; Feng, H.; Ren, X.; Liu, H.; Liu, W. Carbohydrate metabolism and gene regulation during anther development in an androdioecious tree, Tapiscia sinensis. Ann. Bot. 2017, 120, 967–977. [Google Scholar] [CrossRef] [PubMed]
- McCormick, S. Control of male gametophyte development. Plant Cell 2004, 16, S142–S153. [Google Scholar] [CrossRef] [PubMed]
- Piffanelli, P.; Ross, J.H.E.; Murphy, D.J. Biogenesis and function of the lipidic structures of pollen grains. Sex. Plant Reprod. 1998, 11, 65–80. [Google Scholar] [CrossRef]
- Ariizumi, T.; Toriyama, K. Genetic regulation of sporopollenin synthesis and pollen exine development. Annu. Rev. Plant Biol. 2011, 62, 437–460. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Guo, Z.-L.; Zhou, W.-T.; Zhang, C.; Zhang, Z.-Y.; Lou, Y.; Xiong, S.-X.; Yao, X.-Z.; Fan, J.-J.; Zhu, J.; et al. The regulation of sporopollenin biosynthesis genes for rapid pollen wall formation. Plant Physiol. 2018, 178, 283–294. [Google Scholar] [CrossRef] [PubMed]
- Gavrilenko, Т.А.; Klimenko, N.S.; Antonova, O.Y.; Lebedeva, V.A.; Evdokimova, Z.Z.; Gadjiyev, N.M.; Apalikova, O.V.; Alpatyeva, N.V.; Kostina, L.I.; Zoteyeva, N.M.; et al. Molecular screening of potato varieties bred in the northwestern zone of the Russian Federаtion. Vavilov J. Genet. Breed. 2018, 22, 35–45. (In Russian) [Google Scholar] [CrossRef]
- Antonova, O.Y.; Klimenko, N.S.; Evdokimova, Z.Z.; Kostina, L.I.; Gavrilenko, T.A. Finding RB/Rpi-blb1/Rpi-sto1-like sequences in conventionally bred potato varieties. Vavilov J. Genet. Breed. 2018, 22, 693–702. [Google Scholar] [CrossRef]
- Evdokimova, Z.Z.; Kalashnik, M.B. Potentsial slozhnykh mezhvidovykh gibridov kartofelya po ustoichivosti k boleznyam i drugim khozyaistvenno-tsennym priznakam. [The potential of complex interspecific hybrids of potato on resistance to diseases and other economically valuable traits]. Proc. Kuban SAU 2016, 3, 73–76. (In Russian) [Google Scholar]
- Johnsen, L.G.; Skou, P.B.; Khakimov, B.; Bro, R. Gas chromatography—Mass spectrometry data processing made easy. J. Chromatogr. A 2017, 1503, 57–64. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2016. [Google Scholar]
- Hastie, T.; Tibshirani, R.; Friedman, J. The Elements of Statistical Learning. Data Mining, Inference, and Prediction. In Springer Series in Statistics, 2nd ed.; Springer: Berlin, Germany, 2017; 745p. [Google Scholar]
- Gu, Z.; Eils, R.; Schlesner, M. Complex heatmaps reveal patterns and correlations in multidimensional genomic data. Bioinformatics 2016, 32, 2847–2849. [Google Scholar] [CrossRef] [PubMed]
- Stacklies, W.; Redestig, H.; Scholz, M.; Walther, D.; Selbig, J. pcaMethods-a bioconductor package providing PCA methods for incomplete data. Bioinformatics 2007, 23, 1164–1167. [Google Scholar] [CrossRef] [PubMed]
- Schoelkopf, B.; Smola, A.; Mueller, K.-R. Nonlinear component analysis as a kernel eigenvalue problem. Neural Comput. 1998, 10, 1299–1319. [Google Scholar] [CrossRef]
- Karatzoglou, A.; Smola, A.; Hornik, K.; Zeileis, A. kernlab—An S4 package for kernel methods in R. J. Stat. Softw. 2004, 11, 1–20. [Google Scholar] [CrossRef]
- Bartenhagen, C. RDRToolbox: A Package for Nonlinear Reduction with Isomap and LLE. 2004. R Package Version 1.24.0. Available online: https://bioconductor.org/packages/release/bioc/html/RDRToolbox.html (accessed on 30 November 2018).
- Liaw, A.; Wiener, M. Classification and regression by random Forest. R News 2002, 2, 18–22. [Google Scholar]
- Thevenot, E.A.; Roux, A.; Xu, Y.; Ezan, E.; Junot, C. Analysis of the human adult urinary metabolome variations with age, body mass index and gender by implementing a comprehensive workflow for univariate OPLS statistical analyses. J. Proteome Res. 2015, 14, 3322–3335. [Google Scholar] [CrossRef] [PubMed]
- Shannon, P.; Markiel, A.; Ozier, O.; Baliga, N.S.; Wang, J.T.; Ramage, D.; Amin, N.; Schwikowski, B.; Ideker, T. Cytoscape: A software environment for integrated models of biomolecular interaction networks. Genome Res. 2003, 13, 2498–2504. [Google Scholar] [CrossRef]
- Anderson, M.J. A new method for non-parametric multivariate analysis of variance. Austral Ecol. 2001, 26, 32–46. [Google Scholar]
- Oksanen, J.; Blanchet, F.G.; Friendly, M.; Kindt, R.; Legendre, P.; McGlinn, D.; Minchin, P.R.; O’Hara, R.B.; Simpson, G.L.; Solymos, P.; et al. Vegan: Community Ecology Package. R Package Version 2.5-3. 2018. Available online: https://CRAN.R-project.org/package=vegan (accessed on 27 December 2018).
- Alexander, M.P. Differential staining of aborted and nonaborted pollen. Stain Technol. 1969, 44, 117–122. [Google Scholar] [CrossRef]
- Regan, S.M.; Moffatt, B.A. Cytochemical analysis of pollen development in wild-type Arabidopsis and a male-sterile mutant. Plant Cell 1990, 2, 877–888. [Google Scholar] [CrossRef]
- Erdtman, G. The acetolysis method: A revised description. Sven. Bot. Tidskr. 1960, 54, 561–564. [Google Scholar]
- Gavrilova, O.A. Application of confocal laser scanning microscopy to study pollen wall morphology. Bot. Zhurnal 2014, 99, 1139–1147. (In Russian) [Google Scholar]
- Banga, S.S.; Labana, K.S.; Banga, S.K. Male sterility in Indian mustard (Brassica juncea (L.) Coss.)—A biochemical characterization. Theor. Appl. Genet. 1984, 67, 515–519. [Google Scholar] [CrossRef] [PubMed]
- Oliver, S.N.; Van Dongen, J.T.; Alfred, S.C.; Mamun, E.A.; Zhao, X.; Saini, H.S.; Fernandes, S.F.; Blanchard, C.L.; Sutton, B.G.; Geigenberger, P.; et al. Cold-induced repression of the rice anther-specific cell wall invertase gene OSINV4 is correlated with sucrose accumulation and pollen sterility. Plant Cell Environ. 2005, 28, 1534–1551. [Google Scholar] [CrossRef]
- Oliver, S.N.; Dennis, E.S.; Dolferus, R. ABA regulates apoplastic sugar transport and is a potential signal for cold-induced pollen sterility in rice. Plant Cell Physiol. 2007, 48, 1319–1330. [Google Scholar] [CrossRef] [PubMed]
- Dobrovolskaya, A.A.; Rodionova, G.B.; Voronkov, A.S.; Kovaleva, L.V. Sporophyte-gametophyte interactions in the anther-male-gametophyte system of petunia. Russ. J. Plant Physiol. 2009, 56, 394–401. [Google Scholar] [CrossRef]
- Hedhly, A.; Vogler, H.; Schmid, M.W.; Pazmino, D.; Gagliardini, V.; Santelia, D.; Grossniklaus, U. Starch turnover and metabolism during flower and early embryo development. Plant Physiol. 2016, 172, 2388–2402. [Google Scholar] [CrossRef] [PubMed]
- Kong, J.; Li, Z.; Tan, Y.-P.; Wan, C.-X.; Li, S.-Q.; Zhu, Y.-G. Different gene expression patterns of sucrose-starch metabolism during pollen maturation in cytoplasmic male-sterile and male-fertile lines of rice. Physiol. Plant 2007, 130, 136–147. [Google Scholar] [CrossRef]
- Wu, Y.; Min, L.; Wu, Z.; Yang, L.; Zhu, L.; Yang, X.; Yuan, D.; Guo, X.; Zhang, X. Defective pollen wall contributes to male sterility in the male sterile line 1355A of cotton. Sci. Rep. 2015, 5, 9608. [Google Scholar] [CrossRef]
- Jia, S.S.; Wang, Y.; Hu, J.H.; Ding, Z.T.; Liang, Q.; Zhang, Y.F.; Wang, H. Mineral and metabolic profiles in tea leaves and flowers during flower development. Plant Physiol. Biochem. 2016, 106, 316–326. [Google Scholar] [CrossRef]
- Tang, H.; Song, Y.; Guo, J.; Wang, J.; Zhang, L.; Niu, N.; Ma, S.; Zhang, G.; Zhao, H. Physiological and metabolome changes during anther development in wheat (Triticum aestivum L.). Plant Physiol. Biochem. 2018, 132, 18–32. [Google Scholar] [CrossRef] [PubMed]
- Puzanskiy, R.K.; Yemelyanov, V.V.; Shavarda, A.L.; Gavrilenko, T.A.; Shishova, M.F. Age- and organ-specific differences of potato (Solanum phureja) plants metabolome. Russ. J. Plant Physiol. 2018, 65, 813–823. [Google Scholar] [CrossRef]
- Yue, Y.; Tian, S.; Wang, Y.; Ma, H.; Liu, S.; Wang, Y.; Hu, H. Transcriptomic and GC-MS metabolomic analyses reveal the sink strength changes during petunia anther development. Int. J. Mol. Sci. 2018, 9, 955. [Google Scholar] [CrossRef] [PubMed]
- Dobritzsch, S.; Weyhe, M.; Schubert, R.; Dindas, J.; Hause, G.; Kopka, J.; Hause, B. Dissection of jasmonate functions in tomato stamen development by transcriptome and metabolome analyses. BMC Biol. 2015, 13, 28. [Google Scholar] [CrossRef] [PubMed]
- Fragkostefanakis, S.; Mesihovic, A.; Simm, S.; Paupière, M.J.; Hu, Y.; Paul, P.; Mishra, S.K.; Tschiersch, B.; Theres, K.; Bovy, A.; et al. HsfA2 controls the activity of developmentally and stress-regulated heat stress protection mechanisms in tomato male reproductive tissues. Plant Physiol. 2016, 170, 2461–2477. [Google Scholar] [CrossRef] [PubMed]
- Obermeyer, G.; Fragner, L.; Lang, V.; Weckwerth, W. Dynamic adaption of metabolic pathways during germination and growth of lily pollen tubes after inhibition of the electron transport chain. Plant Physiol. 2013, 162, 1822–1833. [Google Scholar] [CrossRef] [PubMed]
- Seki, M.; Umezawa, T.; Urano, K.; Shinizaki, K. Regulatory metabolic networks in drought stress responses. Curr. Opin. Plant Biol. 2007, 10, 296–302. [Google Scholar] [CrossRef] [PubMed]
- Mukaiyama, A.; Koga, Y.; Takano, K.; Kayana, S. Osmolyte effect on the stability and folding of a hyperthermophilic protein. Protein Struct. Funct. Genet. 2008, 71, 110–118. [Google Scholar] [CrossRef]
- Ünal, M.; Vardar, F.; Aytürk, Ö. Callose in plant sexual reproduction. In Current Progress in Biological Research; Silva-Opps, M., Ed.; IntechOpen: London, UK, 2013; Available online: https://www.intechopen.com/books/current-progress-in-biological-research/callose-in-plant-sexual-reproduction (accessed on 30 November 2018).
- Zhang, D.B.; Shi, J.X.; Yang, X.J. Role of lipid metabolism in plant pollen exine development. In Lipids in Plant and Algae Development; Subcellular Biochemistry; Nakamura, Y., Li-Beisson, Y., Eds.; Springer: Berlin, Germany, 2016; Volume 86, pp. 315–337. [Google Scholar]
- Kim, S.S.; Douglas, C.J. Sporopollenin Monomer Biosynthesis in Arabidopsis. J. Plant Biol. 2013, 56, 1–6. [Google Scholar] [CrossRef]
- Mackenzie, G.; Boa, A.N.; Diego-Taboada, A.; Atkin, S.L.; Sathyapalan, T. Sporopollenin, the least known yet toughest natural biopolymer. Front. Mater. 2015, 2, 66. [Google Scholar] [CrossRef]
- Blackmore, S.; Wortley, A.H.; Skvarla, J.J.; Rowley, J.R. Pollen wall development in flowering plants. New Phytol. 2007, 174, 483–498. [Google Scholar] [CrossRef] [PubMed]





| # | Genotype | Designations | Pedigree Information * | Cytoplasm Type * | % of Normal Pollen Grains Stained with Acetocarmine |
|---|---|---|---|---|---|
| Male-sterile genotypes: | |||||
| 1 | cv. Gusar | G | cv. Arosa × cv. Vdokhnoveniye | W/Gamma | 0 |
| 2 | cv. Sudarynja | S | 8889/3 × 89181/6 | W/Gamma | 0 |
| 3 | cv. Evraziya | E | 95100/27 × 943/9 | W/Gamma | 2.3% |
| 4 | 1604/16 | 1604/16 | 95100/27 × 943/6 | W/Gamma | 0 |
| Male-fertile genotypes: | |||||
| 5 | 1101/10 | 1101/10 | cv. Charodej × 943/9 | W/alpha(D) | 76.2% |
| 6 | 211/9 | 211/9 | cv. Charodej × 943/6 | W/alpha(D) | 90.9% |
| 7 | 2103/7 | 2103/7 | - | W/alpha(D) | 89.9% |
| 8 | cv. Lomonosovskij | L | 89287/1 × 8334/20 | W/alpha(D) | 73.0% |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shishova, M.; Puzanskiy, R.; Gavrilova, O.; Kurbanniazov, S.; Demchenko, K.; Yemelyanov, V.; Pendinen, G.; Shavarda, A.; Gavrilenko, T. Metabolic Alterations in Male-Sterile Potato as Compared to Male-Fertile. Metabolites 2019, 9, 24. https://doi.org/10.3390/metabo9020024
Shishova M, Puzanskiy R, Gavrilova O, Kurbanniazov S, Demchenko K, Yemelyanov V, Pendinen G, Shavarda A, Gavrilenko T. Metabolic Alterations in Male-Sterile Potato as Compared to Male-Fertile. Metabolites. 2019; 9(2):24. https://doi.org/10.3390/metabo9020024
Chicago/Turabian StyleShishova, Maria, Roman Puzanskiy, Olga Gavrilova, Shamuhommed Kurbanniazov, Kirill Demchenko, Vladislav Yemelyanov, Galina Pendinen, Alexey Shavarda, and Tatjana Gavrilenko. 2019. "Metabolic Alterations in Male-Sterile Potato as Compared to Male-Fertile" Metabolites 9, no. 2: 24. https://doi.org/10.3390/metabo9020024
APA StyleShishova, M., Puzanskiy, R., Gavrilova, O., Kurbanniazov, S., Demchenko, K., Yemelyanov, V., Pendinen, G., Shavarda, A., & Gavrilenko, T. (2019). Metabolic Alterations in Male-Sterile Potato as Compared to Male-Fertile. Metabolites, 9(2), 24. https://doi.org/10.3390/metabo9020024







